Mosquito Mycobiota: An Overview of Non-Entomopathogenic Fungal Interactions
Abstract
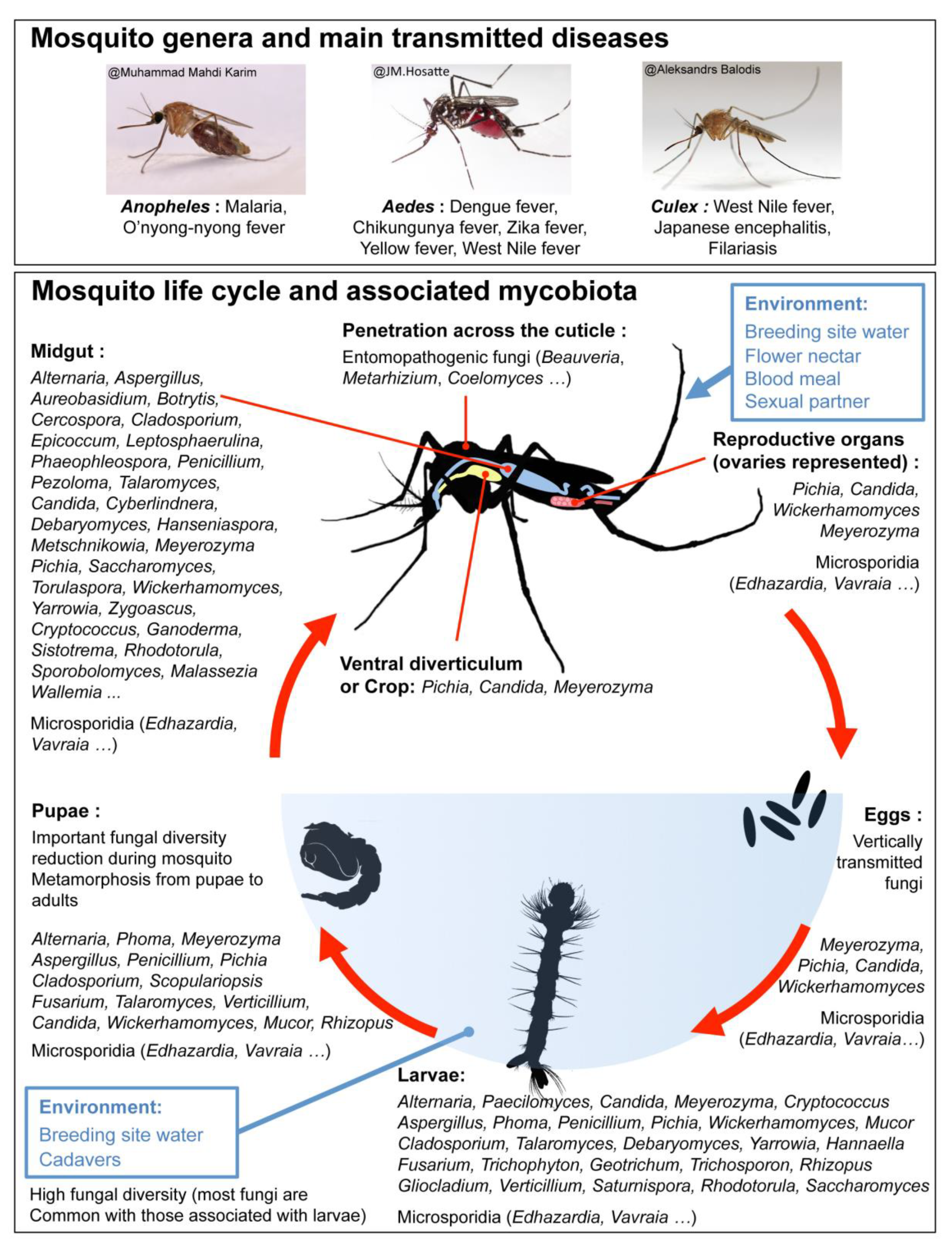
1. Introduction
2. Influence of Mycobiota on Mosquito Life-History Traits and Digestive Processes
2.1. Impact on Development, Survival and Reproduction
2.2. Mutualistic Interactions and Their Role in Digestive Processes
3. Influence of Mosquito-Associated Mycobiota on Vector Competence
3.1. Direct Impact through the Production of Fungal Toxins or the Modulation of Enzymatic Activities
3.2. Indirect Impact through the Modulation of the Immune System
4. Influence of Fungi and Their Associated Volatile Compounds on Mosquito Behavior
4.1. Attractive or Repulsive Effects and Impact on Breeding Site Selection
4.2. Impact on Larval and Adult Feeding Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forster, W.A.; Walker, E.D. Mosquitoes (Culicidae). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press Elsevier: Oxford, UK, 2018; Volume 1, pp. 261–325. [Google Scholar]
- Clements, A.N. (Ed.) Development, nutrition and reproduction. In The Biology of Mosquitoes, 1st ed.; CABI Publishing: Wallingford, UK, 1992; Volume 1, pp. 1–532. [Google Scholar]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito microbiota and implications for disease control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef]
- Ciota, A.T.; Kramer, L.D. Vector-virus interactions and transmission dynamics of West Nile virus. Viruses 2013, 5, 3021–3047. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasit. Vectors 2012, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, V.; Berrang-Ford, L.; Scott, M.E.; Lindsay, L.R. Expanding geographical distribution of the mosquito, Culex pipiens, in Canada under climate change. Appl. Geogr. 2012, 33, 53–62. [Google Scholar] [CrossRef]
- Caminade, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colón-González, F.J.; Stenlund, H.; Martens, P.; Lloyd, S.J. Impact of climate change on global malaria distribution. Proc. Natl. Acad. Sci. USA 2014, 111, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, S.H.P.P.; De Silva, W.A.P.P.; Weeraratne, T.C.; Surendran, S.N. Insecticide resistance in mosquitoes: Development, mechanisms and monitoring. Ceylon. J. Sci. 2018, 47, 299–309. [Google Scholar] [CrossRef]
- Guégan, M.; Zouache, K.; Démichel, C.; Minard, G.; Tran Van, V.; Potier, P.; Mavingui, P.; Valiente Moro, C. The mosquito holobiont: Fresh insight into mosquito-microbiota interactions. Microbiome 2018, 6, 49. [Google Scholar] [CrossRef]
- Minard, G.; Mavingui, P.; Valiente Moro, C. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 2013, 6, 146. [Google Scholar] [CrossRef]
- Scolari, A.; Casiraghi, M.; Bonizzoni, M. Aedes spp. and their microbiota: A review. Front. Microbiol. 2019, 10, 2036. [Google Scholar] [CrossRef]
- Minard, G.; Tran, F.H.; Van, V.T.; Goubert, C.; Bellet, C.; Lambert, G.; Kim, K.L.; Thuy, T.H.; Mavingui, P.; Valiente Moro, C. French invasive Asian tiger mosquito populations harbor reduced bacterial microbiota and genetic diversity compared to Vietnamese autochthonous relatives. Front. Microbiol. 2015, 6, 970. [Google Scholar] [CrossRef] [PubMed]
- Luis, P.; Vallon, L.; Tran, F.-H.; Hugoni, M.; Tran-Van, V.; Mavingui, P.; Minard, G.; Valiente Moro, C. Aedes albopictus mosquitoes host a locally structured mycobiota with evidence of reduced fungal diversity in invasive populations. Fungal Ecol. 2019, 39, 257–266. [Google Scholar] [CrossRef]
- Morales, M.E.; Ocampo, C.B.; Cadena, H.; Copeland, C.S.; Termini, M.; Wesson, D.M. Differential identification of Ascogregarina species (Apicomplexa: Lecudinidae) in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) by Polymerase Chain Reaction. J. Parasitol. 2005, 91, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.A.; Liu, R.M.; Bennett, S.N. RNA shotgun metagenomic sequencing of northern California (USA) mosquitoes uncovers viruses, bacteria, and fungi. Front. Microbiol. 2015, 6, 185. [Google Scholar] [CrossRef]
- Thongsripong, P.; Chandler, J.A.; Green, A.B.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Mosquito vector-associated microbiota: Metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod-borne diseases. Ecol. Evol. 2017, 8, 1352–1368. [Google Scholar] [CrossRef]
- Muturi, E.J.; Bara, J.J.; Rooney, A.P.; Hansen, A.K. Midgut fungal and bacterial microbiota of Aedes triseriatus and Aedes japonicus shift in response to La Crosse virus infection. Mol. Ecol. 2016, 25, 4075–4090. [Google Scholar] [CrossRef]
- Badran, R.A.M.; Aly, M.Z.Y. Studies on the mycotic inhabitants of Culex pipiens collected from fresh water ponds in Egypt. Mycopathologia 1995, 132, 105–110. [Google Scholar] [CrossRef]
- Da Costa, G.L.; Cunha de Oliveira, P. Penicillium species in mosquitoes from two Brazilian regions. J. Basic Microbiol. 1998, 38, 343–347. [Google Scholar] [CrossRef]
- Sur, B.; Bihari, V.; Sharma, A.; Basu, S.K. Survey of termite-inhabited soil and mosquito breeding sites in Lucknow, India for potential mycopathogens of Anopheles stephensi. Mycopathologia 1999, 144, 77–80. [Google Scholar] [CrossRef]
- Agarwala, S.P.; Sagar, S.K.; Sehgal, S.S. Use of mycelial suspension and metabolites of Paecilomyces lilacinus (Fungi: Hyphomycetes) in control of Aedes aegypti larvae. J. Commun. Dis. 1999, 31, 193–196. [Google Scholar]
- Mohanty, S.S.; Prakash, S. Laboratory evaluation of Trichophyton ajelloi, a fungal pathogen of Anopheles stephensi and Culex quinquefasciatus. J. Am. Mosquito Control. 2000, 16, 254–257. [Google Scholar]
- Scholte, E.J.; Knols, B.G.J.; Samson, R.A.; Takken, W. Entomopathogenic fungi for mosquito control: A review. J. Insect. Sci. 2004, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.D.; Sarquis, M.I.; Ferreira-Keppler, R.L.; Hamada, N.; Alencar, Y.B. Filamentous fungi associated with mosquito larvae (Diptera: Culicidae) in municipalities of the Brazilian Amazon. Neotrop. Entomol. 2009, 38, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Lilly, K.A.; Turell, M.J.; Willner, K.M.; Butani, A.; Nolan, N.M.E.; Lentz, S.M.; Akmal, A.; Mateczun, A.I.; Brahmbhatt, T.N.; Sozhamannan, S.; et al. Arbovirus detection in insect vectors by rapid high—throughput pyrosequencing. PLoS Negl. Trop. Dis. 2010, 4, e878. [Google Scholar] [CrossRef]
- Belda, E.; Coulibaly, B.; Fofana, A.; Beavogui, A.H.; Traore, S.F.; Gohl, D.M.; Vernick, K.D.; Riehle, M.M. Preferential suppression of Anopheles gambiae host sequences allows detection of the mosquito eukaryotic microbiome. Sci. Rep. 2017, 7, 3241. [Google Scholar] [CrossRef]
- Krajacich, B.J.; Huestis, D.L.; Dao, A.; Yaro, A.S.; Diallo, M.; Krishna, A.; Xu, J.; Lehmann, T. Investigation of the seasonal microbiome of Anopheles coluzzii mosquitoes in Mali. PLoS ONE 2018, 13, e0194899. [Google Scholar] [CrossRef]
- Bozic, J.; Capone, A.; Pediconi, D.; Mensah, P.; Cappelli, A.; Valzano, M.; Mancini, M.V.; Scuppa, P.; Martin, E.; Epis, S.; et al. Mosquitoes can harbour yeasts of clinical significance and contribute to their environmental dissemination: Identification of yeasts in different mosquito species. Environ. Microbiol. Rep. 2017, 9, 642–648. [Google Scholar] [CrossRef]
- Frants, T.G.; Mertvetsova, O.A. Yeast associations with mosquitoes of the genus Aedes Mg. (Diptera, Culicidae) in the Tom-Ob river region. Nauchnye Doklady Vysshei Shkoly Biologicheskie Nauki 1986, 4, 94–98. [Google Scholar]
- Ignatova, E.A.; Nagomaia, S.S.; Povazhnaia, T.N.; Ianishevskaia, G.S. The yeast flora of blood-sucking mosquitoes. Microbiol. Z 1996, 58, 12–15. [Google Scholar]
- Steyn, A.; Roets, F.; Botha, A. Yeasts associated with Culex pipiens and Culex theileri mosquito larvae and the effect of selected yeast strains on the ontogeny of Culex pipiens. Microb. Ecol. 2016, 71, 747–760. [Google Scholar] [CrossRef]
- Barredo, E.; DeGennaro, M. Not just from blood: Mosquito nutrient acquisition from nectar sources. Trends Parasitol. 2020, 36, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Nieto, L.M.; D’Alessio, C.; Perotti, M.A.; Berón, C.M. Culex pipiens development is greatly influenced by native bacteria and exogenous yeast. PLoS ONE 2016, 11, e0153133. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, D.S.; Santos, A.V.; Marini, D.C.; Russo Ede, S.; Peixoto, A.M.; Bacci Júnior, M.; Berbert-Molina, M.A.; Lemos, F.J. First isolation of microorganisms from the gut diverticulum of Aedes aegypti (Diptera: Culicidae): New perspectives for an insect-bacteria association. Memórias do Instituto Oswaldo Cruz 2007, 102, 919–924. [Google Scholar]
- Gusmão, D.S.; Santos, A.V.; Marini, D.C.; Bacci, M., Jr.; Berbert-Molina, M.A.; Lemos, F.J. Culture-dependent and culture-independent characterization of microorganisms associated with Aedes aegypti (Diptera: Culicidae) (L.) and dynamics of bacterial colonization in the midgut. Acta Trop. 2010, 115, 275–281. [Google Scholar]
- Ricci, I.; Damiani, C.; Scuppa, P.; Mosca, M.; Crotti, E.; Rossi, P.; Rizzi, A.; Capone, A.; Gonella, E.; Ballarini, P.; et al. The yeast Wickerhamomyces anomalus (Pichia anomala) inhabits the midgut and reproductive system of the Asian malaria vector Anopheles stephensi. Environ. Microbiol. 2011, 13, 911–921. [Google Scholar] [CrossRef]
- Bukhari, T.; Takken, W.; Koenraadt, C.J.M. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit. Vectors 2011, 4, 23. [Google Scholar] [CrossRef]
- Becnel, J.J.; White, S.E.; Shapiro, A.M. Review of microsporidia-mosquito relationships: From the simple to the complex. Folia Parasitol. 2005, 52, 41–50. [Google Scholar] [CrossRef]
- Damiani, C.; Ricci, I.; Crotti, E.; Rossi, P.; Rizzi, A.; Scuppa, P.; Capone, A.; Ulissi, U.; Epis, S.; Genchi, M.; et al. Mosquito-bacteria symbiosis: The case of Anopheles gambiae and Asaia. Microb. Ecol. 2010, 60, 644–654. [Google Scholar] [CrossRef]
- Corréa, M.A.; Matusovsky, B.; Brackney, D.E.; Steven, B. Generation of axenic Aedes aegypti demonstrate live bacteria are not required for mosquito development. Nat. Commun. 2018, 9, 4464. [Google Scholar] [CrossRef]
- Valzania, L.; Martinson, V.G.; Harrison, R.E.; Boyd, B.M.; Coon, K.L.; Brown, M.R.; Strand, M.R. Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLoS Negl. Trop. Dis. 2018, 12, e0006638. [Google Scholar] [CrossRef]
- Stefanini, I. Yeast-insect associations: It takes guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.S.; Virginio, F.; Riback, T.I.S.; Suesdek, L.; Barufi, J.B.; Genta, F.A. Microorganism-based larval diets affect mosquito development, size and nutritional reserves in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Front. Physiol. 2019, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Telang, A.; Frame, L.; Brown, M.R. Larval feeding duration affects ecdysteroid levels and nutritional reserves regulating pupal commitment in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). J. Exp. Biol. 2007, 210, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Valzania, L.; Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Hypoxia-induced transcription factor signaling is essential for larval growth of the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2018, 115, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R. Composition and functional roles of the gut microbiota in mosquitoes. Curr. Opin. Insect. Sci. 2018, 28, 59–65. [Google Scholar] [CrossRef]
- Barnard, D.R.; Xue, R.-D.; Rotstein, M.A.; Becnel, J.J. Microsporidiosis (Microsporidia: Culicosporidae) alters blood-feeding responses and DEET repellency in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 1040–1046. [Google Scholar] [CrossRef]
- Lorenz, L.M.; Koella, J.C. The microsporidian parasite Vavraia culicis as a potential late life–acting control agent of malaria. Evol. Appl. 2011, 4, 783–790. [Google Scholar] [CrossRef]
- Guégan, M.; Tran Van, V.; Martin, E.; Minard, G.; Tran, F.; Fel, B.; Hay, A.; Simon, L.; Barakat, M.; Potier, P.; et al. Who is eating fructose within the Aedes albopictus gut microbiota? Environ. Microbiol. 2020, 22, 1193–1206. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Machado, F.P.; Lima, J.B.; Valle, D.; Ribolla, P.E.M. Sugar digestion in mosquitoes: Identification and characterization of three midgut α-glucosidases of the neo-tropical malaria vector Anopheles aquasalis (Diptera: Culicidae). Comp. Biochem. Physiol. A Mol. Int. Physiol. 2007, 147, 993–1000. [Google Scholar] [CrossRef]
- Esquivel, C.J.; Cassone, B.J.; Piermarini, P.M. A de novo transcriptome of the Malpighian tubules in non blood-fed and blood-fed Asian tiger mosquitoes Aedes albopictus: Insights into diuresis, detoxification, and blood meal processing. PeerJ 2016, 4, e1784. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Varotto Boccazzi, I.; De Marco, L.; Bongiorno, G.; Montagna, M.; Sacchi, L.; Mensah, P.; Ricci, I.; Gradoni, L.; Bandi, C.; et al. The mycobiota of the sand fly Phlebotomus perniciosus: Involvement of yeast symbionts in uric acid metabolism. Environ. Microbiol. 2018, 20, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Ulissi, U.; Valzano, M.; Damiani, C.; Epis, S.; Gabrielli, M.G.; Conti, S.; Polonelli, L.; Bandi, C.; Favia, G.; et al. A Wickerhamomyces anomalus killer strain in the malaria vector Anopheles stephensi. PLoS ONE 2014, 9, e95988. [Google Scholar] [CrossRef]
- Valzano, M.; Cecarini, V.; Cappelli, A.; Capone, A.; Bozic, J.; Cuccioloni, M.; Epis, S.; Petrelli, D.; Angeletti, M.; Eleuteri, A.M.; et al. yeast strain associated to Anopheles mosquitoes produces a toxin able to kill malaria parasites. Malar. J. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Valzano, M.; Cecarini, V.; Bozic, J.; Rossi, P.; Mensah, P.; Amantini, C.; Favia, G.; Ricci, I. Killer yeasts exert anti-plasmodial activities against the malaria parasite Plasmodium berghei in the vector mosquito Anopheles stephensi and in mice. Parasit. Vectors 2019, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Angleró-Rodríguez, Y.I.; Talyuli, O.A.; Blumberg, B.J.; Kang, S.; Demby, C.; Shields, A.; Carlson, J.; Jupatanakul, N.; Dimopoulos, G. An Aedes aegypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. Elife 2017, 6, e28844. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, P.; Sirisena, P.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito Innate Immunity. Insects 2018, 9, 95. [Google Scholar] [CrossRef]
- Tawidian, P.; Rhodes, V.L.; Michel, K. Mosquito-fungus interactions and antifungal immunity. Insect Biochem. Mol. Biol. 2019, 111, 103182. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Lanz, H.; Rodrguez, M.H.; González-Ceron, L.; Tsutsumi, V. Cellular-mediated reactions to foreign organisms inoculated into the hemocoel of Anopheles albimanus (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 61–69. [Google Scholar] [CrossRef]
- Da Silva, J.B.; De Albuquerque, C.M.R.; De Araújo, E.C.; Peixoto, C.A.; Hurd, H. Immune defense mechanisms of Culex quinquefasciatus (Diptera: Culicidae) against Candida albicans infection. J. Invertebr. Pathol. 2000, 76, 257–262. [Google Scholar] [CrossRef]
- Bargielowski, I.; Koella, J.C. A possible mechanism for the suppression of Plasmodium berghei development in the mosquito Anopheles gambiae by the microsporidian Vavraia culicis. PLoS ONE 2009, 4, e4676. [Google Scholar] [CrossRef]
- Herren, J.K.; Mbaisi, L.; Mararo, E.; Makhulu, E.E.; Mobegi, V.A.; Butungi, H.; Mancini, M.V.; Oundo, J.W.; Teal, E.T.; Pinaud, S.; et al. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat. Commun. 2020, 11, 2187. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Short, S.M.; Bahia, A.C.; Saraiva, R.G.; Dong, Y.; Kang, S.; Tripathi, A.; Mlambo, G.; Dimopoulos, G. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog. 2014, 10, e1004398. [Google Scholar] [CrossRef]
- Angleró-Rodríguez, Y.I.; Blumberg, B.J.; Dong, Y.; Sandiford, S.L.; Pike, A.; Clayton, A.M.; Dimopoulos, G. A natural Anopheles-associated Penicillium chrysogenum enhances mosquito susceptibility to Plasmodium infection. Sci. Rep. 2016, 6, 34084. [Google Scholar] [CrossRef] [PubMed]
- Wooding, M.; Naudé, Y.; Rohwer, E.; Bouwer, M. Controlling mosquitoes with semiochemicals: A review. Parasit. Vectors 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, N.O.; Takken, W.; Dicke, M.; Schraa, G.; Smallegange, R.C. Chemical ecology of interactions between human skin microbiota and mosquitoes. FEMS Microbiol. Ecol. 2010, 74, 1–9. [Google Scholar] [CrossRef]
- Peach, D.A.H.; Carroll, C.; Meraj, S.; Gomes, S.; Galloway, E.; Balcita, A.; Coatsworth, H.; Young, N.; Uriel, Y.; Gries, R.; et al. Nectar-dwelling microbes of common tansy are attractive to its mosquito pollinator, Culex pipiens L. bioRxiv 2020, 4, 024380. [Google Scholar]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpędowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piškur, J. Chemical signaling and insect attraction is a conserved trait in yeasts. Ecol. Evol. 2018, 8, 2962–2974. [Google Scholar] [CrossRef]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The ecology of insect–yeast relationships and its relevance to human industry. Proc. Biol. Sci. 2018, 285, 20172733. [Google Scholar] [CrossRef]
- Aldridge, R.L.; Britch, S.C.; Allan, S.A.; Tsikolia, M.; Calix, L.C.; Bernier, U.R.; Linthicum, K.J. Comparison of volatiles and mosquito capture efficacy for three carbohydrate sources in a yeast-fermentation CO2 generator. J. Am. Mosq. Control. Assoc. 2016, 32, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Smallegange, R.C.; Schmied, W.H.; van Roey, K.J.; Verhulst, N.O.; Spitzen, J.; Mukabana, W.R.; Takken, W. Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar. J. 2010, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Mweresa, C.K.; Omusula, P.; Otieno, B.; Van Loon, J.J.A.; Takken, W.; Mukabana, W.R. Molasses as a source of carbon dioxide for attracting the malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar. J. 2014, 13, 160. [Google Scholar] [CrossRef]
- Sukumaran, D.; Ponmariappan, S.; Sharma, A.K.; Jha, H.K.; Wasu, Y.H.; Sharma, A.K. Application of biogenic carbon dioxide produced by yeast with different carbon sources for attraction of mosquitoes towards adult mosquito traps. Parasitol. Res. 2016, 115, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Jerry, D.C.T.; Mohammed, T.; Mohammed, A. Yeast-generated CO2: A convenient source of carbon dioxide for mosquito trapping using the BG-Sentinel ® traps. Asian Pac. J. Trop. Biomed. 2017, 7, 896–900. [Google Scholar] [CrossRef]
- George, J.; Jenkins, N.E.; Blanford, S.; Thomas, M.B.; Baker, T.C. Malaria mosquitoes attracted by fatal fungus. PLoS ONE 2013, 8, e62632. [Google Scholar] [CrossRef]
- Reeves, W.K. Oviposition by Aedes aegypti (Diptera: Culicidae) in relation to conspecific larvae infected with internal symbiotes. J. Vector. Ecol. 2004, 29, 159–163. [Google Scholar]
- Geetha, I.; Paily, K.; Padmanaban, V.; Balaraman, K. Oviposition response of the mosquito, Culex quinquefasciatus to the secondary metabolite(s) of the fungus, Trichoderma viride. Memórias do Instituto Oswaldo Cruz 2003, 98, 223–226. [Google Scholar] [CrossRef]
- Eneh, L.K.; Saijo, H.; Borg-Karlson, A.-K.; Lindh, J.M.; Rajarao, G.K. Cedrol, a malaria mosquito oviposition attractant is produced by fungi isolated from rhizomes of the grass Cyperus rotundus. Malar. J. 2016, 15, 478. [Google Scholar] [CrossRef]
- Lindh, J.M.; Okal, M.N.; Herrera-Varela, M.; Borg-Karlson, A.-K.; Torto, B.; Lindsay, S.W.; Fillinger, U. Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex. Malar. J. 2015, 14, 119. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, G.; Buscariollo, D.; Pitts, R.J.; Wenger, H.; Zwiebel, L.J. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 6433–6438. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pitts, R.J.; Bohbot, J.D.; Jones, P.L.; Wang, G.; Zwiebel, L.J. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 2010, 8, e1000467. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malassigné, S.; Valiente Moro, C.; Luis, P. Mosquito Mycobiota: An Overview of Non-Entomopathogenic Fungal Interactions. Pathogens 2020, 9, 564. https://doi.org/10.3390/pathogens9070564
Malassigné S, Valiente Moro C, Luis P. Mosquito Mycobiota: An Overview of Non-Entomopathogenic Fungal Interactions. Pathogens. 2020; 9(7):564. https://doi.org/10.3390/pathogens9070564
Chicago/Turabian StyleMalassigné, Simon, Claire Valiente Moro, and Patricia Luis. 2020. "Mosquito Mycobiota: An Overview of Non-Entomopathogenic Fungal Interactions" Pathogens 9, no. 7: 564. https://doi.org/10.3390/pathogens9070564
APA StyleMalassigné, S., Valiente Moro, C., & Luis, P. (2020). Mosquito Mycobiota: An Overview of Non-Entomopathogenic Fungal Interactions. Pathogens, 9(7), 564. https://doi.org/10.3390/pathogens9070564




