Control of Persistent Salmonella Infection Relies on Constant Thymic Output Despite Increased Peripheral Antigen-Specific T Cell Immunity
Abstract
:1. Introduction
2. Results
2.1. Mice Lacking a Thymus at the Initiation of Infection Succumb to Oral Salmonella Infection
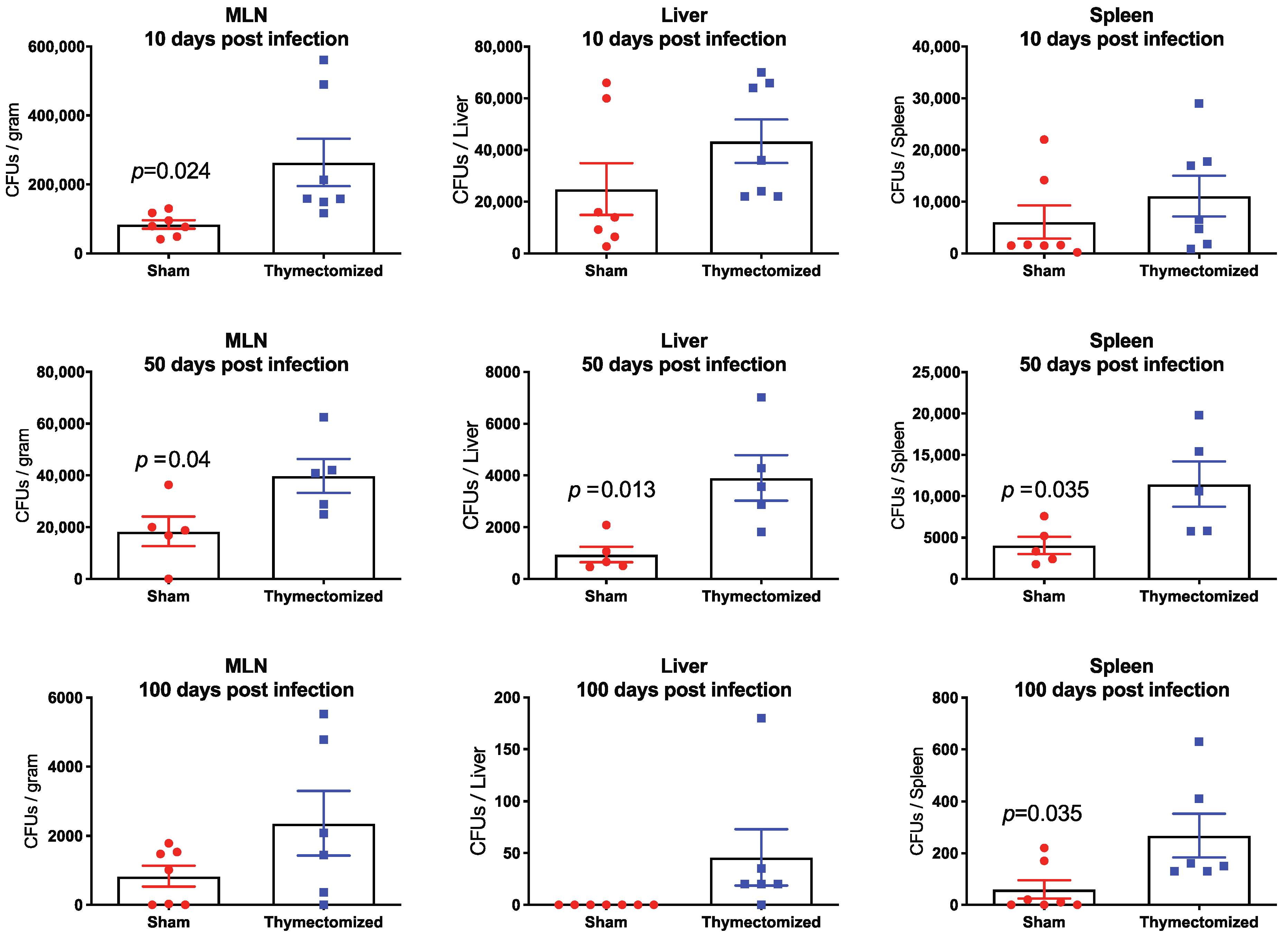
2.2. Preinfection Thymectomy Results in Significantly Increased Salmonella Burdens Systemically Compared to Sham Controls
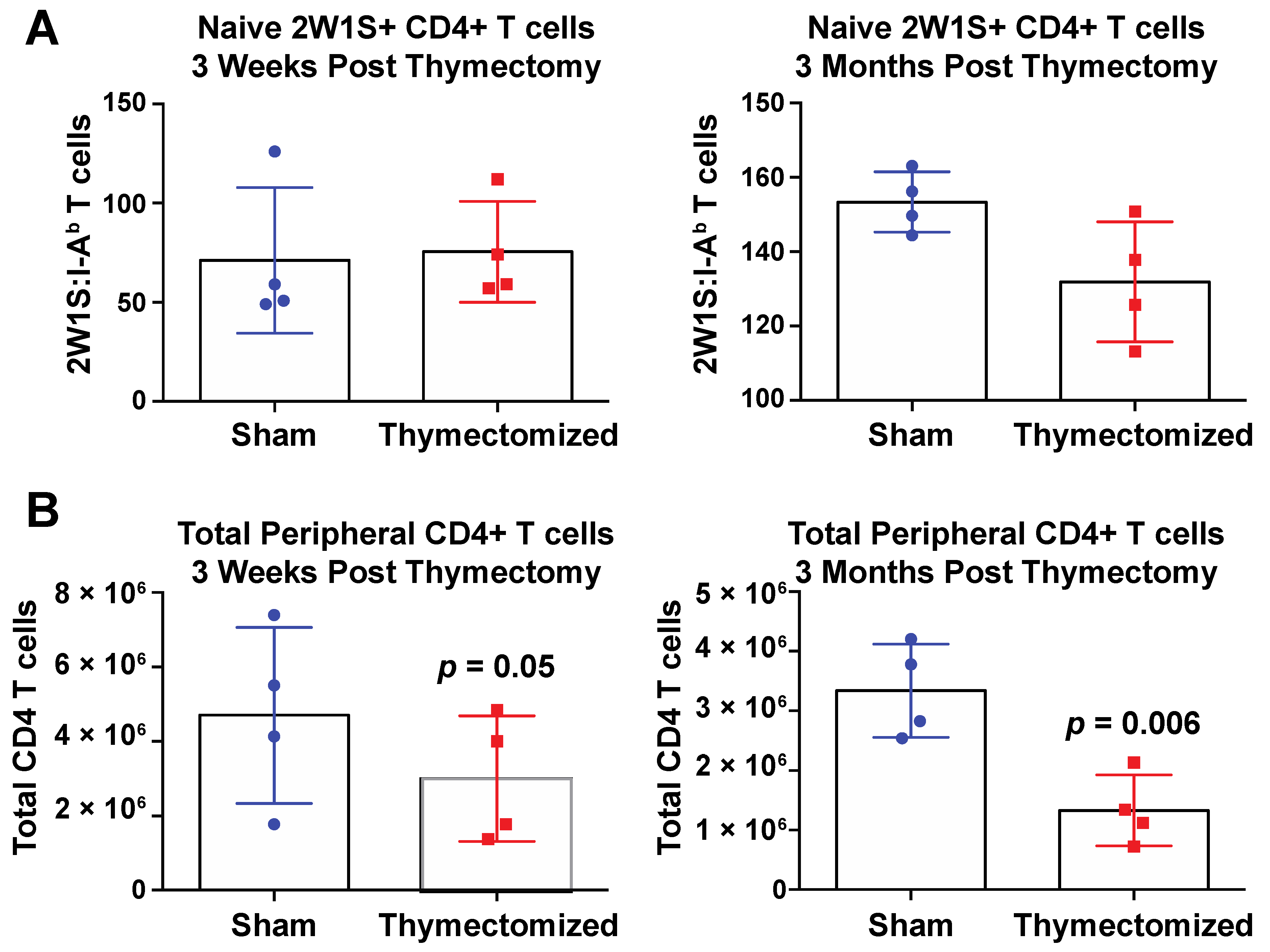
2.3. Preinfection Thymectomy Induces Increased Salmonella-Specific CD4 T Cell Numbers
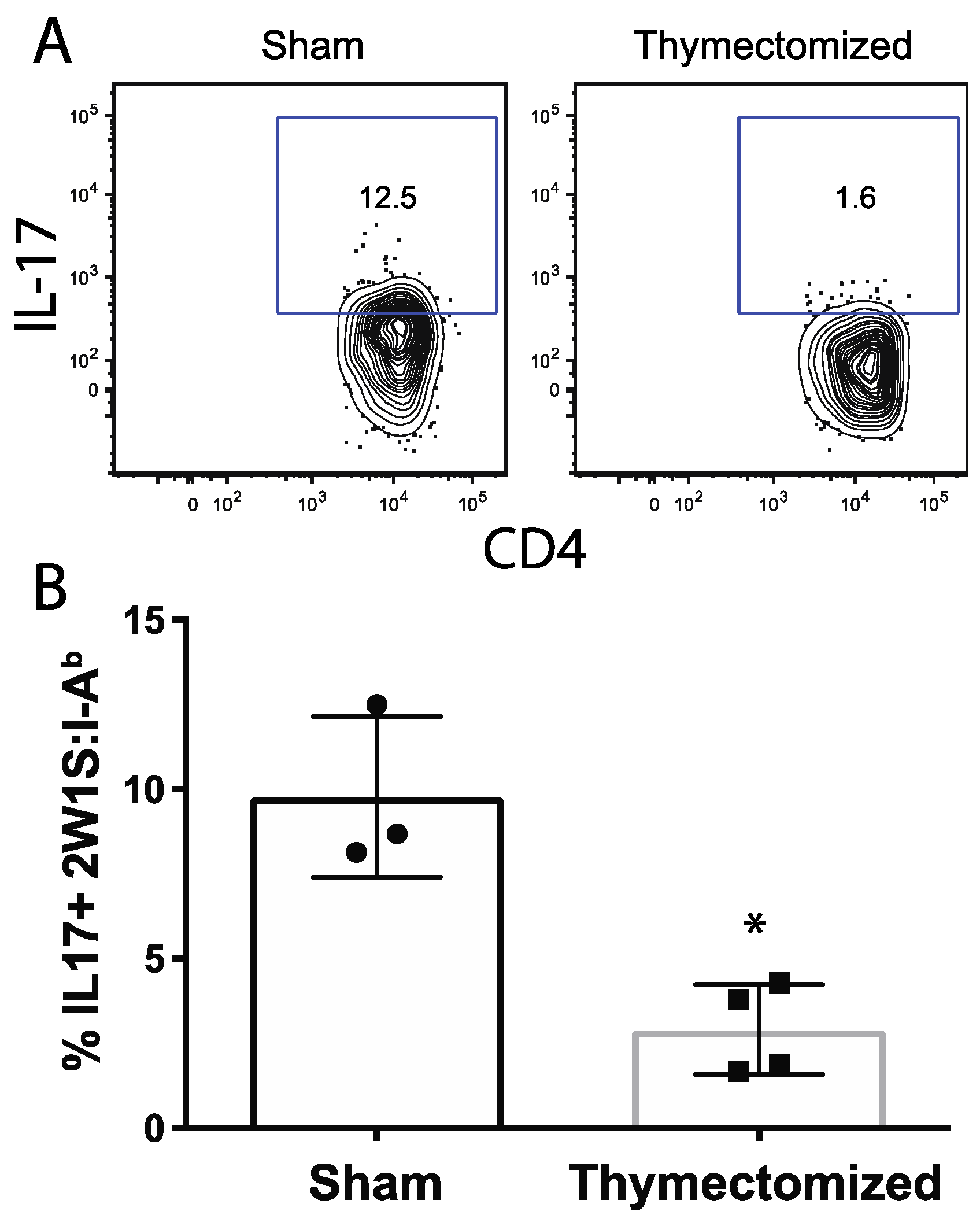
2.4. Peripheral Salmonella-Specific CD4 T Cells in Thymectomized Mice Are Deficient in Production of IL-17
3. Discussion
4. Materials and Methods
4.1. Mouse Line Usage and Rationale
4.2. Thymectomy Procedure
4.3. Bacterial Strains and Infection
4.4. Bacterial Plating
4.5. Cell Preparations
4.6. Tetramer Labeling and Magnetic Activation Cell Sorting
4.7. Flow Cytometry
4.8. In Vivo Cytokine Stimulations
4.9. Intracellular Cytokine Staining
4.10. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boursalian, T.E.; Golob, J.; Soper, D.M.; Cooper, C.J.; Fink, P.J. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 2004, 5, 418–425. [Google Scholar] [CrossRef]
- Hale, J.S.; Boursalian, T.E.; Turk, G.L.; Fink, P.J. Thymic output in aged mice. Proc. Natl. Acad. Sci. USA 2006, 103, 8447–8452. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, M.K.; Chu, H.H.; McLachlan, J.B.; Moon, J.J. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu. Rev. Immunol. 2010, 28, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Billard, M.J.; Gruver, A.L.; Sempowski, G.D. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PLoS ONE 2011, 6, e17940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.D.; Huang, K.J.; Lin, Y.S.; Lei, H.Y. Sepsis-Induced apoptosis of the thymocytes in mice. J. Immunol. 1994, 152, 5014–5021. [Google Scholar]
- Gruver, A.L.; Sempowski, G.D. Cytokines, leptin, and stress-induced thymic atrophy. J. Leukoc. Biol. 2008, 84, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Lord, G.M.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Investig. 1999, 104, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera-Sandoval, C.R.; Yang, O.O.; Jojic, N.; Lovato, P.; Chen, D.Y.; Boechat, M.I.; Cooper, P.; Zuo, J.; Ramirez, C.; Belzer, M.; et al. Supranormal thymic output up to 2 decades after HIV-1 infection. AIDS 2016, 30, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartling, H.J.; Gaardbo, J.C.; Ronit, A.; Salem, M.; Laye, M.; Clausen, M.R.; Skogstrand, K.; Gerstoft, J.; Ullum, H.; Nielsen, S.D. Impaired thymic output in patients with chronic hepatitis C virus infection. Scand. J. Immunol. 2013, 78, 378–386. [Google Scholar] [CrossRef]
- Miller, N.E.; Bonczyk, J.R.; Nakayama, Y.; Suresh, M. Role of thymic output in regulating CD8 T-Cell Homeostasis during acute and chronic viral infection. J. Virol. 2005, 79, 9419–9429. [Google Scholar] [CrossRef] [Green Version]
- Permar, S.R.; Moss, W.J.; Ryon, J.J.; Douek, D.C.; Monze, M.; Griffin, D.E. Increased thymic output during acute measles virus infection. J. Virol. 2003, 77, 7872–7879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, E.A.; Coughlan, R.E.; Flores-Langarica, A.; Lax, S.; Nicholson, J.; Desanti, G.E.; Marshall, J.L.; Bobat, S.; Hitchcock, J.; White, A.; et al. Thymic function is maintained during salmonella-induced atrophy and recovery. J. Immunol. 2012, 189, 4266–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-Y.; Tanaka, K.; Koga, Y.; Wang, Y.; Nomoto, K. The effect of adult thymectomy on immune response to infection with listeria monocytogenes in mice. Immunobiology 1993, 188, 355–369. [Google Scholar] [CrossRef]
- Bach, M.A.; Hoffenbach, A. Strain-Dependent protective effect of adult thymectomy on murine infection by Mycobacterium lepraemurium. Clin. Exp. Immunol. 1987, 68, 521–527. [Google Scholar] [PubMed]
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar] [PubMed]
- Bhan, M.K.; Bahl, R.; Bhatnagar, S. Typhoid and paratyphoid fever. Lancet 2005, 366, 749–762. [Google Scholar] [CrossRef]
- Gunn, J.S.; Marshall, J.M.; Baker, S.; Dongol, S.; Charles, R.C.; Ryan, E.T. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014, 22, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.M.; Black, R.E.; Lanata, C. Precise estimation of the numbers of chronic carriers of salmonella typhi in santiago, chile, an endemic area. J. Infect. Dis. 1982, 146, 724–726. [Google Scholar] [CrossRef]
- Fierer, J.; Guiney, D.G. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Investig. 2001, 107, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.W.; McLachlan, J.B.; Kurtz, J.R.; Jenkins, M.K. CD4+ T cell persistence and function after infection are maintained by low-level peptide: MHC class II presentation. J. Immunol. 2013, 190, 2828–2834. [Google Scholar] [CrossRef] [Green Version]
- González, J.F.; Kurtz, J.; Bauer, D.L.; Hitt, R.; Fitch, J.; Wetzel, A.; Perle, K.L.; White, P.; McLachlan, J.; Gunn, J.S. Establishment of chronic typhoid infection in a mouse carriage model involves a Type 2 immune shift and T and B Cell recruitment to the gallbladder. Mbio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, J.R.; Nieves, W.; Bauer, D.L.; Israel, K.E.; Adcox, H.E.; Gunn, J.S.; Morici, L.A.; McLachlan, J.B. Salmonella persistence and host immunity are dictated by the anatomical microenvironment. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Johanns, T.M.; Ertelt, J.M.; Rowe, J.H.; Way, S.S. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010, 6, e1001043. [Google Scholar] [CrossRef] [PubMed]
- McSorley, S.J.; Cookson, B.T.; Jenkins, M.K. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 2000, 164, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Nauciel, C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J. Immunol. 1990, 145, 1265–1269. [Google Scholar]
- Govoni, G.; Gros, P. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm. Res. 1998, 47, 277–284. [Google Scholar] [CrossRef]
- Eckmann, L.; Fierer, J.; Kagnoff, M.F. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J. Immunol. 1996, 156, 2894–2900. [Google Scholar]
- Frederick, D.R.; Goggins, J.A.; Sabbagh, L.M.; Freytag, L.C.; Clements, J.D.; McLachlan, J.B. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4(+) T cells following parenteral immunization. Mucosal. Immunol. 2017, 171, 1097. [Google Scholar] [CrossRef] [Green Version]
- McLachlan, J.B.; Catron, D.M.; Moon, J.J.; Jenkins, M.K. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 2009, 30, 277–288. [Google Scholar] [CrossRef] [Green Version]
- den Braber, I.; Mugwagwa, T.; Vrisekoop, N.; Westera, L.; Mögling, R.; de Boer, A.B.; Willems, N.; Schrijver, E.H.R.; Spierenburg, G.; Gaiser, K.; et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012, 36, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Vianna, P.H.O.; Canto, F.B.; Nogueira, J.S.; Nunes, C.F.C.G.; Bonomo, A.C.; Fucs, R. Critical influence of the thymus on peripheral T cell homeostasis. Immun. Inflamm. Dis. 2016, 4, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Pagán, A.J.; Igyártó, B.Z.; Taylor, J.J.; Jenkins, M.K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 2011, 35, 583–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianci, R.; Pinti, M.; Nasi, M.; Starnino, S.; Cammarota, G.; Miele, L.; Luca, A.D.; Cauda, R.; Raducci, F.; Grieco, A.; et al. Impairment of recent thymic emigrants in HCV infection. Int. J. Immunopathol. Pharmacol. 2005, 18, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Chen, C.H.; Cooper, M.D. Reversible disruption of thymic function by steroid treatment. J. Immunol. 2002, 168, 6500–6505. [Google Scholar] [CrossRef] [Green Version]
- Staton, T.L.; Habtezion, A.; Winslow, M.M.; Sato, T.; Love, P.E.; Butcher, E.C. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat. Immunol. 2006, 7, 482–488. [Google Scholar] [CrossRef]
- Geddes, K.; Rubino, S.J.; Magalhaes, J.G.; Streutker, C.; Bourhis, L.L.; Cho, J.H.; Robertson, S.J.; Kim, C.J.; Kaul, R.; Philpott, D.J.; et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 2011, 17. [Google Scholar] [CrossRef]
- Lee, S.-J.; McLachlan, J.B.; Kurtz, J.R.; Fan, D.; Winter, S.E.; Bäumler, A.J.; Jenkins, M.K.; McSorley, S.J. Temporal expression of bacterial proteins instructs host CD4 T cell expansion and th17 development. PLoS Pathog. 2012, 8, e1002499. [Google Scholar] [CrossRef]
- Schulz, S.M.; Köhler, G.; Holscher, C.; Iwakura, Y.; Alber, G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 2008, 20, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Raffatellu, M.; Santos, R.L.; Verhoeven, D.E.; George, M.D.; Wilson, R.P.; Winter, S.E.; Godinez, I.; Sankaran, S.; Paixao, T.A.; Gordon, M.A.; et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 2008, 14, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Marks, B.R.; Nowyhed, H.N.; Choi, J.-Y.; Poholek, A.C.; Odegard, J.M.; Flavell, R.A.; Craft, J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat. Immunol. 2009, 10, 1125–1132. [Google Scholar] [CrossRef]
- Massot, B.; Michel, M.-L.; Diem, S.; Ohnmacht, C.; Latour, S.; Dy, M.; Eberl, G.; Leite-de-Moraes, M.C. TLR-Induced cytokines promote effective proinflammatory natural Th17 cell responses. J. Immunol. 2014, 192, 5635–5642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydström, A.; Wick, M.J. Monocyte and neutrophil recruitment during oral Salmonella infection is driven by MyD88-derived chemokines. Eur. J. Immunol. 2009, 39, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.A.; Rydström, A.; Sundquist, M.; Wick, M.J. Early cellular responses to Salmonella infection: Dendritic cells, monocytes, and more. Immunol. Rev. 2008, 225, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Rydström, A.; Wick, M.J. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral salmonella infection. J. Immunol. 2007, 178, 5789–5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlan, J.W. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect. Immun. 1996, 64, 1043–1047. [Google Scholar] [CrossRef] [Green Version]
- Spees, A.M.; Kingsbury, D.D.; Wangdi, T.; Xavier, M.N.; Tsolis, R.M.; Bäumler, A.J. Neutrophils are a source of gamma interferon during acute Salmonella enterica serovar Typhimurium colitis. Infect. Immun. 2014, 82, 1692–1697. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.; Albergante, L.; Blackburn, C.C.; Newman, T.J. Thymic involution and rising disease incidence with age. Proc. Natl. Acad. Sci. USA 2018, 115, 1883–1888. [Google Scholar] [CrossRef] [Green Version]
- Palmer, D.B. The effect of age on thymic function. Front. Immunol. 2013, 4, 316. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, W.A.; Lang, P.O.; Aspinall, R. Tracing thymic output in older individuals. Clin. Exp. Immunol. 2010, 161, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B.; Lambert, N.D.; Kirkland, J.L. A systems biology approach to the effect of aging, immunosenescence and vaccine response. Curr. Opin. Immunol. 2014, 29, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: A review. Gynecol. Endocrinol. 2013, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kato, A.; Sekai, M.; Hamazaki, Y.; Minato, N. Physiologic thymic involution underlies age-dependent accumulation of senescence-associated CD4+ T cells. J. Immunol. 2017, 199, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Stanaway, J.D.; Reiner, R.C.; Blacker, B.F.; Goldberg, E.M.; Khalil, I.A.; Troeger, C.E.; Andrews, J.R.; Bhutta, Z.A.; Crump, J.A.; Im, J.; et al. The global burden of typhoid and paratyphoid fevers: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Gay, R.; Thomas, A.; Pae, M.; Wu, D.; Logsdon, L.; Mecsas, J.; Meydani, S.N. Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J. Med. Microbiol. 2009, 58, 1559–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, S.F.; Kauffman, C.A. Aging and the response to salmonella infection. Exp. Gerontol. 1990, 25, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Glass, K.; Liu, B.; Hope, K.; Kirk, M. Salmonella infection in middle-aged and older adults: Incidence and risk factors from the 45 and up study. Foodborne Pathog. Dis. 2016, 13, 689–694. [Google Scholar] [CrossRef]
- Vugia, D.J.; Samuel, M.; Farley, M.M.; Marcus, R.; Shiferaw, B.; Shallow, S.; Smith, K.; Angulo, F.J.; Emerging Infections Program FoodNet Working Group. Invasive salmonella infections in the United States, FoodNet, 1996–1999: Incidence, serotype distribution, and outcome. Clin. Infect. Dis. 2004, 38 (Suppl. S3), S149–S156. [Google Scholar] [CrossRef] [Green Version]
- Friesen, T.J.; Ji, Q.; Fink, P.J. Recent thymic emigrants are tolerized in the absence of inflammation. J. Exp. Med. 2016, 213, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Uzzau, S.; Figueroa-Bossi, N.; Rubino, S.; Bossi, L. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. USA 2001, 98, 15264–15269. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goggins, J.A.; Kurtz, J.R.; McLachlan, J.B. Control of Persistent Salmonella Infection Relies on Constant Thymic Output Despite Increased Peripheral Antigen-Specific T Cell Immunity. Pathogens 2020, 9, 605. https://doi.org/10.3390/pathogens9080605
Goggins JA, Kurtz JR, McLachlan JB. Control of Persistent Salmonella Infection Relies on Constant Thymic Output Despite Increased Peripheral Antigen-Specific T Cell Immunity. Pathogens. 2020; 9(8):605. https://doi.org/10.3390/pathogens9080605
Chicago/Turabian StyleGoggins, J. Alan, Jonathan R Kurtz, and James B. McLachlan. 2020. "Control of Persistent Salmonella Infection Relies on Constant Thymic Output Despite Increased Peripheral Antigen-Specific T Cell Immunity" Pathogens 9, no. 8: 605. https://doi.org/10.3390/pathogens9080605
APA StyleGoggins, J. A., Kurtz, J. R., & McLachlan, J. B. (2020). Control of Persistent Salmonella Infection Relies on Constant Thymic Output Despite Increased Peripheral Antigen-Specific T Cell Immunity. Pathogens, 9(8), 605. https://doi.org/10.3390/pathogens9080605



