Zebrafish as a Model for Fish Diseases in Aquaculture
Abstract
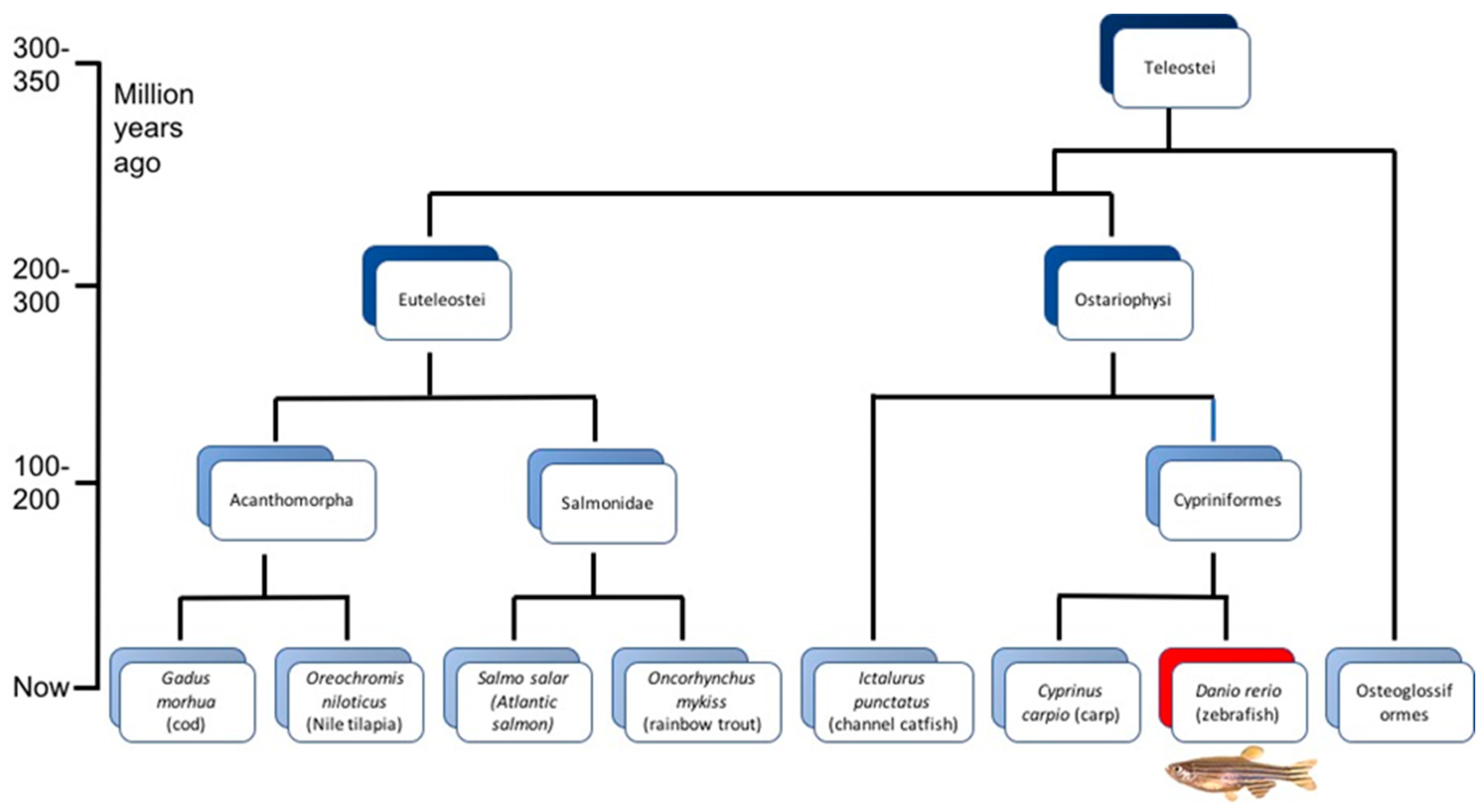
:1. Introduction
1.1. Background
1.2. Prophylactic Approaches
1.3. Immunology
1.4. Infection Biology
2. Fish Diseases in Aquaculture Studied in the Zebrafish Model
2.1. Bacteria
2.1.1. Mycobacterium marinum
2.1.2. Vibrio anguillarum
2.1.3. Aeromonas salmonicida
2.1.4. Yersinia ruckeri
2.1.5. Flavobacterium psychrophilum
2.2. Virus
2.2.1. SVCV
2.2.2. IHNV
2.2.3. VHSV
2.2.4. IPN
2.3. Parasites
2.3.1. I. multifiliis
2.3.2. Trypanosoma carassii
3. Discussion
3.1. Advantages of Using the Zebrafish as a Model
3.2. Drawbacks of Using the Zebrafish as a Model
4. Conclusions
Funding
Conflicts of Interest
References
- Dooley, K.; Zon, L. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- White, R.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieschke, G.; Currie, P. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Bradford, Y.M.; Toro, S.; Ramachandran, S.; Ruzicka, L.; Howe, D.G.; Eagle, A.; Kalita, P.; Martin, R.; Taylor Moxon, S.A.; Schaper, K.; et al. Zebrafish models of human disease: Gaining Insight into Human Disease at ZFIN. ILAR J. 2017, 58, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Carnovali, M.; Banfi, G.; Mariotti, M. Zebrafish Models of human skeletal Disorders: Embryo and Adult Swimming Together. Biomed. Res. Int. 2019, 2019, 1253710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, C.; Kim, C. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008, 25, 341–350. [Google Scholar] [CrossRef]
- Gilbert, M.J.; Zerulla, T.C.; Tierney, K.B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, M.C.; de Castro, I.P.; Ferreira, M.G. Telomeres in aging and disease: Lessons from zebrafish. Dis. Model Mech. 2016, 9, 737–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayuela, M.L.; Claes, K.B.M.; Ferreira, M.G.; Henriques, C.M.; van Eeden, F.; Varga, M.; Vierstraete, J.; Mione, M.C. The Zebrafish as an emerging model to study DNA damage in aging, cancer and other diseases. Front. Cell Dev. Biol. 2018, 6, 178. [Google Scholar] [CrossRef] [Green Version]
- Bagatto, B.; Burggren, W. A three-dimensional functional assessment of heart and vessel development in the larva of the zebrafish (Danio rerio). Physiol. Biochem. Zool. 2006, 79, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Langenau, D.; Ferrando, A.; Traver, D.; Kutok, J.; Hezel, J.; Kanki, J.; Zon, L.; Look, A.; Trede, N. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 2004, 101, 7369–7374. [Google Scholar] [CrossRef] [Green Version]
- Page, D.; Wittamer, V.; Bertrand, J.; Lewis, K.; Pratt, D.; Delgado, N.; Schale, S.; McGue, C.; Jacobsen, B.; Doty, A.; et al. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood 2013, 122, E1–E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto-Teixeira, F.; Muzzopappa, M.; Swoger, J.; Mineo, A.; Sharpe, J.; López-Schier, H. Intravital imaging of hair-cell development and regeneration in the zebrafish. Front. Neuroanat. 2013, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldman, M.; Lin, S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahm, R.; Geisler, R. Learning from small fry: The zebrafish as a genetic model organism for aquaculture fish species. Mar. Biotechnol. 2006, 8, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.; Torroja, C.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Streisinger, G. Attainment of minimal biological variability and measurements of genotoxicity: Production of homozygous diploid zebra fish. Natl. Cancer Inst. Monogr. 1984, 65, 53–58. [Google Scholar]
- Varga, M. The Doctor of Delayed Publications: The remarkable life of George Streisinger (1927–1984). Zebrafish 2018, 15, 314–319. [Google Scholar] [CrossRef]
- Gray, C.; Loynes, C.; Whyte, M.; Crossman, D.; Renshaw, S.; Chico, T. Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb. Haemost. 2011, 105, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Berthelot, C.; Brunet, F.; Chalopin, D.; Juanchich, A.; Bernard, M.; Noël, B.; Bento, P.; Da Silva, C.; Labadie, K.; Alberti, A.; et al. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 2014, 5, 3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef] [PubMed]
- Ribas, L.; Piferrer, F. The zebrafish (Danio rerio) as a model organism, with emphasis on applications for finfish aquaculture research. Rev. Aquac. 2014, 6, 209–240. [Google Scholar] [CrossRef]
- Plaut, I. Effects of fin size on swimming performance, swimming behaviour and routine activity of zebrafish Danio rerio. J. Exp. Biol. 2000, 203, 813–820. [Google Scholar] [PubMed]
- Siccardi, A.J.; Garris, H.W.; Jones, W.T.; Moseley, D.B.; D’Abramo, L.R.; Watts, S.A. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish 2009, 6, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yossa, R.; Sarker, P.K.; Karanth, S.; Ekker, M.; Vandenberg, G.W. Effects of dietary biotin and avidin on growth, survival, feed conversion, biotin status and gene expression of zebrafish Danio rerio. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 160, 150–158. [Google Scholar] [CrossRef]
- Hensley, M.R.; Leung, Y.F. A convenient dry feed for raising zebrafish larvae. Zebrafish 2010, 7, 219–231. [Google Scholar] [CrossRef]
- Kurtzman, M.S.; Craig, M.P.; Grizzle, B.K.; Hove, J.R. Sexually segregated housing results in improved early larval survival in zebrafish. Lab. Anim. (NY) 2010, 39, 183–189. [Google Scholar] [CrossRef]
- Spence, R. Zebrafish ecology and behaviour. In Zebrafish Models in Neurobehavioral Research, Neuromethods 52; Kalueff, A.V., Cachet, J.M., Eds.; Springer Science: New York, NY, USA, 2011; pp. 1–46. [Google Scholar]
- Buchmann, K.; Lindenstrom, T.; Sigh, J. Partial cross protection against Ichthyophthirius multifiliis in Gyrodactylus derjavini immunized rainbow trout. J. Helminthol. 1999, 73, 189–195. [Google Scholar] [CrossRef]
- Jorgensen, L.V.; Nemli, E.; Heinecke, R.D.; Raida, M.K.; Buchmann, K. Immune-relevant genes expressed in rainbow trout following immunisation with a live vaccine against Ichthyophthirius multifiliis. Dis. Aquat. Org. 2008, 80, 189–197. [Google Scholar] [CrossRef]
- Jorgensen, L.; Heinecke, R.; Skjodt, K.; Rasmussen, K.; Buchmann, K. Experimental evidence for direct in situ binding of IgM and IgT to early trophonts of Ichthyophthirius multifiliis (Fouquet) in the gills of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2011, 34, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.L.; Matthews, R.A. Ichthyophthiriasis in carp, Cyprinus-carpio L.—fate of parasites in immunized fish. J. Fish Dis. 1992, 15, 497–505. [Google Scholar] [CrossRef]
- Gonzalez, S.F.; Buchmann, K.; Nielsen, M.E. Complement expression in common carp (Cyprinus carpio L.) during infection with Ichthyophthirius multifiliis. Dev. Comp. Immunol. 2007, 31, 576–586. [Google Scholar] [CrossRef]
- Clark, T.; Dickerson, H.; Gratzek, J.; Findly, R. In vitro response of Ichthyophthirius-multifiliis to sera from immune channel catfish. J. Fish Biol. 1987, 31, 203–208. [Google Scholar] [CrossRef]
- Dickerson, H.; Clark, T. Ichthyophthirius multifiliis: A model of cutaneous infection and immunity in fishes. Immunol. Rev. 1998, 166, 377–384. [Google Scholar] [CrossRef]
- Cabello, F.C. Antibiotics and aquaculture in Chile: Implications for human and animal health. Rev. Med. Chil. 2004, 132, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonheim, T.C.; Bøgwald, J.; Dalmo, R.A. What happens to the DNA vaccine in fish? A review of current knowledge. Fish Shellfish Immunol. 2008, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Samuel-Shaker, D.; Watral, V.; Kent, M.L. Attenuated Mycobacterium marinum protects zebrafish against mycobacteriosis. J. Fish Dis. 2010, 33, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wu, H.; Xiao, J.; Wang, Q.; Liu, Q.; Zhang, Y. Immune responses of zebrafish (Danio rerio) induced by bath-vaccination with a live attenuated Vibrio anguillarum vaccine candidate. Fish Shellfish Immunol. 2012, 33, 36–41. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, C.; Wu, H.; Yang, M.; Liu, Q.; Wang, Q.; Zhang, Y. Transcriptome profiling reveals Th17-like immune responses induced in zebrafish bath-vaccinated with a live attenuated Vibrio anguillarum. PLoS ONE 2013, 8, e73871. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Chang, X.; Tang, Y.; Liu, Q.; Zhang, Y. Notable mucosal immune responses induced in the intestine of zebrafish (Danio rerio) bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol. 2014, 40, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, B.; Wu, H.; Gao, L.; Liu, Q.; Wang, Q.; Xiao, J.; Zhang, Y. Th17-like immune response in fish mucosal tissues after administration of live attenuated Vibrio anguillarum via different vaccination routes. Fish Shellfish Immunol. 2014, 37, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, H.; Wang, Q.; Qu, J.; Liu, Q.; Xiao, J.; Zhang, Y. A live attenuated combination vaccine evokes effective immune-mediated protection against Edwardsiella tarda and Vibrio anguillarum. Vaccine 2014, 32, 5937–5944. [Google Scholar] [CrossRef]
- Korbut, R.; Mehrdana, F.; Kania, P.; Larsen, M.; Frees, D.; Dalsgaard, I.; Jorgensen, L. Antigen uptake during different life stages of zebrafish (Danio rerio) using a GFP-tagged Yersinia ruckeri. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Solís, C.J.; Poblete-Morales, M.; Cabral, S.; Valdés, J.A.; Reyes, A.E.; Avendaño-Herrera, R.; Feijóo, C.G. Neutrophil migration in the activation of the innate immune response to different Flavobacterium psychrophilum vaccines in zebrafish (Danio rerio). J. Immunol. Res. 2015, 2015, 515187. [Google Scholar] [CrossRef] [Green Version]
- García-Valtanen, P.; Martinez-Lopez, A.; Ortega-Villaizan, M.; Perez, L.; Coll, J.M.; Estepa, A. In addition to its antiviral and immunomodulatory properties, the zebrafish β-defensin 2 (zfBD2) is a potent viral DNA vaccine molecular adjuvant. Antivir. Res. 2014, 101, 136–147. [Google Scholar] [CrossRef]
- Briolat, V.; Jouneau, L.; Carvalho, R.; Palha, N.; Langevin, C.; Herbomel, P.; Schwartz, O.; Spaink, H.P.; Levraud, J.P.; Boudinot, P. Contrasted innate responses to two viruses in zebrafish: Insights into the ancestral repertoire of vertebrate IFN-stimulated genes. J. Immunol. 2014, 192, 4328–4341. [Google Scholar] [CrossRef] [Green Version]
- Novoa, B.; Romero, A.; Mulero, V.; Rodriguez, I.; Fernandez, I.; Figueras, A. Zebrafish (Danio rerio) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV). Vaccine 2006, 24, 5806–5816. [Google Scholar] [CrossRef]
- Kavaliauskis, A.; Arnemo, M.; Kim, S.H.; Ulanova, L.; Speth, M.; Novoa, B.; Dios, S.; Evensen, Ø.; Griffiths, G.W.; Gjøen, T. Use of Poly(I:C) Stabilized with chitosan as a vaccine-adjuvant against viral hemorrhagic septicemia virus infection in zebrafish. Zebrafish 2015, 12, 421–431. [Google Scholar] [CrossRef]
- Jorgensen, L.v.G. Infection and immunity against Ichthyophthirius multifiliis in zebrafish (Danio rerio). Fish Shellfish Immunol. 2016, 57, 335–339. [Google Scholar] [CrossRef]
- Jørgensen, L.v.G.; Korbut, R.; Jeberg, S.; Kania, P.; Buchmann, K. Association between adaptive immunity and neutrophil dynamics in zebrafish (Danio rerio) infected by a parasitic ciliate. PLoS ONE 2018, 13, e0203297. [Google Scholar] [CrossRef]
- Chinchilla, B.; Gomez-Casado, E.; Encinas, P.; Falco, A.; Estepa, A.; Coll, J. In vitro neutralisation of viral hemorrhagic septicaemia virus by plasma from immunised zebrafish. Zebrafish 2013, 10, 43–51. [Google Scholar] [CrossRef]
- Hortle, E.; Johnson, K.E.; Johansen, M.D.; Nguyen, T.; Shavit, J.A.; Britton, W.J.; Tobin, D.M.; Oehlers, S.H. Thrombocyte inhibition restores protective immunity to mycobacterial infection in zebrafish. J. Infect. Dis. 2019, 220, 524–534. [Google Scholar] [CrossRef]
- Encinas, P.; Garcia-Valtanen, P.; Chinchilla, B.; Gomez-Casado, E.; Estepa, A.; Coll, J. Identification of multipath genes differentially expressed in pathway-targeted microarrays in zebrafish infected and surviving spring viremia carp virus (SVCV) suggest preventive drug candidates. PLoS ONE 2013, 8, e73553. [Google Scholar] [CrossRef]
- Tort, L.; Blanch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Immunologia 2003, 22, 277–286. [Google Scholar]
- Nakanishi, T.; Shibasaki, Y.; Matsuura, Y. T Cells in Fish. Biology 2015, 4, 640–663. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Takizawa, F.; Parra, D.; Gómez, D.; von Gersdorff Jørgensen, L.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728. [Google Scholar] [CrossRef] [Green Version]
- Mashoof, S.; Criscitiello, M.F. Fish Immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Scapigliati, G.; Fausto, A.M.; Picchietti, S. Fish lymphocytes: An evolutionary equivalent of mammalian innate-like lymphocytes? Front. Immunol. 2018, 9, 971. [Google Scholar] [CrossRef] [Green Version]
- Star, B.; Jentoft, S. Why does the immune system of Atlantic cod lack MHC II? Bioessays 2012, 34, 648–651. [Google Scholar] [CrossRef] [Green Version]
- Villard, J.; Masternak, K.; Lisowska-Grospierre, B.; Fischer, A.; Reith, W. MHC class II deficiency: A disease of gene regulation. Medicine 2001, 80, 405–418. [Google Scholar] [CrossRef]
- Danilova, N.; Bussmann, J.; Jekosch, K.; Steiner, L. The immunoglobulin heavy-chain locus in zebrafish: Identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 2005, 6, 295–302. [Google Scholar] [CrossRef]
- Hansen, J.D.; Landis, E.D.; Phillips, R.B. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc. Natl. Acad. Sci. USA 2005, 102, 6919–6924. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Tang, X.; Zhan, W.; Xing, J.; Sheng, X. Immunoglobulin tau heavy chain (IgT) in flounder, Paralichthys olivaceus: Molecular cloning, characterization, and expression analyses. Int. J. Mol. Sci. 2016, 17, 1571. [Google Scholar] [CrossRef] [Green Version]
- Sunyer, J.O.; Zarkadis, I.K.; Sahu, A.; Lambris, J.D. Multiple forms of complement C3 in trout that differ in binding to complement activators. Proc. Natl. Acad. Sci. USA 1996, 93, 8546–8551. [Google Scholar] [CrossRef] [Green Version]
- Whyte, S. The innate immune response of finfish—A review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Kaattari, I.M.; Ma, C.; Kaattari, S. The teleost humoral immune response. Fish Shellfish Immunol. 2013, 35, 1719–1728. [Google Scholar] [CrossRef]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003, 81, 253–330. [Google Scholar]
- Willett, C.E.; Cherry, J.J.; Steiner, L.A. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics 1997, 45, 394–404. [Google Scholar] [CrossRef]
- Danilova, N.; Steiner, L.A. B cells develop in the zebrafish pancreas. Proc. Natl. Acad. Sci. USA 2002, 99, 13711–13716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilova, N.; Hohman, V.S.; Sacher, F.; Ota, T.; Willett, C.E.; Steiner, L.A. T cells and the thymus in developing zebrafish. Dev. Comp. Immunol. 2004, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Wen, Z.; Lam, T.J.; Sin, Y.M. Morphologic transformation of the thymus in developing zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2002, 225, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Levraud, J.P.; Boudinot, P.; Colin, I.; Benmansour, A.; Peyrieras, N.; Herbomel, P.; Lutfalla, G. Identification of the zebrafish IFN receptor: Implications for the origin of the vertebrate IFN system. J. Immunol. 2007, 178, 4385–4394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggad, D.; Mazel, M.; Boudinot, P.; Mogensen, K.E.; Hamming, O.J.; Hartmann, R.; Kotenko, S.; Herbomel, P.; Lutfalla, G.; Levraud, J.P. The two groups of zebrafish virus-induced interferons signal via distinct receptors with specific and shared chains. J. Immunol. 2009, 183, 3924–3931. [Google Scholar] [CrossRef] [Green Version]
- Aggad, D.; Stein, C.; Sieger, D.; Mazel, M.; Boudinot, P.; Herbomel, P.; Levraud, J.P.; Lutfalla, G.; Leptin, M. In vivo analysis of Ifn-γ1 and Ifn-γ2 signaling in zebrafish. J. Immunol. 2010, 185, 6774–6782. [Google Scholar] [CrossRef] [Green Version]
- Levraud, J.; Palha, N.; Langevin, C.; Boudinot, P. Through the looking glass: Witnessing host-virus interplay in zebrafish. Trends Microbiol. 2014, 22, 490–497. [Google Scholar] [CrossRef]
- Boxx, G.M.; Cheng, G. The roles of type I interferon in bacterial infection. Cell Host Microbe 2016, 19, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Meijer, A.H.; Verbeek, F.J.; Salas-Vidal, E.; Corredor-Adámez, M.; Bussman, J.; van der Sar, A.M.; Otto, G.W.; Geisler, R.; Spaink, H.P. Transcriptome profiling of adult zebrafish at the late stage of chronic tuberculosis due to Mycobacterium marinum infection. Mol. Immunol. 2005, 42, 1185–1203. [Google Scholar] [CrossRef]
- Oyarbide, U.; Iturria, I.; Rainieri, S.; Pardo, M.A. Use of gnotobiotic zebrafish to study Vibrio anguillarum pathogenicity. Zebrafish 2015, 12, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Chen, S.; Cao, Z.; Lin, Y.; Mo, D.; Zhang, H.; Gu, J.; Dong, M.; Liu, Z.; Xu, A. Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: Striking similarities and obvious differences with mammals. Mol. Immunol. 2007, 44, 295–301. [Google Scholar] [CrossRef]
- Lin, B.; Cao, Z.; Su, P.; Zhang, H.; Li, M.; Lin, Y.; Zhao, D.; Shen, Y.; Jing, C.; Chen, S.; et al. Characterization and comparative analyses of zebrafish intelectins: Highly conserved sequences, diversified structures and functions. Fish Shellfish Immunol. 2009, 26, 396–405. [Google Scholar] [CrossRef]
- Sieger, D.; Stein, C.; Neifer, D.; van der Sar, A.M.; Leptin, M. The role of gamma interferon in innate immunity in the zebrafish embryo. Dis. Model. Mech. 2009, 2, 571–581. [Google Scholar] [CrossRef] [Green Version]
- López-Muñoz, A.; Roca, F.J.; Sepulcre, M.P.; Meseguer, J.; Mulero, V. Zebrafish larvae are unable to mount a protective antiviral response against waterborne infection by spring viremia of carp virus. Dev. Comp. Immunol. 2010, 34, 546–552. [Google Scholar] [CrossRef]
- Varela, M.; Diaz-Rosales, P.; Pereiro, P.; Forn-Cuní, G.; Costa, M.M.; Dios, S.; Romero, A.; Figueras, A.; Novoa, B. Interferon-induced genes of the expanded IFIT family show conserved antiviral activities in non-mammalian species. PLoS ONE 2014, 9, e100015. [Google Scholar] [CrossRef]
- Candel, S.; Sepulcre, M.P.; Espín-Palazón, R.; Tyrkalska, S.D.; de Oliveira, S.; Meseguer, J.; Mulero, V. Md1 and Rp105 regulate innate immunity and viral resistance in zebrafish. Dev. Comp. Immunol. 2015, 50, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Guo, X.; Lu, L.F.; Lu, X.B.; Wu, N.; Zhang, Y.A. Regulation pattern of fish irf4 (the gene encoding IFN regulatory factor 4) by STAT6, c-Rel and IRF4. Dev. Comp. Immunol. 2015, 51, 65–73. [Google Scholar] [CrossRef]
- Pereiro, P.; Varela, M.; Diaz-Rosales, P.; Romero, A.; Dios, S.; Figueras, A.; Novoa, B. Zebrafish Nk-lysins: First insights about their cellular and functional diversification. Dev. Comp. Immunol. 2015, 51, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Varela, M.; Forn-Cuní, G.; Dios, S.; Figueras, A.; Novoa, B. Proinflammatory caspase a activation and an antiviral state are induced by a zebrafish perforin after possible cellular and functional diversification from a myeloid ancestor. J. Innate Immun. 2016, 8, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Espín-Palazón, R.; Martínez-López, A.; Roca, F.J.; López-Muñoz, A.; Tyrkalska, S.D.; Candel, S.; García-Moreno, D.; Falco, A.; Meseguer, J.; Estepa, A.; et al. TNFα impairs rhabdoviral clearance by inhibiting the host autophagic antiviral response. PLoS Pathog. 2016, 12, e1005699. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Zhang, Q.M.; Zhang, Y.B.; Li, Z.; Zhang, J.; Xiong, Y.W.; Wu, M.; Gui, J.F. Zebrafish IRF1, IRF3, and IRF7 differentially regulate IFNΦ1 and IFNΦ3 expression through assembly of homo- or heteroprotein complexes. J. Immunol. 2016, 197, 1893–1904. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Y.; Lu, J.; Wang, G. An imidazole coumarin derivative enhances the antiviral response to spring viremia of carp virus infection in zebrafish. Virus Res. 2019, 263, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Encinas, P.; Rodriguez-Milla, M.; Novoa, B.; Estepa, A.; Figueras, A.; Coll, J. Zebrafish fin immune responses during high mortality infections with viral haemorrhagic septicemia rhabdovirus. A proteomic and transcriptomic approach. BMC Genom. 2010, 11, 518. [Google Scholar] [CrossRef] [Green Version]
- Dios, S.; Romero, A.; Chamorro, R.; Figueras, A.; Novoa, B. Effect of the temperature during antiviral immune response ontogeny in teleosts. Fish Shellfish Immunol. 2010, 29, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estepa, A.; Coll, J. Innate multigene family memories are implicated in the viral-survivor zebrafish phenotype. PLoS ONE 2015, 10, e0135483. [Google Scholar] [CrossRef] [PubMed]
- Swaim, L.E.; Connolly, L.E.; Volkman, H.E.; Humbert, O.; Born, D.E.; Ramakrishnan, L. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect. Immun. 2006, 74, 6108–6117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogryzko, N.V.; Lewis, A.; Wilson, H.L.; Meijer, A.H.; Renshaw, S.A.; Elks, P.M. Hif-1α-Induced expression of Il-1β protects against mycobacterial infection in zebrafish. J. Immunol. 2019, 202, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Varela, M.; Romero, A.; Dios, S.; van der Vaart, M.; Figueras, A.; Meijer, A.H.; Novoa, B. Cellular visualization of macrophage pyroptosis and interleukin-1β release in a viral hemorrhagic infection in zebrafish larvae. J. Virol. 2014, 88, 12026–12040. [Google Scholar] [CrossRef] [Green Version]
- Von Gersdorff Jørgensen, L. The dynamics of neutrophils in zebrafish (Danio rerio) during infection with the parasite Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2016, 55, 159–164. [Google Scholar] [CrossRef]
- O’Toole, R.; von Hofsten, J.; Rosqvist, R.; Olsson, P.; Wolf-Watz, H. Visualisation of zebrafish infection by GFP-labelled Vibrio anguillarum. Microb. Pathog. 2004, 37, 41–46. [Google Scholar] [CrossRef]
- Harriff, M.J.; Bermudez, L.E.; Kent, M.L. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: A potential model for environmental mycobacterial infection. J. Fish Dis. 2007, 30, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Takaki, K.; Davis, J.M.; Winglee, K.; Ramakrishnan, L. Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nat. Protoc. 2013, 8, 1114–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, J.G.; Korbut, R.; Ohtani, M.; von Gersdorff Jørgensen, L. Zebrafish (Danio rerio) as a model to visualize infection dynamics of Vibrio anguillarum following intraperitoneal injection and bath exposure. Fish Shellfish Immunol. 2017, 67, 692–697. [Google Scholar] [CrossRef]
- Ludwig, M.; Palha, N.; Torhy, C.; Briolat, V.; Colucci-Guyon, E.; Bremont, M.; Herbomel, P.; Boudinot, P.; Levraud, J. Whole-body analysis of a viral infection: Vascular endothelium is a primary target of infectious hematopoietic necrosis virus in zebrafish larvae. PloS Pathog. 2011, 7, e1001269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, G.; Batts, W.; Winton, J. Susceptibility of zebrafish (Danio rerio) to a model pathogen, spring viremia of carp virus. Comp. Med. 2003, 53, 514–521. [Google Scholar] [PubMed]
- Miller, R.A.; Harbottle, H. Antimicrobial Drug Resistance in Fish Pathogens. Microbiol. Spectr. 2018, 6, 501–520. [Google Scholar] [CrossRef]
- Davis, J.M.; Clay, H.; Lewis, J.L.; Ghori, N.; Herbomel, P.; Ramakrishnan, L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 2002, 17, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Wang, C.; Zhang, K.; Kang, M.; Liang, R.; Zhang, X.; Yan, B. Visualization of macrophage lytic cell death during mycobacterial infection in zebrafish embryos via intravital microscopy. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Prouty, M.G.; Correa, N.E.; Barker, L.P.; Jagadeeswaran, P.; Klose, K.E. Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiol. Lett. 2003, 225, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Cosma, C.L.; Swaim, L.E.; Volkman, H.; Ramakrishnan, L.; Davis, J.M. Zebrafish and frog models of Mycobacterium marinum infection. Curr. Protoc. Microbiol. 2006, 3, 10B.2.1–10B.2.33. [Google Scholar] [CrossRef]
- Johansen, M.D.; Hortle, E.; Kasparian, J.A.; Romero, A.; Novoa, B.; Figueras, A.; Britton, W.J.; de Silva, K.; Purdie, A.C.; Oehlers, S.H. Analysis of mycobacterial infection-induced changes to host lipid metabolism in a zebrafish infection model reveals a conserved role for LDLR in infection susceptibility. Fish Shellfish Immunol. 2018, 83, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Kasparian, J.A.; Hortle, E.; Britton, W.J.; Purdie, A.C.; Oehlers, S.H. Mycobacterium marinum infection drives foam cell differentiation in zebrafish infection models. Dev. Comp. Immunol. 2018, 88, 169–172. [Google Scholar] [CrossRef]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, A.; Calado, R.; Gomes, N.C.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS ONE 2014, 9, e114197. [Google Scholar] [CrossRef] [PubMed]
- Caruffo, M.; Navarrete, N.; Salgado, O.; Díaz, A.; López, P.; García, K.; Feijóo, C.G.; Navarrete, P. Potential probiotic yeasts isolated from the fish gut protect zebrafish (Danio rerio) from a Vibrio anguillarum challenge. Front. Microbiol. 2015, 6, 1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Tian, X.; Kuang, S.; Liu, G.; Zhang, C.; Sun, C. Antagonistic activity and mode of action of phenazine-1-carboxylic acid, produced by Marine Bacterium. Front. Microbiol. 2017, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, H.; Liu, Q.; Wang, Q.; Xiao, J.; Zhang, Y. Skin-injured zebrafish, Danio rerio, are more susceptible to Vibrio anguillarum infection. J. World Aquac. Soc. 2015, 46, 301–310. [Google Scholar] [CrossRef]
- Gudding, R.; Van Muiswinkel, W.B. A history of fish vaccination: Science-based disease prevention in aquaculture. Fish Shellfish Immunol. 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Martin, S.A.; Douglas, A.; Houlihan, D.F.; Secombes, C.J. Starvation alters the liver transcriptome of the innate immune response in Atlantic salmon (Salmo salar). BMC Genom. 2010, 11, 418. [Google Scholar] [CrossRef] [Green Version]
- Da’as, S.; Teh, E.M.; Dobson, J.T.; Nasrallah, G.K.; McBride, E.R.; Wang, H.; Neuberg, D.S.; Marshall, J.S.; Lin, T.J.; Berman, J.N. Zebrafish mast cells possess an FcεRI-like receptor and participate in innate and adaptive immune responses. Dev. Comp. Immunol. 2011, 35, 125–134. [Google Scholar] [CrossRef]
- Kumar, G.; Menanteau-Ledouble, S.; Saleh, M.; El-Matbouli, M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet. Res. 2015, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jank, T.; Eckerle, S.; Steinemann, M.; Trillhaase, C.; Schimpl, M.; Wiese, S.; van Aalten, D.M.; Driever, W.; Aktories, K. Tyrosine glycosylation of Rho by Yersinia toxin impairs blastomere cell behaviour in zebrafish embryos. Nat. Commun. 2015, 6, 7807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, R.; Hannick, L.; Shanmugam, D.; Hostetler, J.; Brami, D.; Joardar, V.; Johnson, J.; Radune, D.; Singh, I.; Badger, J.; et al. Comparative genomics of the pathogenic ciliate Ichthyophthirius multifiliis, its free-living relatives and a host species provide insights into adoption of a parasitic lifestyle and prospects for disease control. Genome Biol. 2011, 12, R100. [Google Scholar] [CrossRef] [Green Version]
- Crim, M.J.; Riley, L.K. Viral diseases in zebrafish: What is known and unknown. ILAR J. 2012, 53, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, M.; Figueras, A.; Novoa, B. Modelling viral infections using zebrafish: Innate immune response and antiviral research. Antivir. Res. 2017, 139, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Baudouy, A.M.; Danton, M.; Merle, G. SVCV infection of Carp (author’s translation). Ann. Rech. Vet. 1980, 11, 245–249. [Google Scholar]
- Ahne, W.; Bjorklund, H.V.; Essbauer, S.; Fijan, N.; Kurath, G.; Winton, J.R. Spring viremia of carp (SVC). Dis. Aquat. Organ 2002, 52, 261–272. [Google Scholar] [CrossRef]
- Ashraf, U.; Lu, Y.; Lin, L.; Yuan, J.; Wang, M.; Liu, X. Spring viraemia of carp virus: Recent advances. J. Gen. Virol. 2016, 97, 1037–1051. [Google Scholar] [CrossRef]
- Ahne, W. The influence of environmental temperature and infection route on the immune response of carp (Cyprinus carpio) to spring viremia of carp virus (SVCV). Vet. Immunol. Immunopathol. 1986, 12, 383–386. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Lu, Y.; Wang, F.; Liu, L.; Liu, J.; Liu, X. Comparative transcriptome analysis of zebrafish (Danio rerio) brain and spleen infected with spring viremia of carp virus (SVCV). Fish Shellfish Immunol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- LaPatra, S.E.; Barone, L.; Jones, G.R.; Zon, L.I. Effects of infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus infection on hematopoietic precursors of the zebrafish. Blood Cells Mol. Dis. 2000, 26, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Collodi, P. Novel form of fibronectin from zebrafish mediates infectious hematopoietic necrosis virus infection. J. Virol. 2002, 76, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzen, N.; Olesen, N.; Koch, C. Immunity to VHS virus in rainbow trout. Aquaculture 1999, 172, 41–61. [Google Scholar] [CrossRef]
- Kim, R.; Faisal, M. Emergence and resurgence of the viral hemorrhagic septicaemia virus (Novirhabdovirus, Rhabdoviridae, Mononegavirales). J. Adv. Res. 2011, 2, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Guo, T.C.; Vakharia, V.N.; Evensen, Ø. Specific nucleotides at the 3′-terminal promoter of viral hemorrhagic septicemia virus are important for virulence in vitro and in vivo. Virology 2015, 476, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeley, R.J.; Perlmutter, A.; Seeley, V.A. Inheritance and longevity of infectious pancreatic necrosis virus in the zebrafish, Barachydanio rerio (Hamilton-Buchanan). Appl. Environ. Microbiol. 1977, 34, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Medina-Gali, R.; Belló-Pérez, M.; Ciordia, S.; Mena, M.C.; Coll, J.; Novoa, B.; Ortega-Villaizán, M.D.M.; Perez, L. Plasma proteomic analysis of zebrafish following spring viremia of carp virus infection. Fish Shellfish Immunol. 2019, 86, 892–899. [Google Scholar] [CrossRef]
- Dickerson, H.W.; Findly, R.C. Immunity to Ichthyophthirius infections in fish: A synopsis. Dev. Comp. Immunol. 2014, 43, 290–299. [Google Scholar] [CrossRef]
- Christoffersen, T.B.; Kania, P.W.; von Gersdorff Jørgensen, L.; Buchmann, K. Zebrafish Danio rerio as a model to study the immune response against infection with Ichthyophthirius multifiliis. J. Fish Dis. 2016, 40, 847–852. [Google Scholar] [CrossRef]
- Gonzalez, S.F.; Buchmann, K.; Nielsen, M.E. Ichthyophthirius multifiliis infection induces massive up-regulation of serum amyloid a in carp (Cyprinus carpio). Vet. Immunol. Immunopathol. 2007, 115, 172–178. [Google Scholar] [CrossRef]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Marnis, H.; Setyawan, A.C.; Buchmann, K. Differential immune gene response in gills, skin, and spleen of rainbow trout Oncorhynchus mykiss infected by Ichthyophthirius multifiliis. PLoS ONE 2019, 14, e0218630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherry, B. Laboratory Infection of Zebrafish (Danio rerio) and Channel Catfish (Ictalurus punctuates) with the Protozoan Parasite Ichthyophthirius multifiliis: A Model for Parasite Persistence. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2003. [Google Scholar]
- Dóró, É.; Jacobs, S.H.; Hammond, F.R.; Schipper, H.; Pieters, R.P.; Carrington, M.; Wiegertjes, G.F.; Forlenza, M. Visualizing trypanosomes in a vertebrate host reveals novel swimming behaviours, adaptations and attachment mechanisms. Elife 2019, 8, e48388. [Google Scholar] [CrossRef]
- Yazawa, R.; Hirono, I.; Aoki, T. Transgenic zebrafish expressing chicken lysozyme show resistance against bacterial diseases. Transgenic Res. 2006, 15, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.C.; Pan, C.Y.; Chen, J.Y. Tilapia hepcidin (TH)2-3 as a transgene in transgenic fish enhances resistance to Vibrio vulnificus infection and causes variations in immune-related genes after infection by different bacterial species. Fish Shellfish Immunol. 2010, 29, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Gesto, M.; Hernández, J.; López-Patiño, M.A.; Soengas, J.L.; Míguez, J.M. Is gill cortisol concentration a good acute stress indicator in fish? A study in rainbow trout and zebrafish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 188, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of pepsinogen gene in the genome of stomachless fish, Takifugu rubripes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 140, 133–140. [Google Scholar] [CrossRef]
- Monson, C.A.; Sadler, K.C. Inbreeding depression and outbreeding depression are evident in wild-type zebrafish lines. Zebrafish 2010, 7, 189–197. [Google Scholar] [CrossRef]
- Ljungfeldt, L.E.R.; Quintela, M.; Besnier, F.; Nilsen, F.; Glover, K.A. A pedigree-based experiment reveals variation in salinity and thermal tolerance in the salmon louse. Evol. Appl. 2017, 10, 1007–1019. [Google Scholar] [CrossRef]
| Focus Area | Bacteria | Virus | Parasites | |||
|---|---|---|---|---|---|---|
| N° | % | N° | % | N° | % | |
| Prophylactic approaches | 12 | 30 | 5 | 14.7 | 0 | 0 |
| Immunology | 17 | 42.5 | 21 | 61.8 | 4 | 57.1 |
| Infection biology | 11 | 27.5 | 8 | 23.5 | 3 | 42.9 |
| Total | 40 | 100 | 34 | 100 | 7 | 100 |
| Pathogen | Focus Area | Zebrafish Stage | Reference |
|---|---|---|---|
| M. marinum | Prophylactic approaches | Adult Larvae | Cui et al. 2010 [39] Hortle et al. 2019 [54] |
| Immunology | Larvae Adult Adult Larvae Larvae Larvae | Davis et al. 2002 [108] Meijer et al. 2005 [80] Swaim et al. 2006 [97] Hortle et al. 2019 [54] Niu et al. 2019 [109] Ogryzko et al. 2019 [98] | |
| Infection biology | Adult Larvae Adult Larvae Larvae Adult, larvae | Prouty et al. 2003 [110] Cosma et al. 2006 [111] Harriff et al. 2007 [102] Takaki et al. 2013 [103] Johansen et al. 2018 [112] Johansen et al. 2018 [113] | |
| V. anguillarum | Prophylactic approaches | Adult Adult Adult Adult Adult Larvae Larvae Larvae | Zhang et al. 2012 [40] Zhang et al. 2013 [41] Liu et al. 2014 [42] Zhang et al. 2014 [43] Gao et al. 2014 [44] Silva et al. 2014 [115] Caruffo et al. 2015 [116] Zhang et al. 2017 [117] |
| Immunology | Adult Adult Adult Adult Adult Larvae | Zhang et al. 2012 [40] Zhang et al. 2013 [41] Liu et al. 2014 [42] Zhang et al. 2014 [43] Gao et al. 2014 [44] Oyarbide et al. 2015 [81] | |
| Infection biology | Larvae Larvae Adult Adult | O’Toole et al. 2004 [101] Oyarbide et al. 2015 [81] Liu et al. 2015 [118] Schmidt et al. 2017 [104] | |
| A. salmonicida | Immunology | Adult Adult Adult, larvae | Lin et al. 2007 [82] Lin et al. 2009 [83] Da’as et al. 2011 [121] |
| Y. ruckeri | Prophylactic approaches | Adult, larvae | Korbut et al. 2016 [45] |
| Immunology | Larvae | Sieger et al. 2009 [84] | |
| Infection biology | Larvae | Jank et al. 2015 [123] | |
| F. psychrophilum | Prophylactic approaches | Larvae | Solís et al. 2015 [46] |
| Immunology | Larvae | Solís et al. 2015 [46] |
| Pathogen | Focus Area | Zebrafish Stage | Reference |
|---|---|---|---|
| SVCV | Prophylactic approaches | Adult Adult | Encinas et al. 2013 [55] García-Valtanen et al. 2014 [47] |
| Immunology | Adult Adult, larvae Adult, larvae Larvae Adult Larvae Adult Larvae Adult Adult, larvae Adult, larvae Larve Adult Adult Adult | Levraud et al. 2007 [75] Aggad et al. 2009 [76] Aggad et al. 2010 [77] López-Muños et al. 2010 [85] Encinas et al. 2013 [55] Varela et al. 2014a [99] Varela et al. 2014b [86] Candel et al. 2015 [87] Li et al. 2015 [88] Pereiro et al. 2015 [89] Varela et al. 2016 [90] Espín-Palazón et al. 2016 [91] Feng et al. 2016 [92] Liu et al. 2019 [93] Medina-Gali et al. 2019 [138] | |
| Infection biology | Adult Adult | Sanders et al. 2003 [106] Wang et al. 2017 [131] | |
| IHNV | Immunology | Adult, larvae Adult, larvae Larvae | Aggad et al. 2009 [76] Aggad et al. 2010 [77] Briolat et al. 2014 [48] |
| Infection biology | Adult Larvae Larvae | LaPatra et al. 2000 [132] Liu et al. 2002 [133] Ludwig et al. 2011 [105] | |
| VHSV | Prophylactic approaches | Adult Adult Adult | Novoa et al. 2006 [49] Chinchilla et al. 2013 [53] Kavaliauskis et al. 2015 [50] |
| Immunology | Adult Adult, Larvae Adult | Encinas et al. 2010 [94] Dios et al. 2010 [95] Estepa and Coll 2015 [96] | |
| Infection biology | Adult | Kim et al. 2015 [136] | |
| IPNV | Infection biology | Adult Adult | Seeley et al. 1977 [137] LaPatra et al. 2000 [132] |
| Pathogen | Focus Area | Zebrafish Stage | Reference |
|---|---|---|---|
| I. multifiliis | Immunology | Adult Adult Adult Adult | Jørgensen 2016 [51] Christoffersen et al. 2016 [142] Jørgensen 2016 [100] Jørgensen et al. 2018 [52] |
| Infection biology | Adult Adult | Cherry 2003 [143] Jørgensen 2016 [51] | |
| T. carassii | Infection biology | Larvae | Dóró et al. 2019 [144] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, L.v.G. Zebrafish as a Model for Fish Diseases in Aquaculture. Pathogens 2020, 9, 609. https://doi.org/10.3390/pathogens9080609
Jørgensen LvG. Zebrafish as a Model for Fish Diseases in Aquaculture. Pathogens. 2020; 9(8):609. https://doi.org/10.3390/pathogens9080609
Chicago/Turabian StyleJørgensen, Louise von Gersdorff. 2020. "Zebrafish as a Model for Fish Diseases in Aquaculture" Pathogens 9, no. 8: 609. https://doi.org/10.3390/pathogens9080609
APA StyleJørgensen, L. v. G. (2020). Zebrafish as a Model for Fish Diseases in Aquaculture. Pathogens, 9(8), 609. https://doi.org/10.3390/pathogens9080609




