Effectiveness of Dunaliella salina Extracts against Bacillus subtilis and Bacterial Plant Pathogens
Abstract
:1. Introduction
2. Results
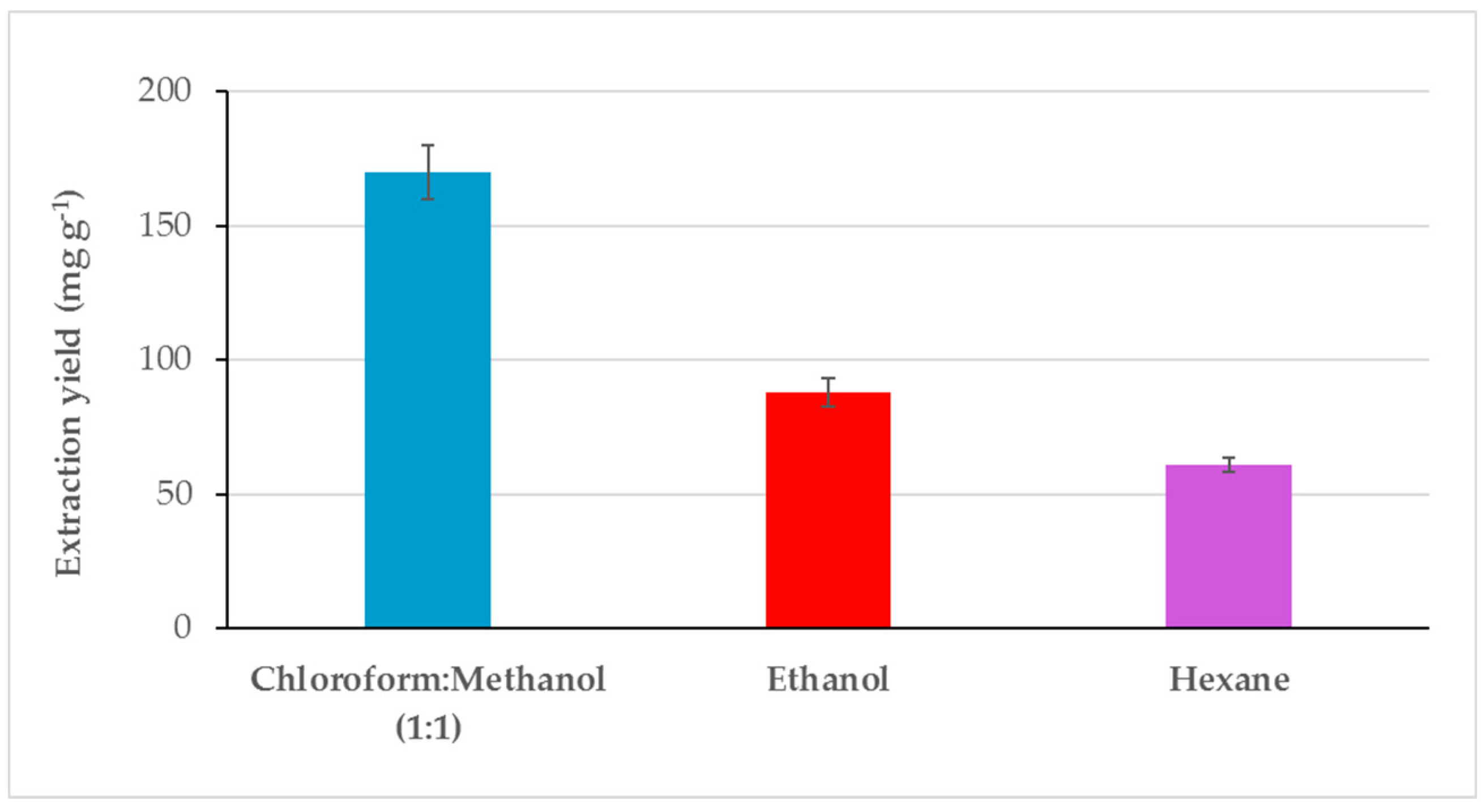
2.1. Extraction Yield and Characterization Pre- and Post-Extraction
2.2. In Vitro Antimicrobial Activity of Different Extracts of Microalgae
2.2.1. Disc Diffusion Method
2.2.2. Minimum Inhibitory Concentration
2.3. Effect of D. salina Extracts on Disease Development in In Vivo Conditions
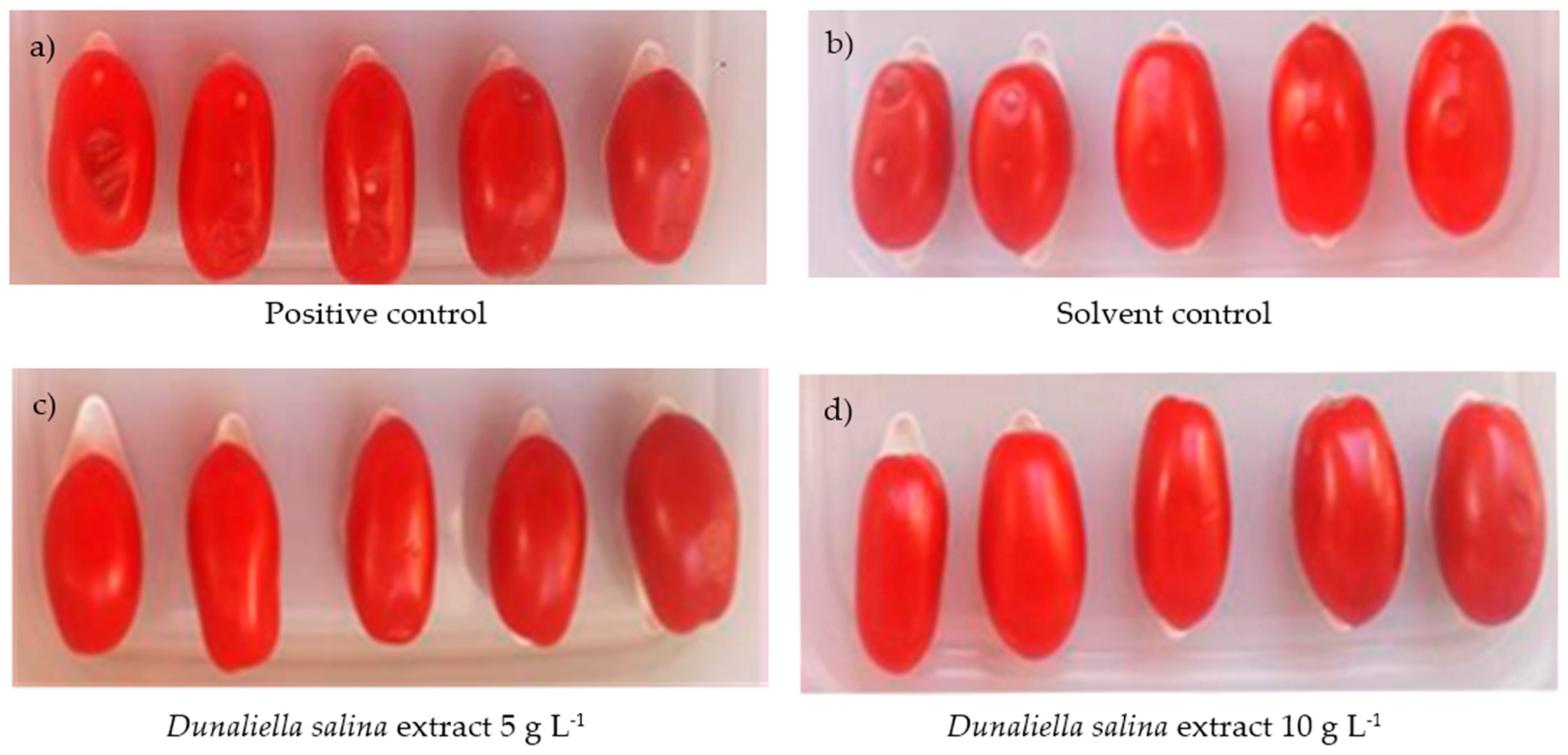
2.3.1. Application of D. salina Extracts to Control Bacterial Speck Spot Caused by P. syringae
2.3.2. Application of D. salina Extracts to Control Bacterial Soft Rot Caused by P. carotovorum subsp. carotovorum on Tomatoes and Zucchini Fruits
2.4. Relationship between β-carotene Concentration and Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Extraction and Chemical Characterization of D. salina Microalgae
4.2. In Vitro Antimicrobial
4.2.1. Agar Disc Diffusion Method
4.2.2. Minimum Inhibitory Concentration (MIC)
4.3. In Vivo Antimicrobial Assay
4.3.1. Application of D. salina Extracts to Bacterial Speck Spot caused by P. syringae
4.3.2. Application of D. salina Extracts to Control Bacterial Soft Rot caused by P. carotovorum
4.4. β-carotene Concentration and Antibacterial Activity
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Fitt, B.D.; McCartney, H.A.; West, J.S. Dispersal of Foliar Plant Pathogens: Mechanisms, Gradients and Spatial Patterns; Jones, D.G., Ed.; The Epidemiology of Plant Diseases; Springer: Dordrecht, the Netherlands, 2006; pp. 159–192. [Google Scholar] [CrossRef]
- Levetin, E. Aerobiology of Agricultural Pathogens. In Manual of Environmental Microbiology; Yates, M.V., Nakatsu, C.H., Miller, R.V., Pillai, S.D., Eds.; ASM Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Fletcher, J.D.; Daly, A.M.; Rodoni, B.C.; Viljanen-Rollinson, S.L.H. Techniques for the treatment, removal and disposal of host material during programmes for plant pathogen eradication. Plant Pathol. 2009, 58, 621–635. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, R.R. Postharvest diseases of fruits and vegetables and their management. In Postharvest Disinfection of Fruits and Vegetables; Academic Press: London, UK, 2018; pp. 1–52. [Google Scholar] [CrossRef]
- Ganeshan, S.; Neetoo, H. Pre-harvest Microbial Contamination of Tomato and Pepper Plants: Understanding the Pre-harvest Contamination Pathways of Mature Tomato and Bell Pepper Plants Using Bacterial Pathogen Surrogates. Adv. Crop Sci. Tech. 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [Green Version]
- Bhat, K.; Masood, N.; Bhat, N.; Ashraf, B.M.; Razvi, S.; Mi, M.; Akhtar, N.; Habib, M.B. Current status of post harvest soft rot in vegetables: A review. Asian J. Plant Sci. 2010, 9, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Pérombelon, M.C.M. Potato diseases caused by soft rot erwinia: An overview of pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar] [CrossRef]
- Gašić, K.; Gavrilović, V.; Dolovac, N.; Trkulja, N.; Živković, S.; Ristić, D.; Obradović, A. Pectobacterium carotovorum subsp. carotovorum: The causal agent of broccoli soft rot in Serbia. Pestic. Phytomed. 2014, 29, 249–255. [Google Scholar] [CrossRef]
- Xin, X.; Kvitko, B.; He, S. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant–pathogen interactions. Plant Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahamoud Ahmed, A.; Lyautey, E.; Bonnineau, C.; Dabrin, A.; Pesce, S. Environmental concentrations of copper, alone or in mixture with arsenic, can impact river sediment microbial community structure and functions. Front Microbiol. 2018, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef] [Green Version]
- Sargent, S.A.; Ritenour, M.A.; Brecht, J.K. Handling, Cooling and Sanitation Techniques for Maintaining Postharvest Quality; University of Florida Cooperative Extension Service, Institute of Food and Agriculturesciences: Gainesville, FL, USA, 2000; pp. 1–17. [Google Scholar]
- Ördög, V.; Stirk, W.A.; Lenobel, R.; Bancířová, M.; Strnad, M.; van Staden, J.; Szigeti, J.; Németh, L. Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J. Appl. Phycol. 2004, 16, 309–314. [Google Scholar] [CrossRef]
- Foteini, K.; Pavlos, M.; Maroudio, K.; Pascal, D. Antibacterial activity in microalgae cultures. Aquac. Res. 2011, 43, 1520–1527. [Google Scholar] [CrossRef]
- Aiyar, P.; Schaeme, D.; García-Altares, M.; Carrasco Flores, D.; Dathe, H.; Hertweck, C.; Sasso, S.; Mittag, M. Antagonistic bacteria disrupt calcium homeostasis and immobilize algal cells. Nat. Commun. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Metzger, P.; Rager, M.-N.; Largeau, C. Botryolins A and B, two tetramethylsqualene triethers from the green microalga. Botryococcus Braunii Phytochem. 2002, 59, 839–843. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Chaieb, K.; Bouraoui, A. Evaluation of antimicrobial activity of organic fractions of six marine algae from Tunisian Mediterranean coasts. Int. J. Pharm. Pharm. Sci. 2012, 4, 534–537. [Google Scholar]
- Bergmann, W.; Feeney, R.J. Contributors to the study of marine products. XXXII. The nucleosides of sponges. I.1. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. The isolation of a new thymine pentoside from sponges. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Baker, J.T. Seaweeds in pharmaceutical studies and applications. In Eleventh International Seaweed Symposium; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 29–40. [Google Scholar]
- Iglesias, M.J.; Soengas, R.; Probert, I.; Guilloud, E.; Gourvil, P.; Mehiri, M.; López, Y.; Cepas, V.; Gutiérrez-del-Río, I.; Redondo-Blanco, S.; et al. NMR characterization and evaluation of antibacterial and antiobiofilm activity of organic extracts from stationary phase batch cultures of five marine microalgae (Dunaliella sp., D. salina, Chaetoceros calcitrans, C. gracilis and Tisochrysis lutea). Phytochemistry 2019, 164, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Amal Maadane, A.; Merghoub, N.; El Mernissi, N.; Ainane, T.; Amzazi, S. Antimicrobial activity of marine microalgae isolated from Moroccan coastlines. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1257–1260. [Google Scholar] [CrossRef] [Green Version]
- Tao, N.; Gao, Y.; Liu, Y.; Ge, F. Carotenoids from the peel of Shatian pummelo (Citrus grandis Osbeck) and its antimicrobial activity. Am. Eurasian J. Agric. Environ. Sci. 2010, 7, 110–115. [Google Scholar]
- Ravikumar, S.; Uma, G.; Gokulakrishnan, R. Antibacterial property of halobacterial carotenoids against human bacterial pathogens. J. Sci. Ind. Res. 2016, 75, 253–257. [Google Scholar]
- Herrero, M.; Ibanez, E.; Cifuentes, A.; Reglero, G.; Santoyo, S. Dunaliella salina microalga pressurized liquid extracts as potential antimicrobials. J. Food Prot. 2006, 69, 2471–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasakumar, K.; Rajashekhar, M. In vitro studies on bactericidal activity and sensitivity pattern of isolated marine microalgae against selective human bacterial pathogens. Indian J. Sci. Technol. 2009, 2, 16–23. [Google Scholar] [CrossRef]
- Widowati, I.; Zainuri, M.; Kusumaningrum, H.P.; Maesaroh, Y.; Hardivillier, Y.; Leignel, V.; Bourgougnon, N.; Mouget, J. Identification of agents causing vibriosis in Litopenaeus vannamei shrimp culture in Kendal, Central Java, Indonesia and application of microalgae Dunaliella salina and Tetraselmis chui as bio-control agents against vibriosis. Aquac. Aquar. Conserv. Legis. 2018, 11, 101–107. [Google Scholar]
- Pane, G.; Cacciola, G.; Giacco, E.; Mariottini, G.L.; Coppo, E. Assessment of the antimicrobial activity of algae extracts on bacteria responsible of external otitis. Mar. Drugs 2015, 13, 6440–6452. [Google Scholar] [CrossRef]
- Sheu, C.W.; Freese, E. Lipopolysaccharide layer protection of Gram-negative bacteria against inhibition by long-chain fatty acids. J. Bacteriol. 1973, 115, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Clelia, A.; Antonio, B.; Daniela, C.; Milena, S. Effectiveness of fatty acids and their monoglycerides against Gram-negative pathogens. Int. J. Food Sci. Technol. 2008, 44, 359–366. [Google Scholar] [CrossRef]
- Jafari, S.; Mobasher, M.A.; Najafipour, S.; Ghasemi, Y.; Mohkam, M.; Ebrahimi, M.A.; Mobasher, N. Antibacterial potential of Chlorella vulgaris and Dunaliella salina extracts against Streptococcus mutans. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e13226. [Google Scholar] [CrossRef]
- Mendola, J.A.; Santoyo, S.; Cifuentes, A.; Reglero, G.; Ibáñez, E.; Señoráns, F.J. Antimicrobial activity of sub- and supercritical CO2 extracts of the green alga Dunaliella salina. J. Food Prot. 2008, 71, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, Y.S.; Kaya, M.; Asan-Ozusaglam, M. Biochemical composition and bioactivity screening of various extracts from Dunaliella salina, a green microalga. EXCLI J. 2014, 13, 679–690. [Google Scholar] [PubMed]
- Manimala, M.R.A.; Murugesan, R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J. Appl. Nat. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Falaise, C.; François, C.; Travers, M.-A.; Morga, B.; Haure, J.; Tremblay, R.; Turcotte, F.; Pasetto, P.; Gastineau, R.; Hardivillier, Y. Antimicrobial compounds from eukaryotic microalgae against human pathogens and diseases in aquaculture. Mar. Drugs. 2016, 14, 159. [Google Scholar] [CrossRef] [Green Version]
- Jena, J.; Subudhi, E. Microalgae: An untapped resource for natural antimicrobials. In The Role of Microalgae in Wastewater Treatment; Springer: Singapore, 2019; pp. 99–114. [Google Scholar] [CrossRef]
- Kirti, K.; Amita, S.; Priti, S.; Mukesh Kumar, A.; Jyoti, A. Colorful World of Microbes: Carotenoids and their applications. Adv. Biol. 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bhagavathy, S.; Sumathi, P.; Jancy Sherene Bell, I. Green algae Chlorococcum humicola- a new source of bioactive compounds with antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs. 2018, 16, 334. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar. Drugs. 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNI EN 12823-2:2000. Foodstuffs Determination of Vitamin a by High Performance Liquid Chromatography Measurement of β-carotene. Available online: http://store.uni.com/catalogo/index.php/uni-en-12823-2- 2000.html?_store=it&_from_store=en (accessed on 13 August 2018).
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2011, 46, 64–70. [Google Scholar] [CrossRef]
- Gullino, M.L.; Gilardi, G.; Sanna, M.; Garibaldi, A. Epidemiology of Pseudomonas syringae pv. syringae on tomato. Phytoparasitica 2009, 37, 461. [Google Scholar] [CrossRef]

| Chemical–Physical Features | |
|---|---|
| Humidity * | 6.63 ± 0.25 |
| Ash # | 48.74 ± 2.50 |
| Proteins # | 10.03 ± 0.57 |
| Carbohydrates # | 25.31 ± 1.55 |
| Lipids # | 3.49 ± 0.10 |
| Total Dietary Fibers # | 8.97 ± 0.50 |
| Carotenoids # | 3.46 ± 0.15 |
| Fatty acids methyl esters composition (mg 100 g−1 on dry basis) | |
| SFAs § | |
| Tridecanoic acid | <Ldl |
| Palmitic acid | 965.00 ± 1.15 |
| Pentadecanoic acid | <Ldl |
| Heptadecanoic acid | <Ldl |
| Stearic acid | 567.68 ± 0.56 |
| Arachidic acid | <Ldl |
| ∑ SFAs | 1532.68 ± 1.70 |
| MUFAs § | |
| Palmitoleic acid | <Ldl |
| cis-9-Octadecenoic acid (oleic acid) | 567.56 ± 1.29 |
| Myristoleic acid | <Ldl |
| Nervonic acid | <Ldl |
| Erucic acid | <Ldl |
| ∑ MUFAs | 567.56 ± 1.29 |
| PUFAs § | |
| cis-8,11,14-Eicosatrienoic acid | <Ldl |
| Linoelaidic acid | <Ldl |
| Linoleic acid | 519.75 ± 0.63 |
| γ-Linolenic acid | 536.22 ± 0.12 |
| Arachidonic acid | <Ldl |
| cis-5,8,11,14,17-Eicosapentaenoic acid | <Ldl |
| ∑ PUFAs | 1055.97 ± 0.75 |
| Compounds | Chloroform:Methanol (1:1) | Ethanol | Hexane |
|---|---|---|---|
| Ash | 30.3% | 32.5% | 36.2% |
| Protein | 26.4% | 24.8% | 14.1% |
| Carbohydrates | 3.0% | 2.6% | 1.4% |
| TDF | 9.1% | 8.5% | 5.6% |
| Carotenoids | 16.4% | 12.8% | 36.6% |
| of which: | |||
| Beta-carotene | 85.0% | 90.0% | 98.0% |
| Lutein | 15.0% | 10.0% | 2.0% |
| Lipids | 14.8% | 18.8% | 6.1% |
| of which FAMEs: | 90.1% | 85.3% | 90.0% |
| FAMEs composition: | |||
| SFAs | 30.1% | 35.0% | 32.0% |
| MUFAs | 60.1% | 56.7% | 65.9% |
| PUFAs | 9.8% | 8.3% | 2.1% |
| Samples | Concentration (mg mL−1) | Inhibition Zone (mm) | ||
|---|---|---|---|---|
| P. carotovorum subsp. carotovorum DSM30168 | P. syringae pv. tomato EPS3 | B. subtilis ET-1 | ||
| Chloroform:Methanol extract | 350.0 | 10.0 ± 0.1 | 8.0 ± 0.1 | 13.0 ± 0.1 |
| Ethanol extract | 214.0 | 11.0 ± 0.1 | 9.0 ± 0.1 | 21.0 ± 0.2 |
| Hexane extract | 97.0 | 9.0 ± 0.1 | 12.0 ± 0.1 | 20.0 ± 0.2 |
| Ciprofloxacin | 0.15 | 20.0 ± 0.2 | 24.0 ± 0.2 | 32.0 ± 0.3 |
| MIC Value (mg mL−1) | |||
|---|---|---|---|
| P. carotovorum | P. syringae | B. subtilis | |
| Chloroform:Methanol | >3.0 | >3.0 | 3.0 |
| Ethanol | >3.0 | >3.0 | 3.0 |
| Hexane | 3.0 | 3.0 | 0.3 |
| Treatments | 5 | 10 | 15 | |||
|---|---|---|---|---|---|---|
| DI (%) | DS (%) | DI (%) | DS (%) | DI (%) | DS (%) | |
| Positive Control | 3.2 ± 0.1 b* | 0.82 ± 0.3 b | 25.2 ± 0.1 b | 1.8 ± 0.3 b | 37.9 ± 0.2 b | 2.2 ± 0.1 b |
| Solvent Control | 3.5 ± 0.1 b | 0.93 ± 0.2 b | 24.2 ± 0.1 b | 1.85 ± 0.1 b | 36.7 ± 0.9 b | 2.9 ± 0.2 b |
| Hexane extract | 0.0 a | 0.0 a | 7.2 ± 0.5 a | 0.02 ± 0.1 a | 13.2 ± 0.4 a | 0.505 ± 0.1 a |
| Treatments | Disease Incidence (%) | |||
|---|---|---|---|---|
| Tomatoes Fruits | Zucchini Fruits | |||
| Incubation Time (hours) | ||||
| 48 | 96 | 48 | 96 | |
| Positive control | 33.4 ± 0.32 a* | 80.6 ± 0.56 b | 90.4 ± 0.33 a | 100.0 b |
| Solvent control | 27.7 ± 0.32 a | 77.9 ± 0.43 b | 86.2 ± 0.23 a | 100.0 b |
| Extract 10 mg mL−1 | 0.0 c ± 0.0 | 5.3 ± 0.23 c | 0.0 c ± 0.0 | 12.6 ± 0.15 c |
| Extract 5 mg mL−1 | 0.0 c ± 0.0 | 12.7 ± 0.12 a | 0.0 c ± 0.0 | 26.1 ± 0.22 a |
| Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|
| Sample | Sample Concentration (mg mL−1) | β-carotene Concentration (mg mL−1) | P. carotovorum subsp. carotovorum | P. syringae pv. | B. subtilis |
| β-carotene SD | 10 | 9.8 ± 0.5 a* | 10.5 ± 0.4 a | 18.1 ± 0.2 a | |
| β-carotene SD | 5 | 5.1 ± 0.3 b | 8.4 ± 0.3 b | 9.3 ± 0.2 c | |
| Chloroform:Methanol extract | 100 | 13.9 | 6.2 ± 0.2 b | 7.7 ± 0.3 b | 9.1 ± 0.6 c |
| Hexane extract | 100 | 35.9 | 10.5 ± 0.8 a | 11.2 ±0.7 a | 19.7 ± 0.2 a |
| Ethanol extract | 100 | 11.5 | 7.2 ± 0.2 b | 6.4 ± 0.4 c | 14.2 ± 0.8 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrico, A.; Trupo, M.; Magarelli, R.; Balducchi, R.; Ferraro, A.; Hristoforou, E.; Marino, T.; Musmarra, D.; Casella, P.; Molino, A. Effectiveness of Dunaliella salina Extracts against Bacillus subtilis and Bacterial Plant Pathogens. Pathogens 2020, 9, 613. https://doi.org/10.3390/pathogens9080613
Ambrico A, Trupo M, Magarelli R, Balducchi R, Ferraro A, Hristoforou E, Marino T, Musmarra D, Casella P, Molino A. Effectiveness of Dunaliella salina Extracts against Bacillus subtilis and Bacterial Plant Pathogens. Pathogens. 2020; 9(8):613. https://doi.org/10.3390/pathogens9080613
Chicago/Turabian StyleAmbrico, Alfredo, Mario Trupo, Rosaria Magarelli, Roberto Balducchi, Angelo Ferraro, Evangelos Hristoforou, Tiziana Marino, Dino Musmarra, Patrizia Casella, and Antonio Molino. 2020. "Effectiveness of Dunaliella salina Extracts against Bacillus subtilis and Bacterial Plant Pathogens" Pathogens 9, no. 8: 613. https://doi.org/10.3390/pathogens9080613










