Rickettsia africae an Agent of African Tick Bite Fever in Ticks Collected from Domestic Animals in Eastern Cape, South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Clearance
2.2. Study Area, Tick Collection and Identifications.
2.3. Ticks Collection and Studied Animals from Where Ticks Have Been Collected
2.4. Total Genomic DNA Extraction from Ticks
2.5. Molecular Identification of Tick and Detection of Bacteria in Ticks
2.6. DNA Sequencing, Sequence Editing and BLASTn Search
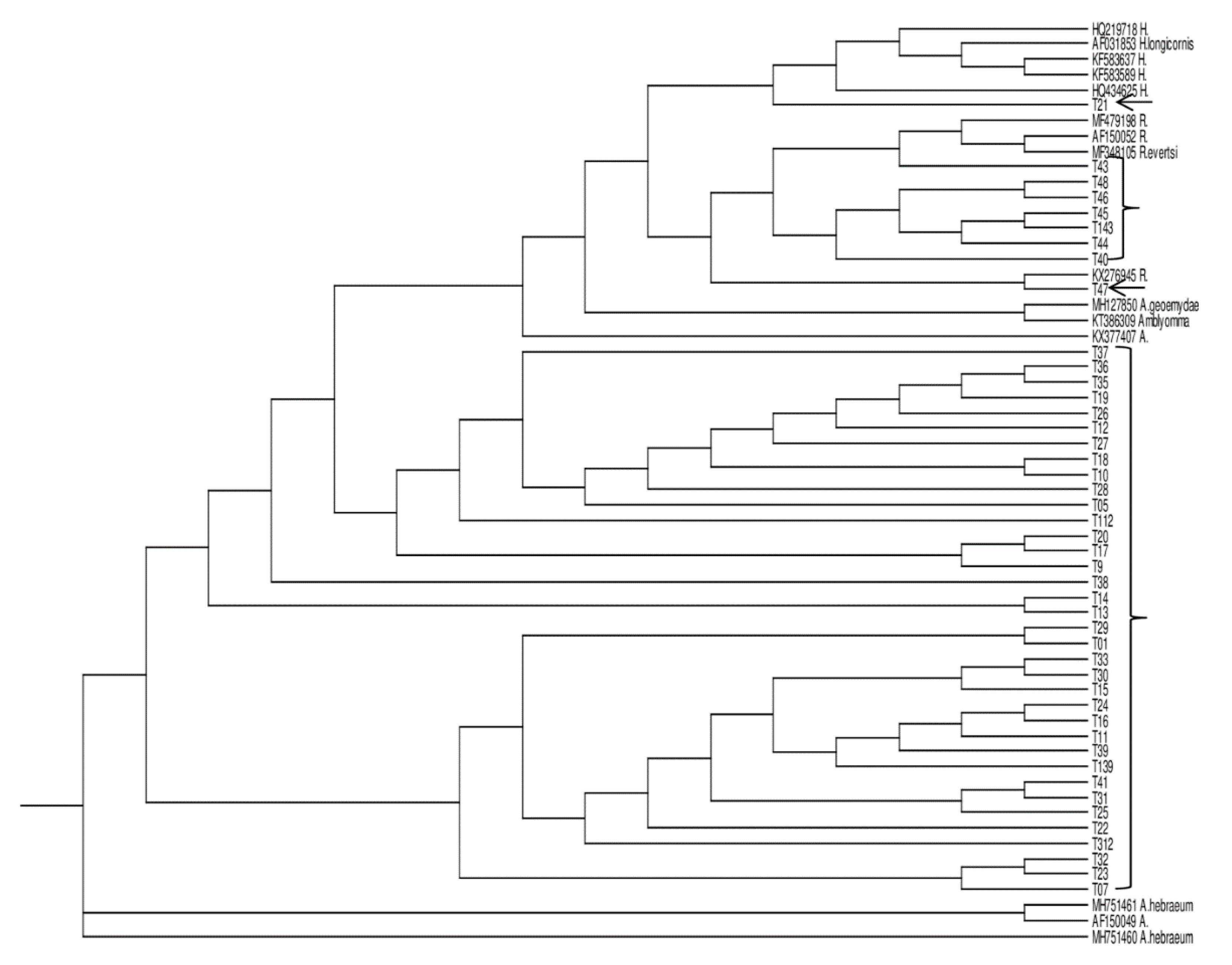
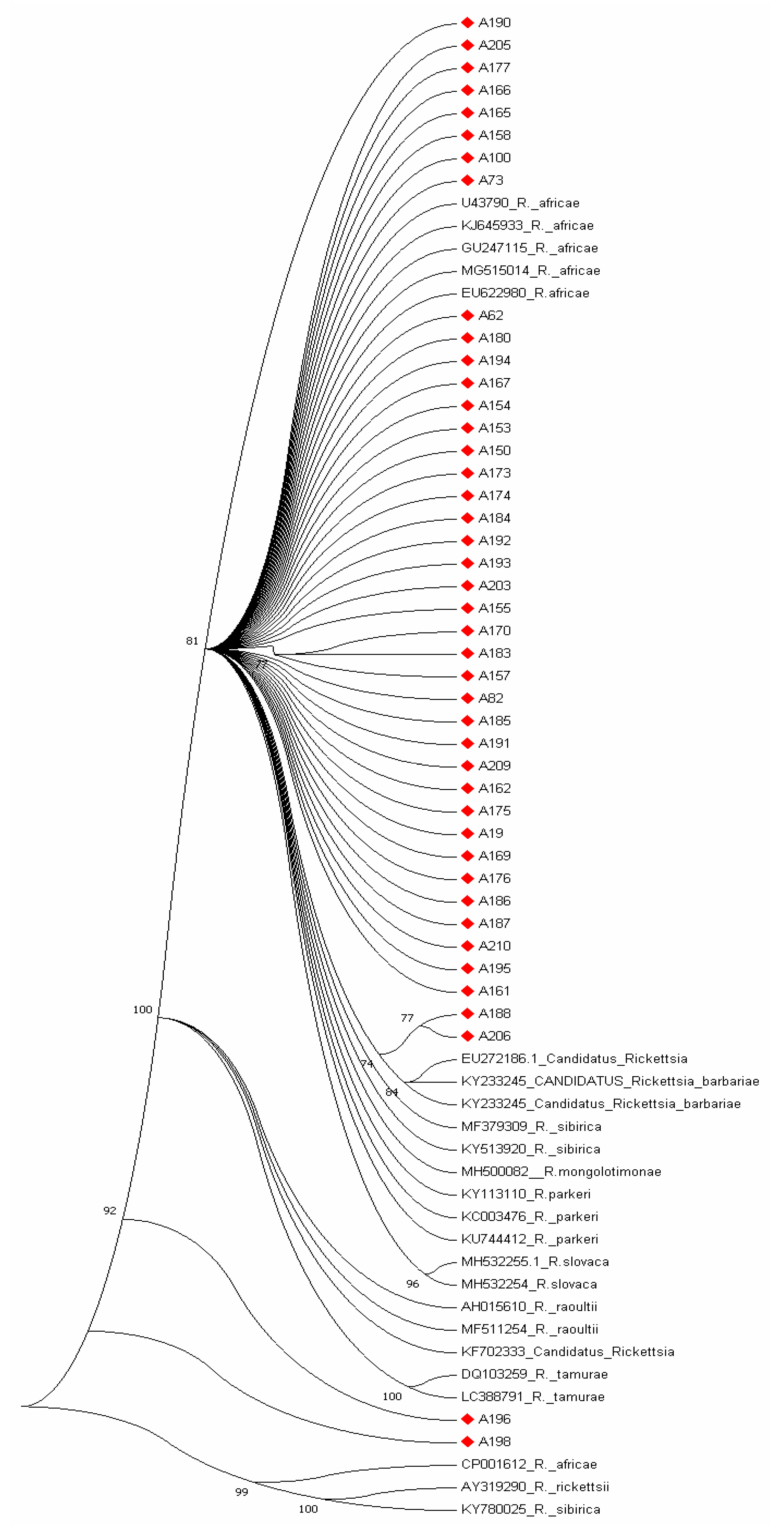
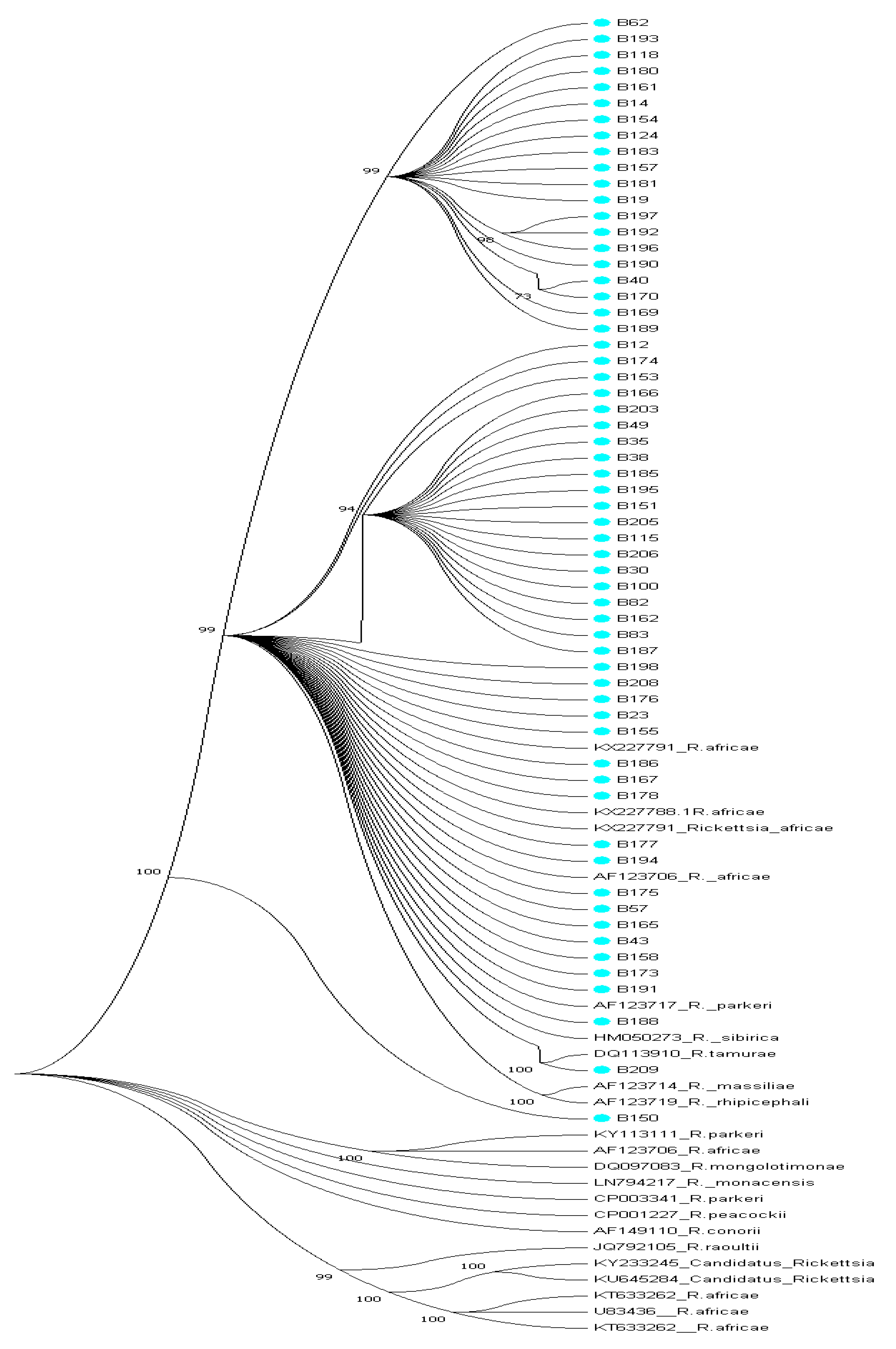
2.7. Phylogenetic Analysis
3. Results
3.1. Tick Prevalence within the Two Study Sites
3.2. Prevalence of Rickettsia spp in the Study Sites
3.3. GenBank Accession Numbers
4. Discussion
5. Conclusions and Recommendations
Endnote
| GenBank Accession numbers of Rickettsia reference sequences used in phylogenetic analyses of the ompA and ompB genes |
| OMPB |
| DQ113910_R.tamurae, CP003341_R.parkeri, AF123706_R.africae, KX227791_R.africae, AF149110_R.conorii, KX227788_R.africae, KY113111_R.parkeri, LN794217_R. monacensis, CP001227_R.peacockii, JQ792105_R.raoultii, U83436_R.africae, KY233245_Candidatus Rickettsia, AF123714_R. massiliae, AF123719_R. rhipicephali, HM050273_R. sibirica, KU645284_Candidatus Rickettsia, KT633262_R.africae, DQ097083_R. mongolotimonae, KX227791_R. africae, KT633262.1 R.africae, KY113111_R_ parkeri, AF123706_R. africae, AF123717_ R. parkeri |
| OmpA |
| CP001612_R. africae, AH015610_R. raoultii, AY319290_R. rickettsia, KF702333_Candidatus Rickettsia, KY780025_R. sibirica, EU272186_ Candidatus Rickettsia, MF511254_R. raoultii, MH500082 _R. mongolotimonae, MH532255_R.slovaca, KY113110_R.parkeri, KY233245_CANDIDATUS Rickettsia barbariae, MF379309_R. sibirica, KY513920_R. sibirica, EU622980_R.africae, MH532254_R.slovaca, MG515014_R. africae GU247115_R. africae, KJ645933_R. africae, U43790_R. africae, DQ103259_R. tamurae, LC388791_R. tamurae, KC003476_R. parkeri, KU744412_R. parkeri |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Britam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 657–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef] [PubMed]
- Bogovic, P.; Lotric-Furlan, S.; Korva, M.; Avsic-Zupanc, T. African Tick-Bite Fever in Traveler Returning to Slovenia from Uganda. Emerg. Infect. Dis. 2016, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shpynov, S.N.; Fournier, P.E.; Pozdnichenko, N.N.; Gumenuk, A.S.; Skiba, A.A. New approaches in the systematics of rickettsiae. New Microbe New Infect. 2018, 23, 93–102. [Google Scholar] [CrossRef]
- Portillo, A.; de Sousa, R.; Santibáñez, S.; Duarte, A.; Edouard, S.; Ifonseca, I.P.; Marques, C.; Novakova, M.; Palomar, A.M.; Santos, M. Guidelines for the detection of Rickettsia spp. Vector Borne Zoonotic Dis. 2017, 17, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. The relationship between spotted fever group Rickettsiae and ixodid ticks. Vet. Res. 2009, 40, 34. [Google Scholar] [CrossRef] [Green Version]
- Tomassone, L.; Portillo, A.; Nováková, M.; Sousa, R.; Oteo, J.A. Neglected aspects of tick-borne rickettsioses. Parasit. Vectors 2018, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Iweriebor, B.C.; Mmbaga, E.J.; Adegborioye, A.; Igwaran, A.; Obi, L.C.; Okoh, A.I. Genetic profiling for Anaplasma and Ehrlichia species in ticks collected in the Eastern Cape Province of South Africa. BMC Microbiol. 2017, 17, 45. [Google Scholar] [CrossRef] [Green Version]
- Yawa, M.; Nyangiwe, N.; Kadzere, C.T.; Muchenje, V.; Mpendulo, T.C.; Marufu, M.C. In search of the Rhipicephalus (Boophilus) microplus in the western-central regions of the Eastern Cape Province, South Africa. Ticks Tick-Borne Dis. 2019, 10, 564–567. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [Green Version]
- Marks, M.; Johnson, V.; Brown, M. Fever in the returned traveler. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1077–1086. [Google Scholar]
- Walker, A.R.; Bouattour, A.; Camicas, J.L.; Estrada-Pana, A.; Horak, I.G.; Latif, A.A. Ticks of Domestic Animals in Africa; A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; ISBN 0-9545173-0-X. [Google Scholar]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eremeeva, M.; Yu, X.; Raoult, D. Differentiation among spotted fever group rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J. Clin. Microbiol. 1994, 32, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Kollars, T.M., Jr.; Kengluecha, A. Spotted fever group Rickettsia in Dermacentor variabilis (Acari: Ixodidae) infesting raccoons (Carnivora:Procyonidae) and opossums (Marsupialia: Didelphimorphidae) in Tennessee. J. Med. Entomol. 2001, 38, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.C.; Billingsley, P.M.; Teltow, G.J.; Seals, J.P.; Turnbough, M.A.; Atkinson, S.F. Borrelia, Ehrlichia, and Rickettsia spp. in Ticks Removed from Persons, Texas, USA. Emerg. Infect. Dis. 2010, 16, 3. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.T.; Satjanadumrong, J.; Hughes, T.; Stenos, J.; Blacksell, S.D. Diagnosis of spotted fever group Rickettsia infections: The Asian perspective. Epidemiol. Infect. 2019, 147, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fournier, P.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene Sequence-Based Criteria for Identification of New Rickettsia Isolates and Description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef] [Green Version]
- Ndip, L.M.; Fokam, E.B.; Bouyer, D.H.; Ndip, R.N.; Titanji, V.P.; Walker, D.H. Detection of Rickettsia africae inpatients and ticks along the coastal region of Cameroon. Am. J. Trop. Med. Hyg. 2004, 71, 363–366. [Google Scholar] [CrossRef]
- Mediannikov, O.; Diatta, G.; Fenollar, F.; Sokhna, C.; Trape, J.F.; Raoult, D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl. Trop. Dis. 2010, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consigny, P.H.; Rolain, J.; Mizzi, D.; Raoult, D. African Tick-bite Fever in French Travelers. Emerg. Infect. Dis. 2005, 11, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, M.; Nicholson, W.L.; Roche, A.J.; Kersh, G.J.; Fitzpatrick, K.A.; Oliver, L.D.; Massung, R.F.; Morrissey, A.B.; Bartlett, J.A.; Onyango, J.J.; et al. Q Fever, Spotted Fever Group, and Typhus Group Rickettsioses Among Hospitalized Febrile Patients in Northern Tanzania. Clin. Infect. Dis. 2011, 53, e8–e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensenius, M.; Fournier, P.E.; Vene, S.; Hoel, T.; Hasle, G.; Henriksen, A.Z.; Hellum, K.B.; Raoult, D.; Myrvang, B.; Norwegian African Tick Bite Fever Study Group. Norwegian African Tick Bite Fever Study Group. African tick bite fever in travelers to rural sub-Equatorial Africa. Clin. Infect. Dis. 2003, 36, 1411–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, K.; Wallménius, K.; Rundlöf-Nygren, P.; Strömdah, S.; Påhlso, C. African tick bite fever in returning Swedish travelers. Report of two cases and aspects of diagnostics. Infect. Ecol. Epidemiol. 2017, 7, 1343081. [Google Scholar] [CrossRef]
- Socolovschi, C.; Matsumoto, K.; Marie, J.L.; Davoust, B.; Raoult, D.; Parola, P. Identification of rickettsiae, Uganda and Djibouti. Emerg. Infect. Dis. 2007, 13, 1508–1510. [Google Scholar] [CrossRef]
- Lorusso, V.; Gruszka, K.A.; Majekodunmi, A.; Igweh, A.; Welburn, S.C.; Picozzi, K. Rickettsia africae in Amblyomma variegatum ticks, Uganda and Nigeria. Emerg. Infect. Dis. 2013, 19, 1705–1707. [Google Scholar] [CrossRef]
- Angerami, R.N.; Krawczak, F.S.; Nieri-Bastos, F.A.; Santos, F.; Medorima, C.; Resende, M.R. First report of African tick-bite fever in a South American traveler. SAGE Open Med. Case Rep. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Harrison, N.; Burgmann, H.; Forstner, C.; Ramharter, M.; Széll, M.; Schötta, A. Molecular diagnosis of African tick bite fever using eschar swabs in a traveler returning from Tanzania. Wien. Klin. Wochenschr. 2016, 128, 602–605. [Google Scholar] [CrossRef] [Green Version]
- Nakao, R.; Qiu, Y.; Igarashi, M.; Magona, J.W.; Zhou, L.; Ito, K.; Sugimoto, C. High prevalence of spotted fever group rickettsiae in Amblyomma variegatum from Uganda and their identification using sizes of intergenic spacers. Ticks Tick-Borne Dis. 2013, 4, 506–512. [Google Scholar] [CrossRef]
- Waner, T.; Keysary, A.; Eremeeva, M.E.; Din, A.B.; Mumcuoglu, K.Y.; King, R.; Atiya-Nasagi, Y. Rickettsia africae and Candidatus Rickettsia barbariae in Ticks in Israel. Am. Soc. Trop. Med. Hyg. 2014, 90, 920–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yssouf, A.; Socolovschi, C.; Kernif, T.; Temmam, S.; Elagadec, E.; Tortosa, P.; Parola, P. First molecular detection of Rickettsia africae in ticks from the Union of the Comoros. Parasit. Vectors 2014, 7, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maina, A.N.; Jiang, J.; Omulo, S.A.; Cutler, S.J.; Ade, F.; Ogola, E.; Feikin, D.R.; Njenga, M.K.; Cleaveland, S.; Mpoke, S.; et al. High Prevalence of Rickettsia africae Variants in Amblyomma variegatum Ticks from Domestic Mammals in Rural Western Kenya: Implications for Human Health. Vector-Borne Zoonotic Dis. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehounoud, C.B.; Yao, K.P.; Dahmani, M.; Achi, Y.L.; Amanzougaghene, N.; N’Douba, A.K.; N’Guessan, J.D.; Raoult, D.; Fenollar, F.; Mediannikov, O. Multiple Pathogens Including Potential New Species in Tick Vectors in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2016. [Google Scholar] [CrossRef] [Green Version]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimita, G.; Mutai, B.; Nyanjom, S.G.; Wamunyokoli, F.; Waitumbi, J. Phylogenetic Variants of Rickettsia africae, and Incidental Identification of “Candidatus Rickettsia Moyalensis” in Kenya. PLoS Negl. Trop. Dis. 2016. [Google Scholar] [CrossRef]
- Paddock, C.D.; Finley, R.W.; Wright, C.S.; Robinson, H.N.; Schrodt, B.J.; Lane, C.C.; Ekenna, O.; Blass, M.A.; Tamminga, C.L.; Ohl, C.A.; et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 2008, 47, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Cowdry, E.V. The distribution of rickettsia in the tissues of insects and arachnids. J. Exp. Med. 1993, 37, 431–459. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.R.; Kohls, G.M.; Cox, G.W.; Davis, G.E. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939, 54, 1482–1484. [Google Scholar] [CrossRef]
- Chen, L.H.; Wilson, M.E. Tick-Borne Rickettsiosis in Traveler Returning from Honduras. Emerg. Infect. Dis. 2009, 15, 1321–1323. [Google Scholar] [CrossRef]
- Romer, Y.; Seijo, A.C.; Crudo, F.; Nicholson, W.L.; Varela-Stokes, A.; Lash, R.R.; Paddock, C.D. Rickettsia parkeri Rickettsiosis, Argentina. Emerg. Infect. Dis. 2011, 17, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Whitman, T.J.; Richards, A.L.; Paddock, C.D.; Tamminga, C.L.; Sniezek, P.J.; Jiang, J.; Byers, D.K.; Sanders, J.W. Rickettsia parkeri infection after tick bite, Virginia. Emerg. Infect. Dis. 2007, 13, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Halajian, A.; Palomar, A.M.; Portillo, A.; Heyne, H.; Romero, L.; Oteo, J.A. Detection of zoonotic agents and a new Rickettsia strain in ticks from donkeys from South Africa: Implications for travel medicine. Travel Med. Infect. Dis. 2018, 26, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Imaokaa, K.; Kanekoa, S.; Tabarab, K.; Kusatakea, K.; Morita, E. The First Human Case of Rickettsia tamurae Infection in Japan. Case Rep. Dermatol. 2011, 3, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Thu, M.J.; Qiu, Y.; Matsuno, K.; Kajihara, M.; Mori-Kajihara, A.; Omori, R.; Monma, N.; Chiba, K.; Seto, J.; Gokuden, M.; et al. Diversity of spotted fever group rickettsiae and their association with host ticks in Japan. Sci. Rep. 2019, 9, 1500. [Google Scholar] [CrossRef] [Green Version]
- Motoi, Y.; Asano, M.; Inokuma, H.; Ando, S.; Kawabata, H.; Takano, A.; Suzuki, M. Detection of Rickettsia tamurae DNA in ticks and wild boar (Sus scrofa leucomystax) skins in Shimane Prefecture, Japan. J. Vet. Med. Sci. 2013, 75, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Gaowa, N.O.; Aochi, M.; Wuritu, W.D.; Yoshikawa, Y.; Kawamori, F.; Honda, T.; Fujita, H.; Takada, N.; Oikawa, Y.; Kawabata, H.; et al. Rickettsiae in Ticks, Japan, 2007–2011. Emerg. Infect. Dis. 2013, 19, 338–340. [Google Scholar] [CrossRef]
- Blanco, C.M.; Teixeira, B.R.; da Silva, A.G.; de Oliveira, R.C.; Strecht, L.; Ogrzewalska, M.; de Lemos, E.R.S. Microorganisms in ticks (Acari: Ixodidae) collected on marsupials and rodents from Santa Catarina, Paraná and Mato Grosso do Sul states, Brazil. Ticks Tick-Borne Dis. 2017, 8, 90–98. [Google Scholar] [CrossRef]


| Primer Name | Gene Primer Sequence (5′ to 3′) | Amplicon bp | TM | Ref | |
|---|---|---|---|---|---|
| Tick DNA | 85F | 12S TTAAGCTTTTCAGAGGAATTTGCTC | 110 | 54.0 | [12] |
| 225R | 12S TTTWWGCTGCACCTTGACTTAA | 52.7 | |||
| Rickettsia spp. | Rr.190 70P | rompA ATGGCGAATATTTCTCCAAAA | 610 | 52.5 | [13] |
| Rr.190 602N | rompA AGTGCAGCATTCGCTCCCCCT | 64.9 | |||
| BG1-21 | rompB GGCAATTAATATCGCTGACGG | 511 | 55.6 | [14] | |
| BG2-20 | rompB GCATCTGCACTAGCACTTTC | 55.2 | |||
| RrCS372 | gltA TTTGTAGCTCTTCTCATCCTATGGC | 410 | 59.0 | [15] | |
| RrCS989 | gltA CCAAGTTCCTTTAATACTTCTTTGC | 57.4 |
| Animals | Tick Species | Developmental Stage | Number of Ticks | Rickettisa Positive Samples |
|---|---|---|---|---|
| CATTLE | R. eversti | Adult | 77 | 5 |
| A. hebraeum | Adult | 170 | 25 | |
| R. microplus | Adult | 27 | ||
| R. appendiculatus | Adult | 94 | 3 | |
| R. simus | Adult | 67 | ||
| H. truncatum | Adult | 55 | ||
| GOAT | R. eversti | Adult | 0 | |
| A. hebraeum | Adult | 133 | 12 | |
| R. microplus | Nymph | 79 | ||
| R. simus | Adult | 24 | ||
| H. truncatum | Adult | 20 | ||
| R. appendiculatus | Adult | 62 | 2 | |
| SHEEP | R. eversti | Adult | 9 | 9 |
| A. hebraeum | Adult | 65 | 4 | |
| R. microplus | Adult | 27 | ||
| R. simus | Adult | 21 | ||
| H. truncatum | Adult | 18 | ||
| HORSE | A. hebraeum | Adult | 25 |
| Strain Accession Number | Species | Geographical Origin |
|---|---|---|
| KU284929 | A. trigrinum | Brazil |
| KU284920 | A. triste | Uruguay |
| KU284864 | A. parvitarsum | Argentina |
| KY676832 | R. annulatus | Israel |
| KY676839 | R. australis | South Africa |
| MF479198 | R. evertsi | DRC |
| AF150043 | Boophilus | Jordan |
| EU921766 | R. microplus | Mozambique |
| KU568502 | R. geigyi | Guinea-Bissau |
| MK332391 | R. microplus | Uganda |
| MG076938 | A. maculatum | Mexico |
| KX377407 | A. gemma | Ethiopia |
| AF150049 | A. hebraeum | Zimbabwe |
| AF031865 | R. punctatus | Australia |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iweriebor, B.C.; Nqoro, A.; Obi, C.L. Rickettsia africae an Agent of African Tick Bite Fever in Ticks Collected from Domestic Animals in Eastern Cape, South Africa. Pathogens 2020, 9, 631. https://doi.org/10.3390/pathogens9080631
Iweriebor BC, Nqoro A, Obi CL. Rickettsia africae an Agent of African Tick Bite Fever in Ticks Collected from Domestic Animals in Eastern Cape, South Africa. Pathogens. 2020; 9(8):631. https://doi.org/10.3390/pathogens9080631
Chicago/Turabian StyleIweriebor, Benson Chuks, Ayabulela Nqoro, and Chikwelu Larry Obi. 2020. "Rickettsia africae an Agent of African Tick Bite Fever in Ticks Collected from Domestic Animals in Eastern Cape, South Africa" Pathogens 9, no. 8: 631. https://doi.org/10.3390/pathogens9080631





