Characterization of Cutaneous Bacterial Microbiota from Superficial Pyoderma Forms in Atopic Dogs
Abstract
:1. Introduction
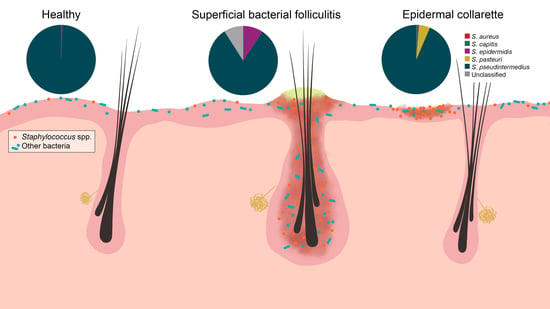
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bloom, P. Canine superficial bacterial folliculitis: Current understanding of its etiology, diagnosis and treatment. Vet. J. 2014, 199, 217–222. [Google Scholar] [CrossRef]
- Hill, P.B.; Lo, A.; Eden, C.A.N.; Huntley, S.; Morey, V.; Ramsey, S.; Richardson, C.; Smith, D.J.; Sutton, C.; Taylor, M.D.; et al. Survey of the prevalence, diagnosis and treatment of dermatological conditions in small animals in general practice. Vet. Rec. 2006, 158, 533–539. [Google Scholar] [CrossRef]
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Kolar, L.M.; Klausner, J.S. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J. Am. Vet. Med. Assoc. 1999, 214, 1336–1341. [Google Scholar]
- Mason, I.S. Canine pyoderma. J. Small Anim. Pr. 1991, 32, 381–386. [Google Scholar] [CrossRef]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Muller & Kirk’s Small Animal Dermatology, 7th ed.; Elsevier: St. Louis, MO, USA, 2013. [Google Scholar]
- Gross, T.L.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Pustular Diseases of the Epidermis in SKIN Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis, 2nd ed.; Blackwell Science Ltd.: Oxford, UK, 2005; Volume 2, pp. 4–26. [Google Scholar]
- Banovic, F.; Linder, K.; Olivry, T. Clinical, microscopic and microbial characterization of exfoliative superficial pyoderma-associated epidermal collarettes in dogs. Vet. Dermatol. 2016, 28, 107-e123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazakerley, J.; Nuttall, T.; Sales, D.; Schmidt, V.; Carter, S.; Hart, C.A.; McEwan, N.A. Staphylococcal colonization of mucosal and lesional skin sites in atopic and healthy dogs. Vet. Dermatol. 2009, 20, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23, 253–266, e251–252. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Kania, S.A.; Hnilica, K.A.; Wilkes, R.P.; Bemis, D.A. Isolation of Staphylococcus schleiferi from dogs with pyoderma. J. Am. Vet. Med. Assoc. 2003, 222, 451–454. [Google Scholar] [CrossRef] [PubMed]
- May, E.R.; Hnilica, K.A.; Frank, L.A.; Jones, R.D.; Bemis, D.A. Isolation of Staphylococcus schleiferi from healthy dogs and dogs with otitis, pyoderma, or both. J. Am. Vet. Med. Assoc. 2005, 227, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Ravens, P.; Vogelnest, L.; Ewen, E.; Bosward, K.; Norris, J.; Norris, J. Canine superficial bacterial pyoderma: Evaluation of skin surface sampling methods and antimicrobial susceptibility of causalStaphylococcusisolates. Aust. Vet. J. 2014, 92, 149–155. [Google Scholar] [CrossRef]
- Bean, D.C.; Wigmore, S. Carriage rate and antibiotic susceptibility of coagulase-positive staphylococci isolated from healthy dogs in Victoria, Australia. Aust. Vet. J. 2016, 94, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.W.; Morris, D.O.; Rankin, S.C.; Cain, C.L.; Misic, A.M.; Houser, T.; Mauldin, E.A.; Grice, E.A. Longitudinal Evaluation of the Skin Microbiome and Association with Microenvironment and Treatment in Canine Atopic Dermatitis. J. Investig. Dermatol. 2016, 136, 1182–1190. [Google Scholar] [CrossRef] [Green Version]
- Pierezan, F.; Olivry, T.; Paps, J.S.; Lawhon, S.D.; Wu, J.; Steiner, J.M.; Suchodolski, J.S.; Rodrigues-Hoffman, A. The skin microbiome in allergen-induced canine atopic dermatitis. Vet. Dermatol. 2016, 27, 332-e82. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Genet. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Kobayashi, T.; Glatz, M.; Horiuchi, K.; Kawasaki, H.; Akiyama, H.; Kaplan, D.H.; Kong, H.H.; Amagai, M.; Nagao, K. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 2015, 42, 756–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugger, S.D.; Bomar, L.; Lemon, K.P. Commensal–Pathogen Interactions along the Human Nasal Passages. PLoS. Pathog. 2016, 12, e1005633. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome. Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Hoffman, A.; Patterson, A.P.; Diesel, A.; Lawhon, S.D.; Ly, H.J.; Stephenson, C.E.; Mansell, J.; Steiner, J.M.; Dowd, S.E.; Olivry, T.; et al. The Skin Microbiome in Healthy and Allergic Dogs. PLoS ONE 2014, 9, e83197. [Google Scholar] [CrossRef] [Green Version]
- Torres, S.; Clayton, J.B.; Danzeisen, J.L.; Ward, T.; Huang, H.; Knights, D.; Johnson, T.J. Diverse bacterial communities exist on canine skin and are impacted by cohabitation and time. PeerJ 2017, 5, e3075. [Google Scholar] [CrossRef] [Green Version]
- Cuscó, A.; Sánchez, A.; Altet, L.; Ferrer, L.; Francino, O. Individual Signatures Define Canine Skin Microbiota Composition and Variability. Front. Vet. Sci. 2017, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Webster, G.F.; Ruggieri, M.R.; McGinley, K.J. Correlation of Propionibacterium acnes Populations with the Presence of Triglycerides on Nonhuman Skin. Appl. Environ. Microbiol. 1981, 41, 1269–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyden, J.J.; Marples, R.R.; Kligman, A.M.; And, R.R.M. Staphylococcus aureus in the lesions of atopic dermatitis. Br. J. Dermatol. 1974, 90, 525. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Tong, Y.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Sugimoto, S.; Iwamoto, T.; Takada, K.; Okuda, K.-I.; Tajima, A.; Iwase, T.; Mizunoe, Y. Staphylococcus epidermidis Esp Degrades Specific Proteins Associated with Staphylococcus aureus Biofilm Formation and Host-Pathogen Interaction. J. Bacteriol. 2013, 195, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef]
- Myles, I.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, M.M.; Freire, M.; Gabrilska, R.A.; Rumbaugh, K.P.; Lemon, K.P. Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species. Front. Microbiol. 2016, 7, 1230. [Google Scholar] [CrossRef] [Green Version]
- Conlan, S.; Kong, H.H.; Segre, J.A. Species-Level Analysis of DNA Sequence Data from the NIH Human Microbiome Project. PLoS ONE 2012, 7, e47075. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Older, C.E.; Ocana, R.; Dominguez, B.; Lawhon, S.D.; Wu, J.; Patterson, A.P.; Rodrigues Hoffmann, A. Novel association of Psychrobacter and Pseudomonas with malodor in Bloodhound dogs and effects of a topical product composed of essential oils and plant-derived essential fatty acids in a randomized, blinded, placebo-controlled study. Vet. Dermatol. 2018, 29, 465-e158. [Google Scholar] [CrossRef] [PubMed]
- Bannoehr, J.; Ben Zakour, N.L.; Waller, A.; Guardabassi, L.; Thoday, K.L.; Broek, A.H.M.V.D.; Fitzgerald, J.R. Population Genetic Structure of the Staphylococcus intermedius Group: Insights into agr Diversification and the Emergence of Methicillin-Resistant Strains. J. Bacteriol. 2007, 189, 8685–8692. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR Method for Species Identification of Coagulase-Positive Staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misic, A.M.; Davis, M.F.; Tyldsley, A.S.; Hodkinson, B.P.; Tolomeo, P.; Hu, B.; Nachamkin, I.; Lautenbach, E.; Morris, D.O.; Grice, E.A. The shared microbiota of humans and companion animals as evaluated from Staphylococcus carriage sites. Microbiome 2015, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salter, S.J.; Cox, M.J.; Turek, E.; Calus, S.; Cookson, W.O.C.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; I Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.J.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2011, 6, 610–618. [Google Scholar] [CrossRef]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine learning for neuroimaging with scikit-learn. Front. Aging Neurosci. 2014, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.S.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, F.; Picard, F.J.; Ke, D.; Paradis, S.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR Assay for Identification of Staphylococci at Genus and Species Levels. J. Clin. Microbiol. 2001, 39, 2541–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Control vs. Pyoderma | Control vs. EC | Control vs. SBF | EC vs. SBF | |
|---|---|---|---|---|
| Alpha diversity | ||||
| Chao1 diversity index | <0.001 | 0.001 | <0.001 | 0.337 |
| Faith’s phylogenetic diversity | <0.001 | 0.009 | 0.008 | 0.915 |
| Observed OTUs | <0.001 | 0.007 | 0.003 | 1.000 |
| Pielou’s evenness | <0.001 | 0.007 | 0.012 | 0.145 |
| Shannon diversity index | <0.001 | 0.001 | <0.001 | 0.337 |
| Beta diversity | ||||
| Bray-Curtis | R = 0.908, p = 0.001 | R = 1.000, p = 0.002 | R = 0.923, p = 0.001 | R = 0.168, p = 0.030 |
| Jaccard | R = 0.923, p = 0.001 | R = 0.959, p = 0.001 | R = 0.983, p = 0.001 | R = 0.154, p = 0.023 |
| Unweighted UniFrac | R = 0.956, p = 0.001 | R = 0.974, p = 0.001 | R = 0.966, p = 0.001 | R = 0.023, p = 0.321 |
| Weighted UniFrac | R = 0.866, p = 0.001 | R = 1.000, p = 0.001 | R = 0.854, p = 0.001 | R = 0.135, p = 0.055 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Older, C.E.; Rodrigues Hoffmann, A.; Hoover, K.; Banovic, F. Characterization of Cutaneous Bacterial Microbiota from Superficial Pyoderma Forms in Atopic Dogs. Pathogens 2020, 9, 638. https://doi.org/10.3390/pathogens9080638
Older CE, Rodrigues Hoffmann A, Hoover K, Banovic F. Characterization of Cutaneous Bacterial Microbiota from Superficial Pyoderma Forms in Atopic Dogs. Pathogens. 2020; 9(8):638. https://doi.org/10.3390/pathogens9080638
Chicago/Turabian StyleOlder, Caitlin E., Aline Rodrigues Hoffmann, Kathleen Hoover, and Frane Banovic. 2020. "Characterization of Cutaneous Bacterial Microbiota from Superficial Pyoderma Forms in Atopic Dogs" Pathogens 9, no. 8: 638. https://doi.org/10.3390/pathogens9080638
APA StyleOlder, C. E., Rodrigues Hoffmann, A., Hoover, K., & Banovic, F. (2020). Characterization of Cutaneous Bacterial Microbiota from Superficial Pyoderma Forms in Atopic Dogs. Pathogens, 9(8), 638. https://doi.org/10.3390/pathogens9080638