Coordinated In Vitro Release of Granulysin, Perforin and IFN-γ in TB and HIV/TB Co-Infection Associated with Clinical Outcomes before and after Anti-TB Treatment
Abstract
:1. Introduction
2. Results
2.1. Higher Releases of In Vitro Perforin, Granzyme-B, Granulysin and IFN-γ from Stimulated PBMCs with PPD and H37Ra in Active TB Patients before and after Anti-TB Treatment
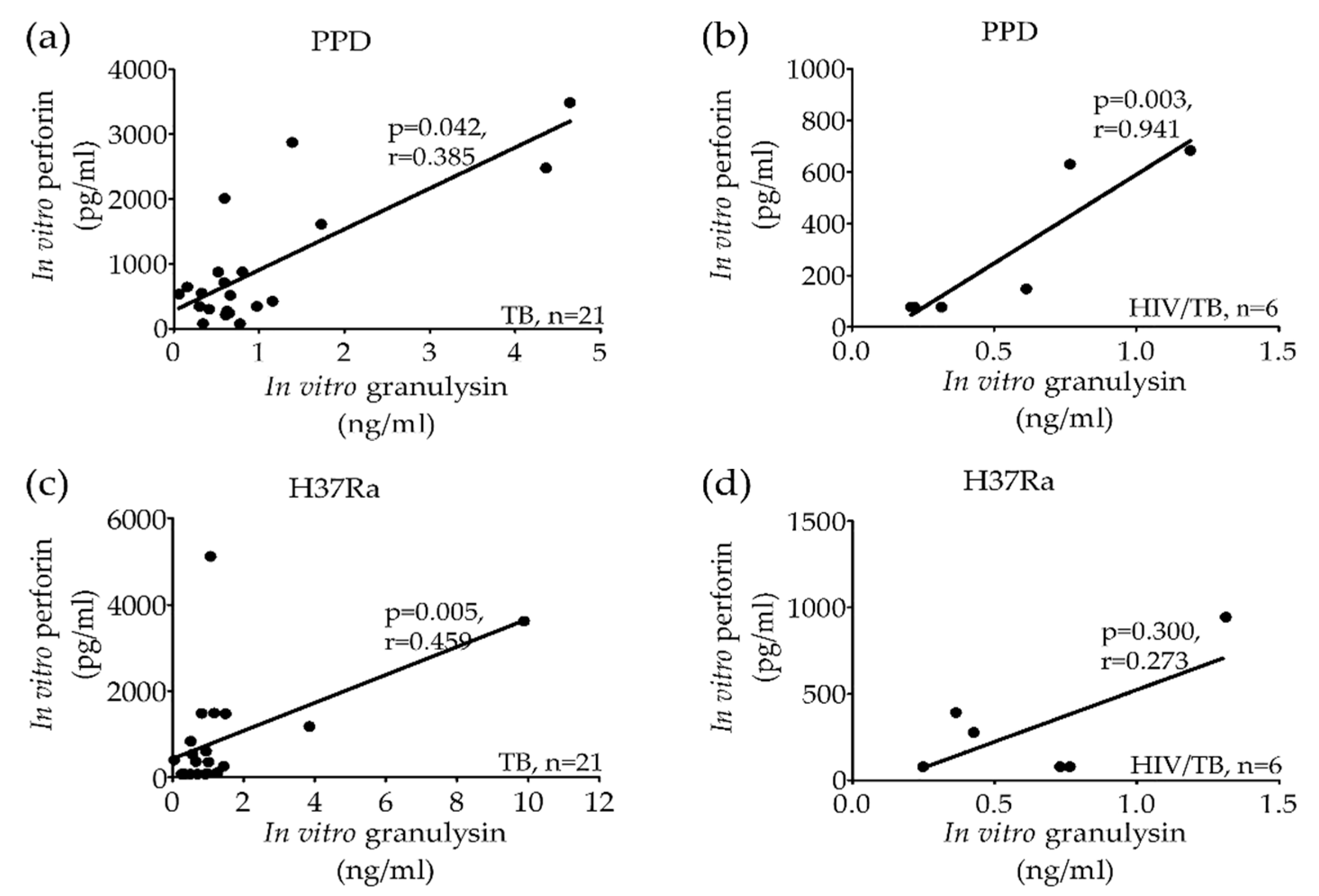
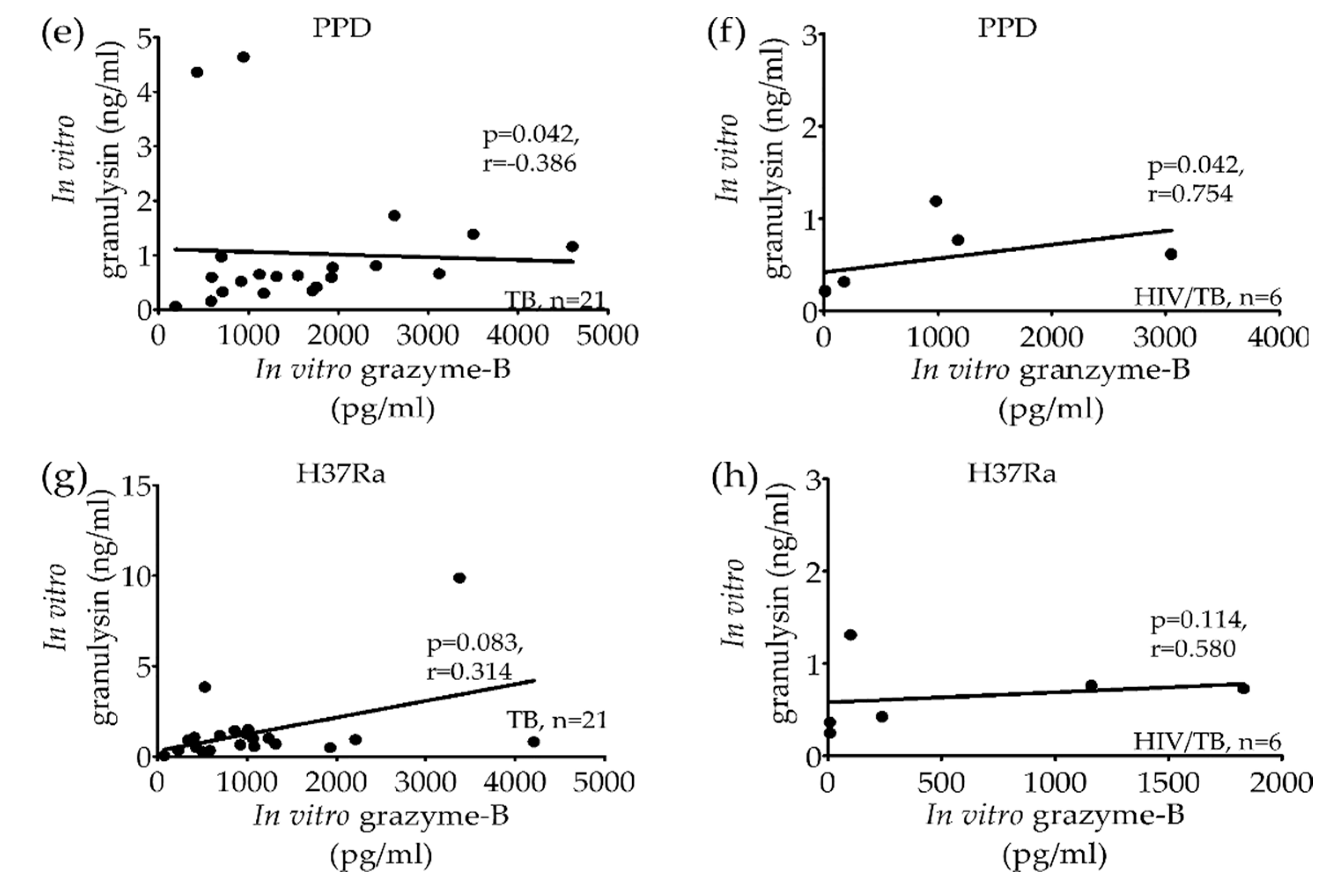
2.2. Significant Correlation of Granulysin, Perforin and Granzyme-B Levels from Stimulated PBMCs in Active TB and HIV/TB Coinfection before Anti-TB Treatment
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Peripheral Blood Mononuclear Cells (PBMCs)
4.3. Stimulation of PBMCs with Purified Protein Derivative (PPD) and Avirulent Heat Killed Mtb (H37Ra) Strain
4.4. Determination of Granulysin, Perforin, Granzyme-B and IFN-γ Releases from Stimulated PBMCs In Vitro
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report. 2016. Available online: https://apps.who.int/iris/handle/10665/250441 (accessed on 15 July 2019).
- Freeman, S.; Post, F.A.; Bekker, L.-G.; Harbacheuski, R.; Steyn, L.M.; Ryffel, B.; Connell, N.D.; Kreiswirth, B.N.; Kaplan, G. Mycobacterium tuberculosis H37Ra and H37Rv Differential Growth and Cytokine/Chemokine Induction in Murine Macrophages In vitro. J. Interf. Cytokine Res. 2006, 26, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Meinken, C.; Bastian, M.; Bruns, H.; Legaspi, A.; Ochoa, M.T.; Krutzik, S.R.; Bloom, B.R.; Ganz, T.; Modlin, R.L.; et al. Macrophages Acquire Neutrophil Granules for Antimicrobial Activity against Intracellular Pathogens. J. Immunol. 2006, 177, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The Immune Response in Tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Lowrie, D.B. Identification and Characterization of Murine Cytotoxic T Cells That Kill Mycobacterium tuberculosis. Infect. Immun. 2000, 68, 3269–3274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacMicking, J.D.; Taylor, G.A.; McKinney, J.D. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 2003, 302, 654–659. [Google Scholar] [CrossRef]
- Jacobs, M.; Togbe, D.; Fremond, C.; Samarina, A.; Allie, N.; Botha, T.; Carlos, D.; Parida, S.K.; Grivennikov, S.; Nedospasov, S.; et al. Tumor necrosis factor is critical to control tuberculosis infection. Microbes Infect. 2007, 9, 623–628. [Google Scholar] [CrossRef]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; Fountain, J.J.; Rangel-Moreno, J.; Cilley, G.E.; Shen, F.; Eaton, S.M.; Gaffen, S.L.; Swain, S.L.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef]
- Andersson, J.; Samarina, A.; Fink, J.; Rahman, S.; Grundström, S. Impaired Expression of Perforin and Granulysin in CD8+ T Cells at the Site of Infection in Human Chronic Pulmonary Tuberculosis. Infect. Immun. 2007, 75, 5210–5222. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.L.; Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001, 19, 93–129. [Google Scholar] [CrossRef]
- Kaufmann, S.H. Protection against tuberculosis: Cytokines, T cells, and macrophages. Ann. Rheum. Dis. 2002, 61, ii54–ii58. [Google Scholar] [CrossRef]
- Gansert, J.L.; Kieβler, V.; Engele, M.; Wittke, F.; Röllinghoff, M.; Krensky, A.M.; Porcelli, S.A.; Modlin, R.L.; Stenger, S.; Kiessler, V. Human NKT cells express granulysin and exhibit antimycobacterial activity. J. Immunol. 2003, 170, 3154–3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serbina, N.V.; Liu, C.-C.; Scanga, C.A.; Flynn, J.L. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J. Immunol. 2000, 165, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenger, S.; Hanson, D.A.; Teitelbaum, R.; Dewan, P.; Niazi, K.R.; Froelich, C.J.; Ganz, T.; Thoma-Uszynski, S.; Melián, A.; Bogdan, C.; et al. An Antimicrobial Activity of Cytolytic T Cells Mediated by Granulysin. Science 1998, 282, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewinsohn, D.M.; Bement, T.T.; Xu, J.; Lynch, D.H.; Grabstein, K.H.; Reed, S.G.; Alderson, M.R. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J. Immunol. 1998, 160, 2374–2379. [Google Scholar] [PubMed]
- Keefe, D.; Shi, L.; Feske, S.; Massol, R.; Navarro, F.; Kirchhausen, T.; Lieberman, J. Perforin Triggers a Plasma Membrane-Repair Response that Facilitates CTL Induction of Apoptosis. Immunity 2005, 23, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Guipouy, D.; Gertner-Dardenne, J.; Pfajfer, L.; German, Y.; Belmonte, N.; Dupré, L. Granulysin- and granzyme-dependent elimination of myeloid cells by therapeutic ova-specific type 1 regulatory T cells. Int. Immunol. 2019, 31, 239–250. [Google Scholar] [CrossRef]
- Zhang, D.; Pasternack, M.S.; Beresford, P.J.; Wagner, L.; Greenberg, A.H.; Lieberman, J. Induction of Rapid Histone Degradation by the Cytotoxic T Lymphocyte Protease Granzyme A. J. Boil. Chem. 2000, 276, 3683–3690. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Li, Q.; Whitin, J.C.; Clayberger, C.; Krensky, A.M. Intracellular mediators of granulysin-induced cell death. J. Immunol. 2003, 171, 2556–2562. [Google Scholar] [CrossRef] [Green Version]
- Walch, M.; Dotiwala, F.; Mulik, S.; Thiery, J.; Kirchhausen, T.; Clayberger, C.; Krensky, A.M.; Martinvalet, D.; Lieberman, J. Cytotoxic Cells Kill Intracellular Bacteria through Granulysin-Mediated Delivery of Granzymes. Cell 2014, 157, 1309–1323. [Google Scholar] [CrossRef] [Green Version]
- Thuong, P.H.; Tam, D.B.; Sakurada, S.; Le Hang, N.T.; Hijikata, M.; Hong, L.T.; Ngoc, P.T.M.; Anh, P.T.; Cuong, V.C.; Matsushita, I.; et al. Circulating granulysin levels in healthcare workers and latent tuberculosis infection estimated using interferon-gamma release assays. BMC Infect. Dis. 2016, 16, 580. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.M.; Raja, A. Cytotoxicity responses to selected ESAT-6 and CFP-10 peptides in tuberculosis. Cell. Immunol. 2010, 265, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, S.T.; Place, S.; Debrie, A.-S.; Locht, C.; Mascart, F. Effector Functions of Heparin-Binding Hemagglutinin–Specific CD8+T Lymphocytes in Latent Human Tuberculosis. J. Infect. Dis. 2005, 192, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Nunnari, G.; Pinzone, M.R.; Vancheri, C.; Palermo, F.; Cacopardo, B. Interferon-γ and interleukin-17 production from PPD-stimulated PBMCs of patients with pulmonary tuberculosis. Clin. Investig. Med. 2013, 36, E64–E71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, R.J.; Walker, N.F.; Meintjes, G.; Deffur, A.; Nicol, M.P.; Skolimowska, K.H.; Matthews, K.; Tadokera, R.; Seldon, R.; Maartens, G.; et al. Cytotoxic Mediators in Paradoxical HIV–Tuberculosis Immune Reconstitution Inflammatory Syndrome. J. Immunol. 2015, 194, 1748–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Laorden, M.I.; Blok, D.C.; Kager, L.M.; Hoogendijk, A.J.; Van Mierlo, G.; Lede, I.O.; Rahman, W.; Afroz, R.; Ghose, A.; Visser, C.E.; et al. Increased intra- and extracellular granzyme expression in patients with tuberculosis. Tuberculosis 2015, 95, 575–580. [Google Scholar] [CrossRef]
- Manca, C.; Reed, M.B.; Freeman, S.; Mathema, B.; Kreiswirth, B.; Barry, C.E.; Kaplan, G. Differential Monocyte Activation Underlies Strain-Specific Mycobacterium tuberculosis Pathogenesis. Infect. Immun. 2004, 72, 5511–5514. [Google Scholar] [CrossRef] [Green Version]
- Manca, C.; Paul, S.; Barry, C.E.; Freedman, V.H.; Kaplan, G. Mycobacterium tuberculosis Catalase and Peroxidase Activities and Resistance to Oxidative Killing in Human Monocytes In vitro. Infect. Immun. 1999, 67, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Divangahi, M.; Chen, M.; Gan, H.; Desjardins, D.; Hickman, T.; Lee, D.M.; Fortune, S.; Behar, S.M.; Remold, H.G.; Dejardins, D. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat. Immunol. 2009, 10, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Bhavanam, S.; Rayat, G.R.; Keelan, M.; Kunimoto, D.; Drews, S.J. Characterization of immune responses of human PBMCs infected with Mycobacterium tuberculosis H37Ra: Impact of donor declared BCG vaccination history on immune responses and M. tuberculosis growth. PLoS ONE 2018, 13, e0203822. [Google Scholar] [CrossRef] [Green Version]
- Pitabut, N.; Sakurada, S.; Tanaka, T.; Ridruechai, C.; Tanuma, J.; Aoki, T.; Kantipong, P.; Piyaworawong, S.; Kobayashi, N.; Dhepakson, P.; et al. Potential Function of Granulysin, Other Related Effector Molecules and Lymphocyte Subsets in Patients with TB and HIV/TB Coinfection. Int. J. Med. Sci. 2013, 10, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Mueller, H.; Faé, K.C.; Magdorf, K.; Ganoza, C.; Wahn, U.; Guhlich, U.; Feiterna-Sperling, C.; Kaufmann, S.H. Granulysin-Expressing CD4+ T Cells as Candidate Immune Marker for Tuberculosis during Childhood and Adolescence. PLoS ONE 2011, 6, e29367. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, M.; Ohminami, H.; Arai, J.; Kasahara, Y.; Ishida, Y.; Fujita, S. Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans. Blood 2000, 95, 2352–2355. [Google Scholar] [CrossRef] [PubMed]
- Booty, M.G.; Nunes-Alves, C.; Carpenter, S.M.; Jayaraman, P.; Behar, S.M. Multiple Inflammatory Cytokines Converge To Regulate CD8+ T Cell Expansion and Function during Tuberculosis. J. Immunol. 2016, 196, 1822–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canaday, D.H.; Wilkinson, R.J.; Li, Q.; Harding, C.V.; Silver, R.F.; Boom, W.H. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J. Immunol. 2001, 167, 2734–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, K.; Takamori, Y.; Suzuki, K.; Nagasawa, M.; Takano, S.; Kasahara, Y.; Nakamura, Y.; Kondo, S.; Sugamura, K.; Nakamura, M.; et al. Granulysin in human serum as a marker of cell-mediated immunity. Eur. J. Immunol. 2003, 33, 1925–1933. [Google Scholar] [CrossRef]
- Brahmbhatt, S.; Black, G.F.; Carroll, N.M.; Beyers, N.; Salker, F.; Kidd, M.; Lukey, P.T.; Duncan, K.; Van Helden, P.; Walzl, G. Immune markers measured before treatment predict outcome of intensive phase tuberculosis therapy. Clin. Exp. Immunol. 2006, 146, 243–252. [Google Scholar] [CrossRef]
- Silva, B.D.D.S.; Trentini, M.M.; Da Costa, A.C.; Kipnis, A.; Junqueira-Kipnis, A.P. Different phenotypes of CD8+ T cells associated with bacterial load in active tuberculosis. Immunol. Lett. 2014, 160, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Gong, H.; Zhang, Q.; Gu, J.; Liang, L.; Zhang, J. Decreased expression of perforin in CD8+ T lymphocytes in patients with Mycobacterium tuberculosis infection and its potential value as a marker for efficacy of treatment. J. Thorac. Dis. 2017, 9, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Pitabut, N.; Mahasirimongkol, S.; Yanai, H.; Ridruechai, C.; Sakurada, S.; Dhepakson, P.; Kantipong, P.; Piyaworawong, S.; Moolphate, S.; Hansudewechakul, C.; et al. Decreased granulysin and increased IFN-γ levels in plasma of patients with newly diagnosed and relapse tuberculosis. Microbiol. Immunol. 2011, 55, 565–573. [Google Scholar] [CrossRef]
 ) and H37Ra (
) and H37Ra (  ) in comparison with unstimulated PBMCs (
) in comparison with unstimulated PBMCs (  ) of TB, HIV/TB coinfection, HIV+HAART−, HIV+HAART+ and healthy controls before anti-TB treatment. A horizontal bar indicated the median of each group. Statistical significant by Mann Whitney U test, p < 0.05. (PPD = purified protein derivative, H37Ra = avirulent stain heat killed Mtb; HIV+HAART− = HIV patients without receiving highly active antiretroviral therapy (HAART); and HIV+HAART+ = HIV patients receiving HAART.
) of TB, HIV/TB coinfection, HIV+HAART−, HIV+HAART+ and healthy controls before anti-TB treatment. A horizontal bar indicated the median of each group. Statistical significant by Mann Whitney U test, p < 0.05. (PPD = purified protein derivative, H37Ra = avirulent stain heat killed Mtb; HIV+HAART− = HIV patients without receiving highly active antiretroviral therapy (HAART); and HIV+HAART+ = HIV patients receiving HAART.
 ) and H37Ra (
) and H37Ra (  ) in comparison with unstimulated PBMCs (
) in comparison with unstimulated PBMCs (  ) of TB, HIV/TB coinfection, HIV+HAART−, HIV+HAART+ and healthy controls before anti-TB treatment. A horizontal bar indicated the median of each group. Statistical significant by Mann Whitney U test, p < 0.05. (PPD = purified protein derivative, H37Ra = avirulent stain heat killed Mtb; HIV+HAART− = HIV patients without receiving highly active antiretroviral therapy (HAART); and HIV+HAART+ = HIV patients receiving HAART.
) of TB, HIV/TB coinfection, HIV+HAART−, HIV+HAART+ and healthy controls before anti-TB treatment. A horizontal bar indicated the median of each group. Statistical significant by Mann Whitney U test, p < 0.05. (PPD = purified protein derivative, H37Ra = avirulent stain heat killed Mtb; HIV+HAART− = HIV patients without receiving highly active antiretroviral therapy (HAART); and HIV+HAART+ = HIV patients receiving HAART.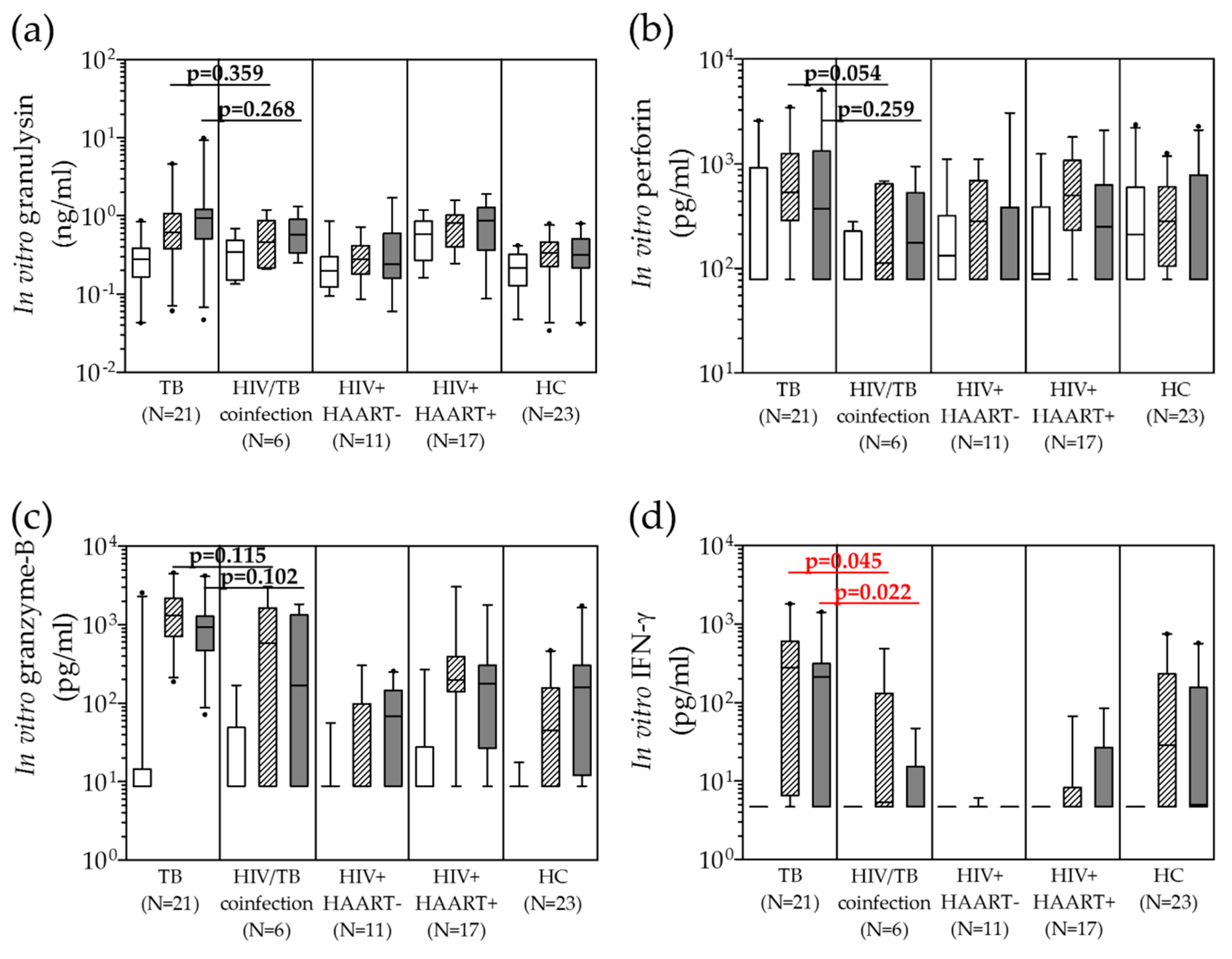
 ) and H37Ra (
) and H37Ra (  ) in comparison with unstimulated (
) in comparison with unstimulated (  ) in active TB and HIV/TB coinfection before and after anti-TB treatment. Wilcoxon Signed Rank test was used to compare different levels before and after completion of anti-TB treatment. p value < 0.05 was statistical significant.
) in active TB and HIV/TB coinfection before and after anti-TB treatment. Wilcoxon Signed Rank test was used to compare different levels before and after completion of anti-TB treatment. p value < 0.05 was statistical significant.
 ) and H37Ra (
) and H37Ra (  ) in comparison with unstimulated (
) in comparison with unstimulated (  ) in active TB and HIV/TB coinfection before and after anti-TB treatment. Wilcoxon Signed Rank test was used to compare different levels before and after completion of anti-TB treatment. p value < 0.05 was statistical significant.
) in active TB and HIV/TB coinfection before and after anti-TB treatment. Wilcoxon Signed Rank test was used to compare different levels before and after completion of anti-TB treatment. p value < 0.05 was statistical significant.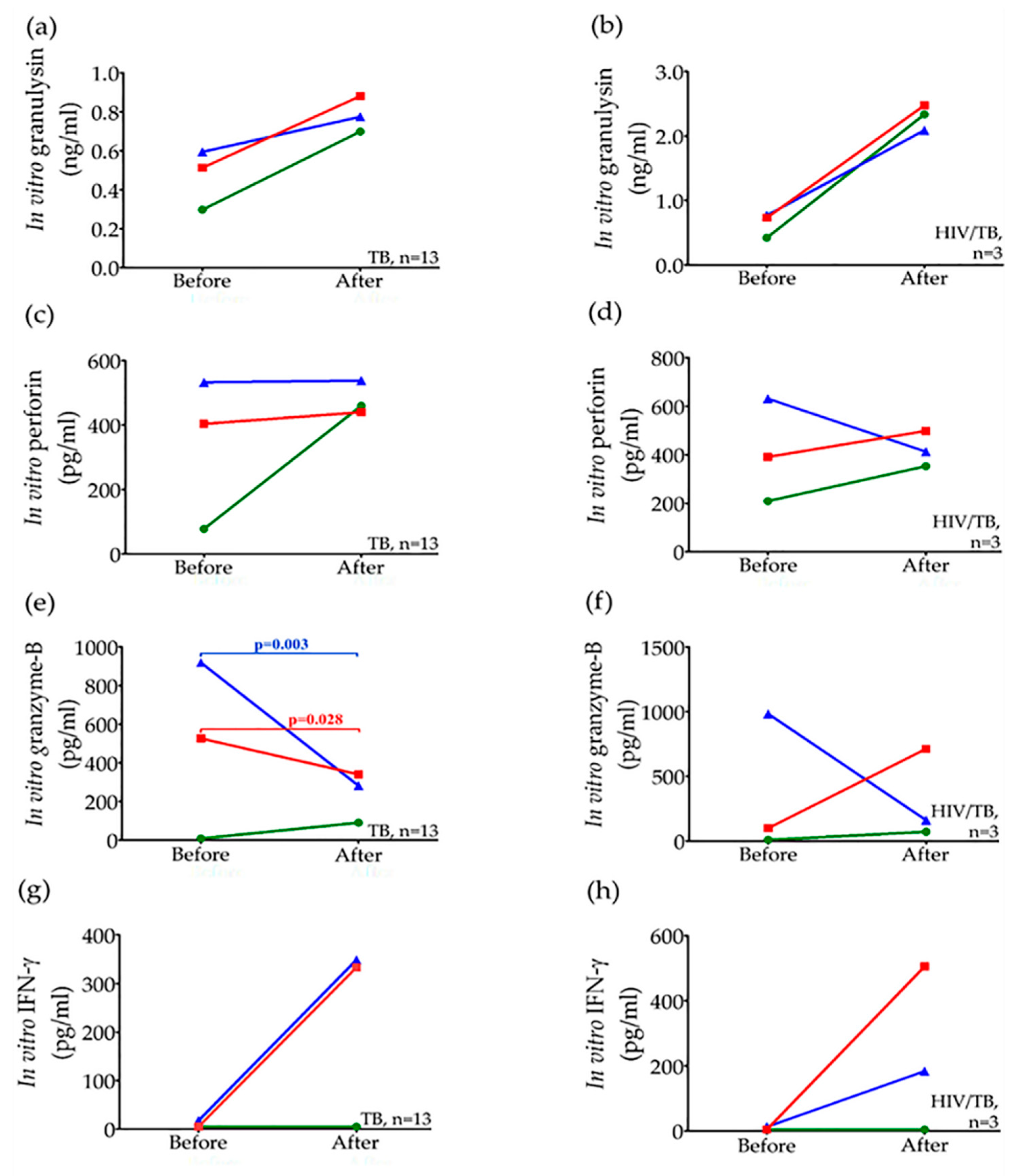
| Measurement | Stimulation with | PBMCs from | ||||
|---|---|---|---|---|---|---|
| Active TB 1 (n = 21) | HIV/TB Coinfection 2 (n = 6) | HIV+ HAART− 3 (n = 11) | HIV+ HAART+ 4 (n = 17) | HC 5 (n = 23) | ||
| Granulysin (ng/mL) (median, IQR) | Medium alone | 0.281, 0.166–0.389 | 0.346, 0.149–0.488 | 0.197, 0.123–0.300 | 0.584, 0.271–0.856 | 0.217, 0.127–0.320 |
| PPD 6 | 0.623, 0.380–1.066 | 0.464, 0.217–0.872 | 0.278, 0.180–0.417 | 0.817, 0.397–1.026 | 0.334, 0.226–0.460 | |
| H37Ra 7 | 0.931, 0.509–1.218 | 0.578, 0.335–0.901 | 0.243, 0.159–0.595 | 0.864, 0.365–1.269 | 0.315, 0.216–0.505 | |
| Perforin (pg/mL) (median, IQR) | Medium alone | <78, <78–918.5 | <78, <78–227 | 132, <78–321 | 89, <78–386 | 210, <78–593 |
| PPD 6 | 533, 286–1246 | 113, <78–644 | 284, <78–694 | 499, 231–1075 | 283, 105–605 | |
| H37Ra 7 | 370, <78–1330 | 177, <78–529 | 78, <78–384 | 249, <78–630 | <78, <78–783 | |
| Granzyme-B (pg/mL) (median, IQR) | Medium alone | <8.79, <8.79–14.40 | <8.79, <8.79–49.01 | <8.79 | <8.79, <8.79–27.5 | <8.79 |
| PPD 6 | 1313, 704–2178 | 587.7, <8.79–1644.5 | <8.79, <8.79–98.33 | 197, 140.7–395.3 | 44.67, <8.79–156.3 | |
| H37Ra 7 | 924.67, 467.33–1279 | 168.7, <8.79–1327.4 | 67.67, <8.79–146 | 176.67, 26.89–305.83 | 159, 12.0–306.33 | |
| IFN-γ (pg/mL) (median, IQR) | Medium alone | <4.7 | <4.7 | <4.7 | <4.7 | <4.7 |
| PPD 6 | 279.25, 6.57–607.5 | 5.4, <4.7–131.34 | <4.7 | <4.7, <4.7–8.245 | 28.6, <4.7–230.73 | |
| H37Ra 7 | 211.85, <4.7–313.1 | <4.7, <4.7–15.30 | <4.7 | <4.7, <4.7–26.98 | 5.04, <4.7–156.51 | |
| Circulating Concentration (Median, IQR) | Active TB (n = 13) | p-Value 1 | HIV/TB Coinfection (n = 3) | p-Value 1 | ||
|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Before Treatment | After Treatment | |||
| * Granulysin (ng/mL) | 1.050 (0.635–1.350) | 1.860 (1.190–4.645) | 0.007 | 7.37 (3.75–10.07) | 9.50 (8.54–10.24) | 0.109 |
| Perforin (pg/mL) | 5538, (4243–8480) | 8575, (7195–12,980) | 0.001 | 10,763, (10,305–13,255) | 13,865, (11,315–19,290) | 0.285 |
| Granzyme-B (pg/mL) | <8.79, <8.79–31.0 | 118, 56.67–145.5 | 0.006 | 26, <8.79–41.33 | 83.33, 71–171.33 | 0.109 |
| * IFN-γ (pg/mL) | 10.04 (<4.70–21.12) | 5.04 (<4.7–8.435) | 0.139 | 53.04 (6.72–89.54) | <4.7 (<4.7–15.08) | 0.285 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitabut, N.; Dhepakson, P.; Sakurada, S.; Keicho, N.; Khusmith, S. Coordinated In Vitro Release of Granulysin, Perforin and IFN-γ in TB and HIV/TB Co-Infection Associated with Clinical Outcomes before and after Anti-TB Treatment. Pathogens 2020, 9, 655. https://doi.org/10.3390/pathogens9080655
Pitabut N, Dhepakson P, Sakurada S, Keicho N, Khusmith S. Coordinated In Vitro Release of Granulysin, Perforin and IFN-γ in TB and HIV/TB Co-Infection Associated with Clinical Outcomes before and after Anti-TB Treatment. Pathogens. 2020; 9(8):655. https://doi.org/10.3390/pathogens9080655
Chicago/Turabian StylePitabut, Nada, Panadda Dhepakson, Shinsaku Sakurada, Naoto Keicho, and Srisin Khusmith. 2020. "Coordinated In Vitro Release of Granulysin, Perforin and IFN-γ in TB and HIV/TB Co-Infection Associated with Clinical Outcomes before and after Anti-TB Treatment" Pathogens 9, no. 8: 655. https://doi.org/10.3390/pathogens9080655





