Does Curcumin Have a Role in the Interaction between Gut Microbiota and Schistosoma mansoni in Mice?
Abstract
:1. Introduction
2. Results
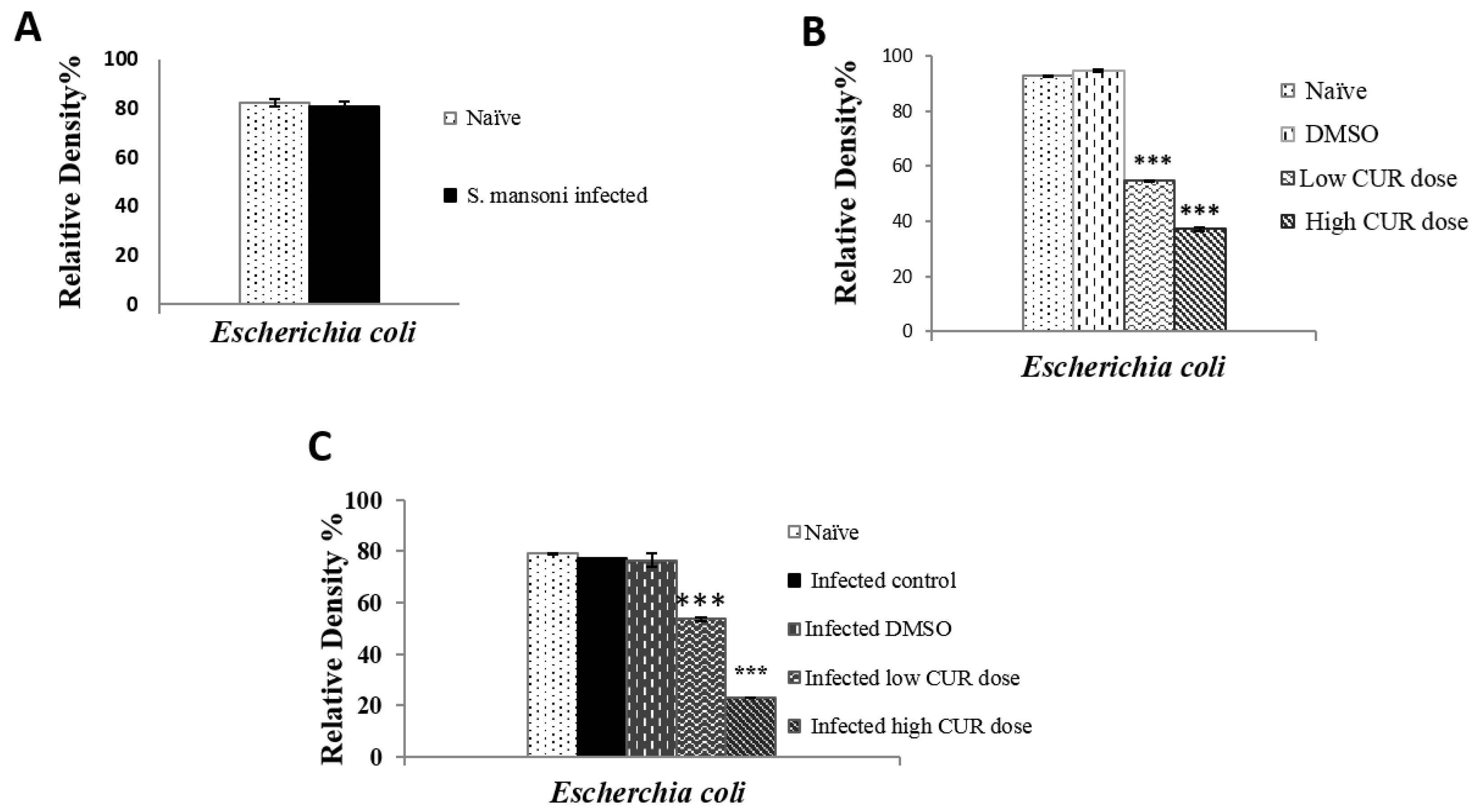
2.1. Impact of S. Mansoni Infection on the Composition of Gut Microbiota
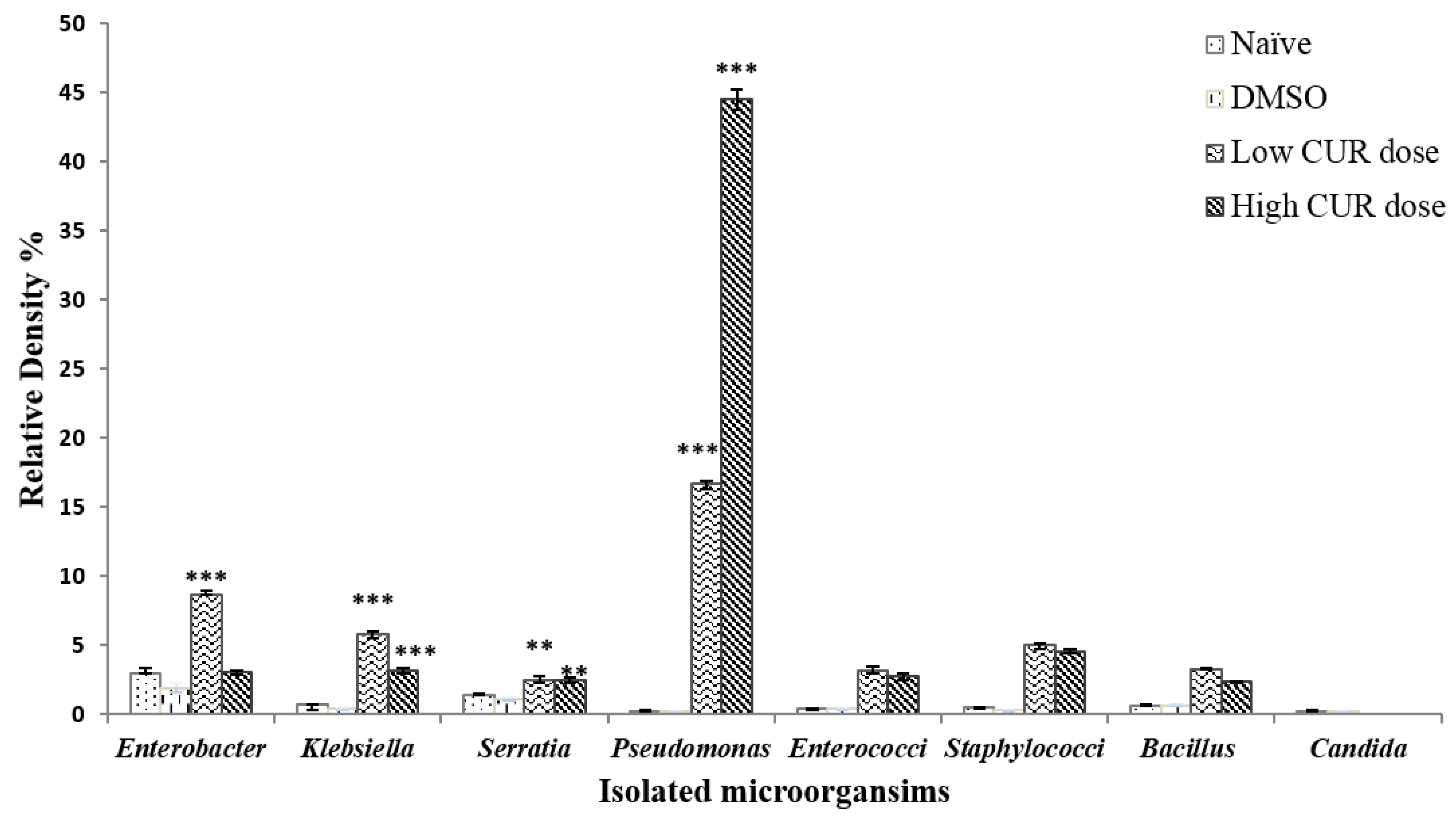
2.2. Influence of Curcumin on the Composition of Mice Gut Microbiota
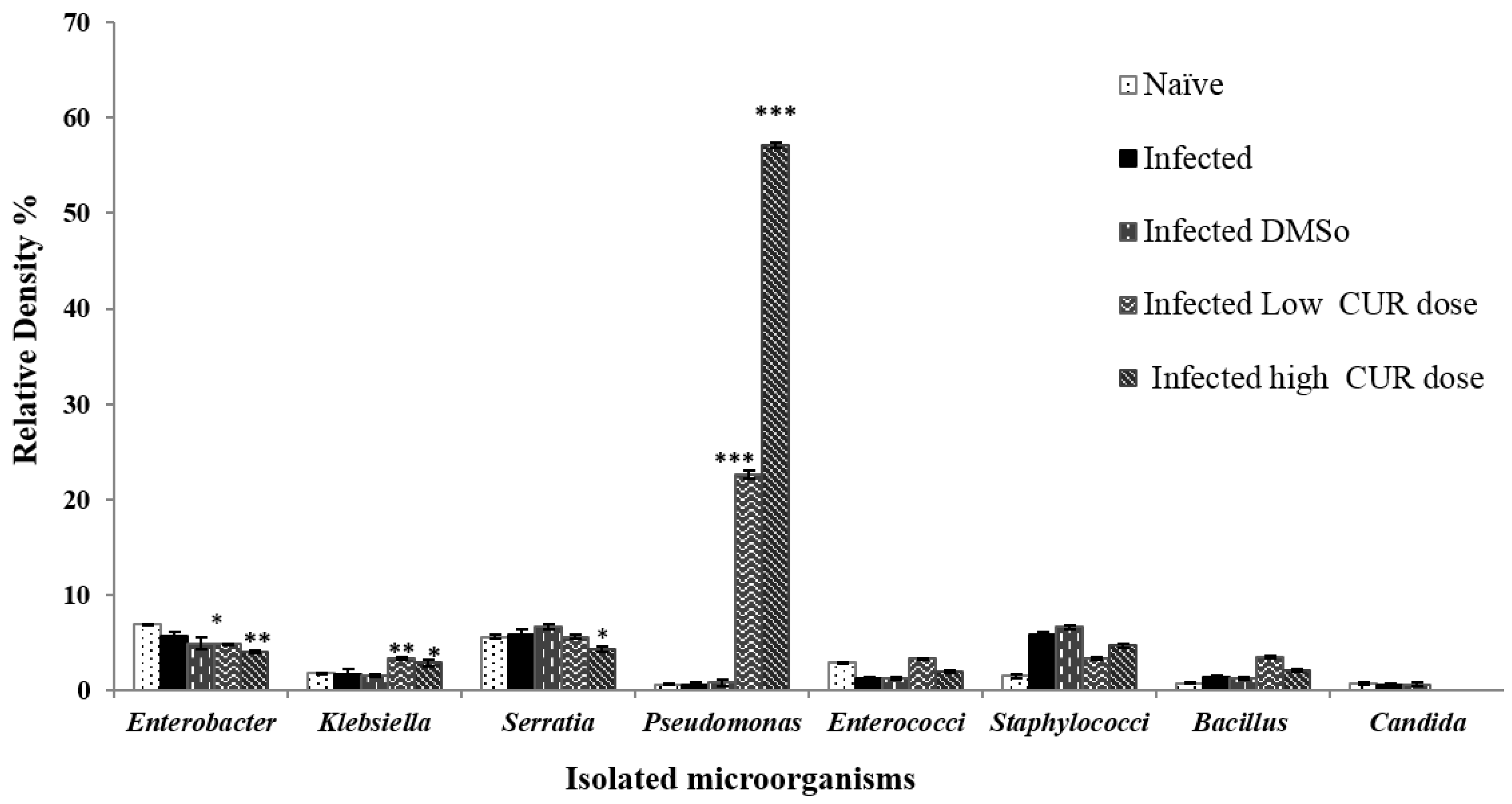
2.3. Schistosomes, CUR, and Gut Microbiota Interactions
2.3.1. Effects on the Microbiota
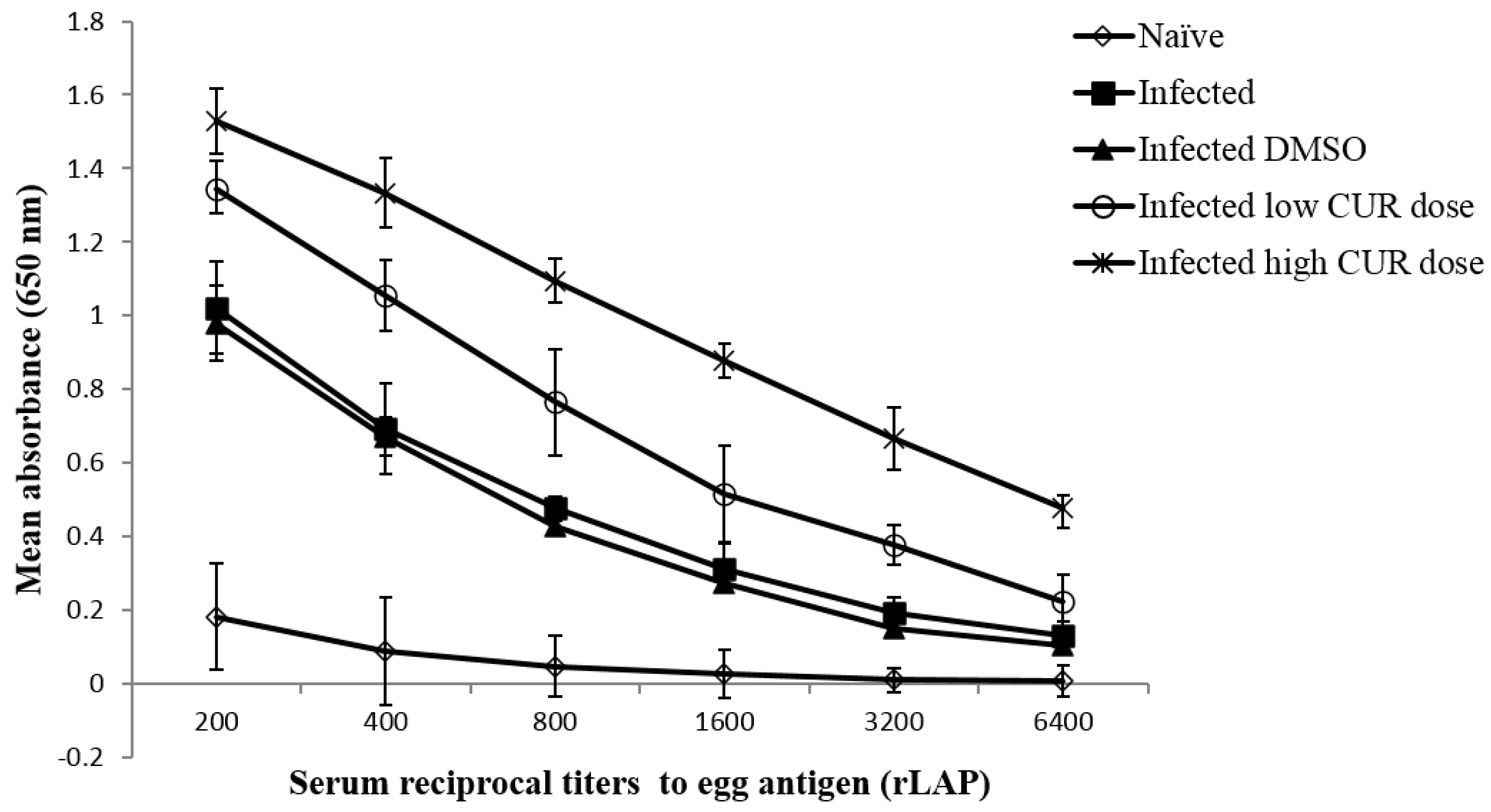
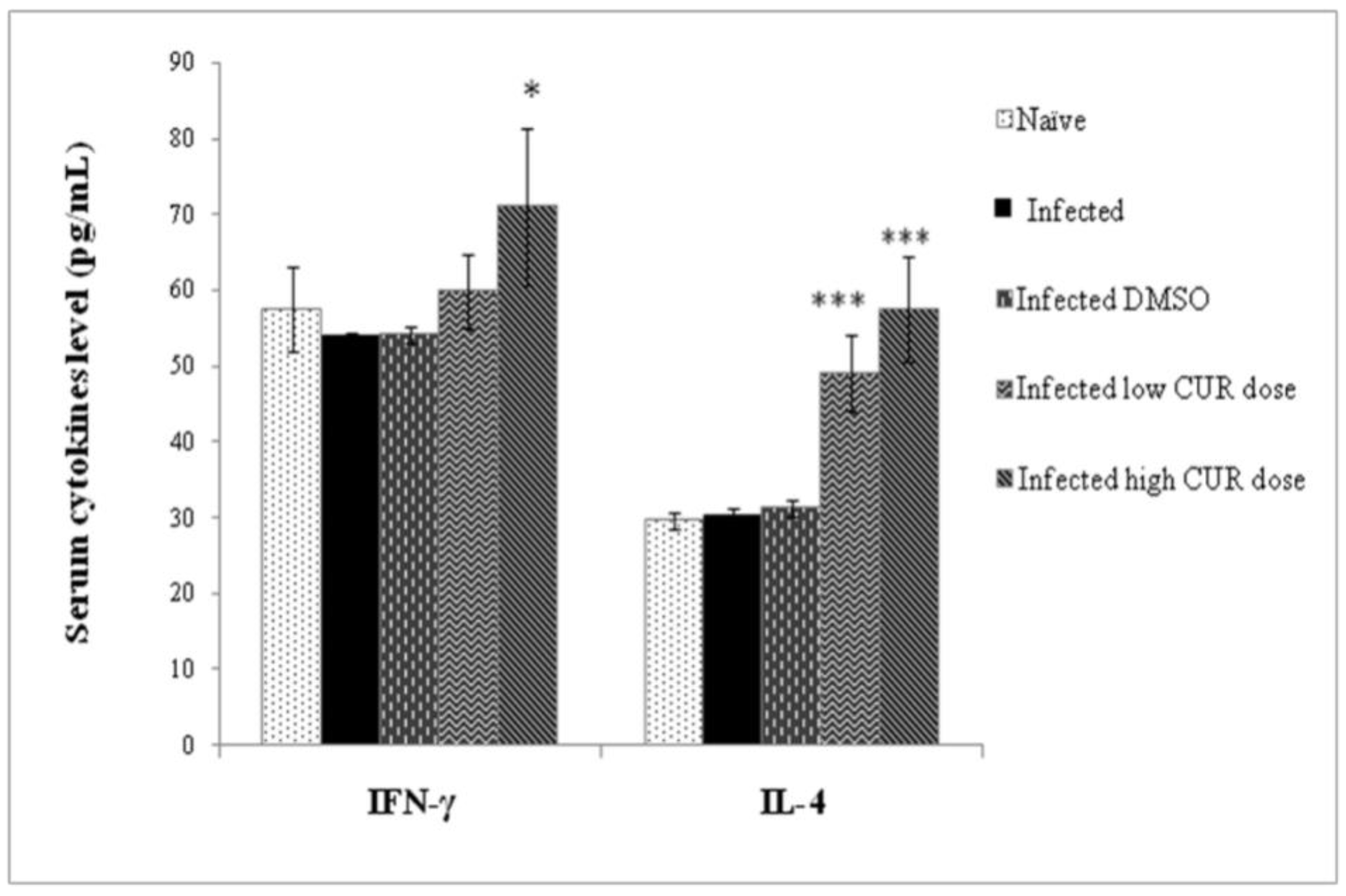
2.3.2. Effects on Immune Responses against Schistosome Egg Antigens
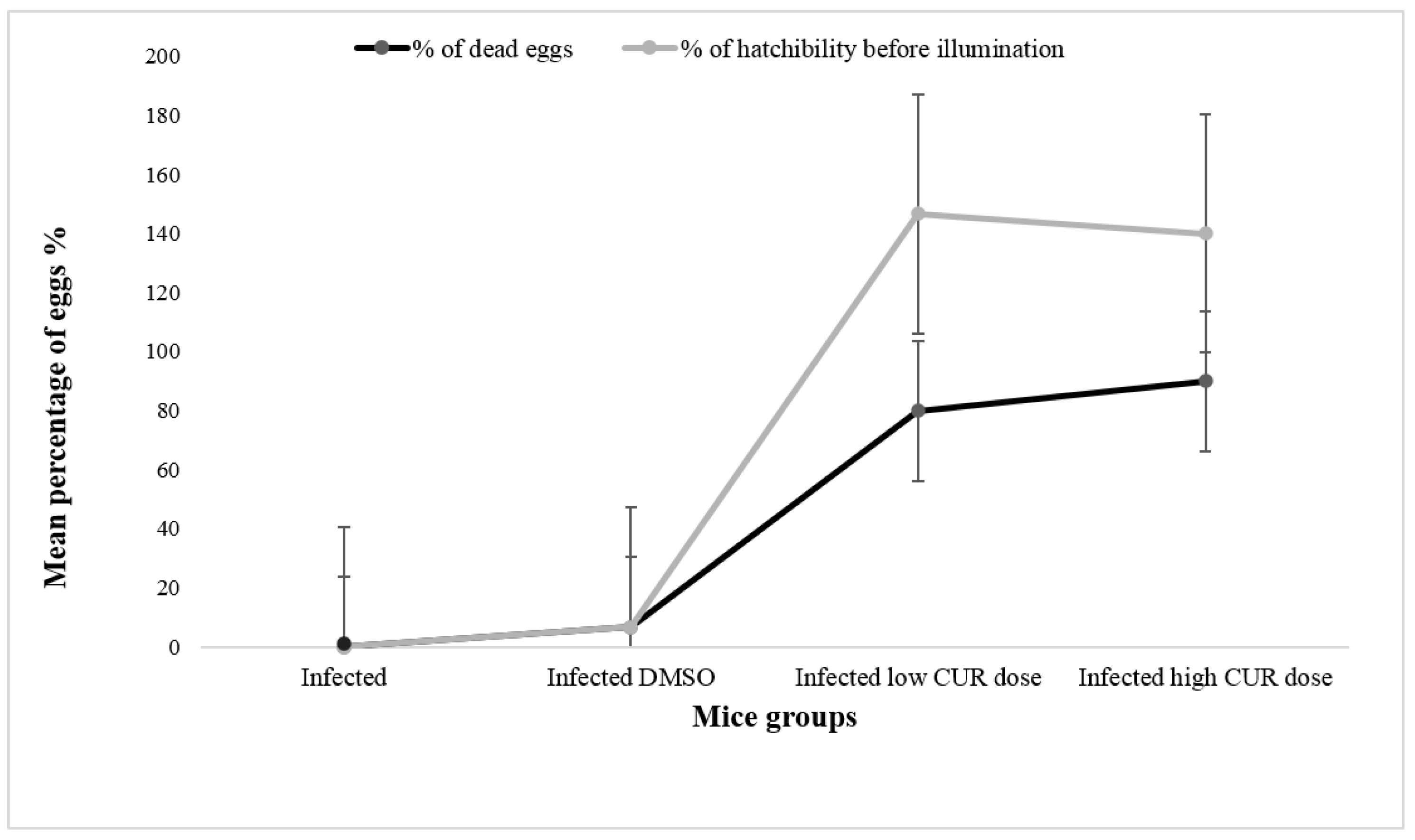
2.3.3. Effects on Parasitological Parameters
3. Discussion
4. Materials and Methods
4.1. Curcumin
4.2. Animals
4.3. Ethical Consideration
4.4. Experimental Design
4.5. Bleeding and Sample Collection
4.6. Microbiological Parameters
4.7. Immunological Parameters
4.7.1. Antibody Assay
4.7.2. Cytokines Assay
4.8. Parasitological Parameters
4.8.1. Worm Burden
4.8.2. Oogram Map
4.8.3. Total Egg Counts
4.8.4. Egg Hatchability
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berrilli, F.; Di Cave, D.; Cavallero, S.; D’Amelio, S. Interactions between parasites and microbial communities in the human gut. Front. Cell Infect. Microbiol. 2012, 2, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Edward, J.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, S.; Li, B.; Li, W.; Wang, J.; Chen, Z.; Yang, J.; Tan, H.; Li, J. Alterations of the Mice Gut Microbiome via Schistosoma japonicum Ova-Induced Granuloma. Front. Microbiol. 2019, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Van der Werf, M.J.; de Vlas, S.J.; Brooker, S.; Looman, C.W.; Nagelkerke, N.J.; Habbema, J.D.; Engels, D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003, 86, 125–139. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Wilson, M.S.; Mentink-Kane, M.M.; Pesce, J.T.; Ramalingam, T.R.; Thompson, R.; Wynn, T.A. Immunopathology of schistosomiasis. Immunol. Cell Biol. 2007, 85, 148–154. [Google Scholar] [CrossRef]
- Schwartz, C.; Fallon, P.G. Schistosoma “eggs-itching” the host: Granuloma formation and egg excretion. Front. Immunol. 2018, 9, 2492. [Google Scholar] [CrossRef] [Green Version]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef]
- Layland, L.E.; Mages, J.; Loddenkemper, C.; Hoerauf, A.; Wagner, H.; Lang, R.; da Costa, C.U. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J. Immunol. 2010, 184, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, T.P.; Peachey, L.E.; Ajami, N.J.; MacDonald, A.S.; Hsieh, M.H.; Brindley, P.J.; Cantacessi, C.; Rinaldi, G. Schistosoma mansoni infection is associated with quantitative and qualitative modifications of the mammalian intestinal microbiota. Sci. Rep. 2018, 8, 12072. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Holzscheiter, M.; Layland, L.E.; Loffredo-verde, E. Lack of host gut Microbiota alters immune responses and intestinal granuloma formation during schistosomiasis. Clin. Exp. Immunol. 2013, 175, 246–257. [Google Scholar] [CrossRef]
- Vuong, H.E.; Yano, J.M.; Fung, T.C.; Hsiao, E.Y. The microbiome and host behavior. Annu. Rev. Neurosci. 2017, 40, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Spor, A.; Koren, O.; Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Ubeda, C.; Djukovic, A.; Isaac, S. Roles of the intestinal microbiota in pathogen protection. Clin. Transl. Immunol. 2017, 6, e128. [Google Scholar] [CrossRef] [PubMed]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kiderlen, A.F.; Croft, S.L. Natural products as antiparasitic drugs. Parasitol. Res. 2003, 90, S55–S62. [Google Scholar] [CrossRef] [PubMed]
- de Melo, N.I.; Magalhães, L.G.; Carvalho, C.E.; Wakabayashi, K.A.L.; Aguiar, G.P.; Ramos, R.C.; Mantovani, A.L.L.; Turatti, I.C.C.; Rodrigues, V.; Groppo, M.; et al. Schistosomicidal activity of the essential oil of Ageratum conyzoides L. (Asteraceae) against Schistosoma mansoni adult worms. Molecules 2011, 16, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2013, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, R.; Arumugam, S.; Thandavarayan, R.A.; Karuppagounder, V.; Watanabe, K. Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discov. Today. 2016, 21, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Sauvain, M.; Deharo, E. Curcuma as a parasiticidal agent: A review. Planta Med. 2011, 77, 672–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou El Dahab, M.M.; Shahat, S.M.; Mahmoud, S.S.M.; Mahana, N.A. In vitro effect of curcumin on Schistosoma species viability, tegument ultrastructure and egg hatchability. Exp. Parasitol. 2019, 199, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin Uptake and Metabolism. Biofactors 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [Green Version]
- Grencis, R.K. Immunity to helminths: Resistance, regulation, and susceptibility to gastrointestinal nematodes. Ann. Rev. Immunol. 2015, 33, 201–225. [Google Scholar] [CrossRef]
- Floudas, A.; Aviello, G.; Schwartz, C.; Jeffery, L.B.; O’Toole, P.W.; Fallon, P.G. Schistosoma mansoni worm iInfection regulates the Intestinal microbiota and susceptibility to colitis. Infect. Immun. 2019, 87, e00275-19. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The intestinal microbiota in colorectal cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Su, L.; Li, Y.; Long, S.R.; Chang, J.; Zhang, W.; Walker, W.A.; Xavier, R.J.; Cheravil, B.J.; Shi, H.N. Helminth induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018, 11, 144–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, G.L.; Millard, A.; Sergeant, M.J.; Midzi, N.; Gwisai, R.; Mduluza, T.; Jvens, A.; Nausch, N.; Mutapi, F.; Pallen, M. Differences in the faecal microbiome in Schistosoma haematobium infected children vs uninfected children. PLoS Negl. Trop. Dis. 2015, 9, e0003861. [Google Scholar] [CrossRef]
- Schneeberger, P.H.; Coulibaly, J.T.; Panic, G.; Daubenberger, C.; Gueuning, M.; Frey, J.; Frey, J.E.; Keiser, J. Investigations on the interplays between Schistosoma mansoni, praziquantel and the gut microbiome. Parasites Vectors. 2018, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Inohara, N.; Nuñez, G. Mechanisms of inflammation driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta 2017, 1861, 1801–1812. [Google Scholar] [CrossRef]
- Gul, P.; Bakht, J. Antimicrobial activity of turmeric extract and its potential use in food industry. J. Food Sci. Technol. 2015, 52, 2272–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, D.G.; Lee, D.G. Antibacterial activity of curcumin via apoptosis like response in Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2011, 10, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Dhamgaye, S.; Singh, A.; Prasad, R. Lipidome analysis reveals antifungal polyphenol curcumin affects membrane lipid homeostasis. Front. Biosci. 2012, 4, 1195–1209. [Google Scholar] [CrossRef]
- Kumar, A.; Dhamgaye, S.; Maurya, I.K.; Singh, A.; Sharma, M.; Prasad, R. Curcumin targets cell wall integrity via calcineurin-mediated signaling in Candida albicans. Antimicrob. Agents Chemother. 2014, 58, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Breternitz, R.; Kusel, J.R.; Lutz, F.; Buehrle, C.; Ruppel, A. Schistosoma mansoni: Stage-dependent formation and repair of membrane pores induced by a cytotoxin from Pseudomonas aeruginosa. Exp. Parasitol. 1992, 74, 340–347. [Google Scholar] [CrossRef]
- Entwistle, L.J.; Pelly, V.S.; Coomes, S.M.; Kannan, Y.; Perez-Lloret, J.; Czieso, S.; Silva Dos Santos, M.; MacRae, J.I.; Collinson, L.; Sesay, A.; et al. Epithelial-cell-derived phospholipase A 2 group 1B is an endogenous anthelmintic. Cell Host Microbe 2017, 22, 484–493. [Google Scholar] [CrossRef] [Green Version]
- Brestoff, J.R.; Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Mutapi, F.; Winborn, G.; Midzi, N.; Taylor, M.; Mduluza, T.; Maizels, R.M. Cytokine responses to Schistosoma haematobium in a Zimbabwean population: Contrasting profiles for IFN-gamma, IL-4, IL-5 and IL-10 with age. BMC Infect. Dis. 2007, 7, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, L.A.; Finlay, B.B.; Maizels, R.M. Cohabitation in the intestine: Interactions among helminth parasites, bacterial microbiota, and host immunity. J. Immunol. 2015, 195, 4059–4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maizels, R.M.; Smith, K.A. Regulatory T cells in infection. Adv. Immunol. 2011, 112, 73–136. [Google Scholar] [PubMed]
- Allam, G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 2009, 214, 712–727. [Google Scholar] [CrossRef]
- Fallon, P.G.; Richardson, E.J.; McKenzie, G.J.; McKenzie, A.N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 2000, 164, 2585–2591. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.-K.; Dunay, I.R.; Moter, A.; Gescher, D.M.; et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Kiuchi, F.; Goto, Y.; Sugimoto, N.; Akao, N.; Kondo, K.; Tsuda, Y. Nematocidal activity of turmeric, synergistic action of curcuminoids. Chem. Pharm. Bull. 1993, 41, 1640–1643. [Google Scholar] [CrossRef] [Green Version]
- Araújo, C.A.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz. 2001, 96, 723–728. [Google Scholar] [CrossRef]
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultraperformance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 985, 38–47. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Ahmed, S.; Aly, S. Antischistosomal and liver protective effects of Curcuma longa extract in Schistosoma mansoni infected mice. Indian J. Expt. Biol. 2007, 45, 791–801. [Google Scholar]
- Magalhães, L.G.; Machado, C.B.; Morais, E.R.; Moreira, E.B.; Soares, C.S.; da Silva, S.H.; Filho, A.A.; Rodrigues, V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2009, 104, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.R.; Oliveira, K.C.; Magalhães, L.G.; Moreira, E.B.; Verjovski Almeida, S.; Rodrigues, V. Effects of curcumin on the parasite Schistosoma mansoni: A transcriptomic approach. Mol. Biochem. Parasitol. 2013, 187, 91–97. [Google Scholar] [CrossRef]
- Guerra-Sá, R.; Castro-Borges, W.; Evangelista, E.A.; Kettelhut, I.C.; Rodrigues, V. Schistosoma mansoni: Functional proteasomes are required for development in the vertebrate host. Exp. Parasitol. 2005, 109, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual two-way interactions of curcumin and gut microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.S.; Karunaratne, L.B.; Lewis, F.A.; Freitas, T.C.; Liang, Y.S. Schistosomiasis. Curr. Protoc. Immunol. 2013, 103, 19.1.1–19.1.58. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic laboratory technique: Plating methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Prasad, A.; Sarfraz, A.; Jaiswal, N.K.; Kumar, R. Evaluation of a new method for Gram staining of bacteria. Med. Sci. 2015, 18, 16–17. [Google Scholar]
- Mahon, C.R.; Lehman, D.C.; Manuselis, G. Textbook of Diagnostic Microbiology, 4th ed.; Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- McDonald, C.L.; Chapin, K. Rapid identification of Staphylococcus aureus from blood culture bottles by a classic 2-hour tube coagulase test. J. Clin. Microbiol. 1995, 33, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, J.H.; Pfaller, M.A.; Carroll, K.C.; Funke, G.; Landry, M.L.; Richter, S.S.; Warnock, D.W. Manual of Clinical Microbiology, 11th ed.; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Chan, P.C.K.; Porschen, R.K. Evaluation of Kanamycin-Esculin Bile Agar for Isolation and Presumptive Identification of Bacteroides fragilis Group. J. Clin. Microbiol. 1977, 6, 528–529. [Google Scholar] [PubMed]
- Gaby, W.L.; Hadley, C. Practical laboratory test for the identification of Pseudomonas aeruginosa. J. Bacteriol. 1957, 74, 356–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ederer, G.M.; Clark, M. Motility-indoleornithine medium. Appl. Microbiol. 1970, 20, 849–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, W.H.; Edwards, P.R. The principal divisions and groups of Enterobacteriaceae and their differentiation. Intern. Bull. Bacteriol. Nomen. Taxon. 1960, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Christensen, W.B. Urea Decomposition as a Means of Differentiating Proteus and Paracolon Cultures from Each Other and from Salmonella and Shigella Types. J. Bacteriol. 1946, 52, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.R.; Ewing, W.H. Identification of Enterobacteriacea, 3rd ed.; Burgess Publishing Co.: Minneapolis, MN, USA, 1972; pp. 351–352. [Google Scholar]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Tallima, H.; Montash, M.; Veprek, P.; Velek, J.; Jezek, J.; El Ridi, R. Differences in immunogenicity and vaccine potential of peptides from Schistosoma mansoni glyceraldehyde 3-phosphate dehydrogenase. Vaccine 2003, 21, 3290–4300. [Google Scholar] [CrossRef]
- Pellegrino, J.; Faria, J. The oogram method for the screening of drugs in schistosomiasis mansoni. Am. J. Trop. Med. Hyg. 1965, 14, 363–369. [Google Scholar] [CrossRef]
- Dalton, J.P.; Day, S.R.; Drew, A.C.; Brindley, P.J. A method for the isolation of schistosome eggs and miracidia free of contaminating host tissues. Parasitology 1997, 115, 29–32. [Google Scholar] [CrossRef] [Green Version]

| Groups | Mean ± SD | |||||
|---|---|---|---|---|---|---|
| IgM | IgG1 | IgG2a | IgG2b | IgA | IgE | |
| Naïve | 0.089 ± 0.010 | 0.032 ± 0.017 | 0.066 ± 0.033 | 0.069 ± 0.008 | 0.037 ± 0.045 | 0.004 ± 0.002 |
| (Cut off) | (0.109) | (0.066) | (0.132) | (0.085) | (0.127) | (0.008) |
| I | 0.284 ± 0.038 * | 0.180 ± 0.024 * | 0.063 ± 0.018 | 0.070 ± 0.017 | 0.045 ± 0.013 | 0.006 ± 0.005 |
| ID | 0.219 ± 0.067 * | 0.112 ± 0.076 * | 0.049 ± 0.036 | 0.138 ± 0.045 * | 0.143 ± 0.019 * | 0.007 ± 0.003 |
| IL | 0.289 ± 0.101 * | 0.189 ± 0.056 * | 0.041 ± 0.021 | 0.053 ± 0.034 | 0.056 ± 0.025 | 0.007 ± 0.003 |
| IH | 0.274 ± 0.075 * | 0.598 ± 0.130 * | 0.036 ± 0.033 | 0.062 ± 0.020 | 0.120 ± 0.040 | 0.007 ± 0.003 |
| Parameter Counts | I | ID | IL | IH |
|---|---|---|---|---|
| Total worm burden | ||||
| Mean ± SD | 43.34 ± 8.44 | 42.0 ± 2.65 | 42.0 ± 15.25 | 15.60 ± 10.01 |
| p value | 0.81 | 0.9 | 0.007 *** | |
| Reduction (%) | 3.10% | 3.10% | 64.00% | |
| Male worm burden | ||||
| Mean ± SD | 24.67 ± 3.95 | 23.0 ± 3.61 | 26.25 ± 11.15 | 8.40 ± 5.90 |
| p value | 0.62 | 0.83 | 0.006 *** | |
| Reduction (%) | 6.70% | 65.90% | ||
| Female worm burden | ||||
| Mean ± SD | 18.67 ± 4.46 | 19.0 ± 4.58 | 15.75 ± 5.12 | 7.20 ± 4.87 |
| p value | 0.93 | 0.47 | 0.005 *** | |
| Reduction (%) | 77.70% | 15.60% | 61.40% | |
| Liver egg counts | ||||
| Mean ± SD | 40333 ± 17616 | 48333.3 ± 14433.76 | 63750 ± 17100.2 | 48333 ± 22317 |
| p value | 0.58 | 0.14 | 0.61 | |
| Reduction (%) | - | - | ||
| Intestine egg counts | ||||
| Mean ± SD | 68500 ± 2121.3 | 52750 ± 30774.7 | 53500 ± 33426.5 | 39666 ± 14982.2 |
| p value | 0.53 | 0.58 | 0.042 *** | |
| Reduction (%) | 21.90% | 42.10% | ||
| % Immature ova | ||||
| Mean ± SD | 33.70 ± 7.42 | 35.48 ± 12.5 | 24.9 ± 6.1 | 20.86 ± 5.22 |
| p value | 0.84 | 0.19 | 0.02 *** | |
| Reduction (%) | 27.30% | 39.40% | ||
| % Mature ova | ||||
| Mean ± SD | 58.38 ± 8.85 | 57.57 ± 11.03 | 62.97 ± 5.2 | 50.25 ± 11.5 |
| p value | 0.92 | 0.48 | 0.32 | |
| Reduction (%) | 1.70% | 13.70% | ||
| % Dead ova | ||||
| Mean ± SD | 7.83 ± 4.51 | 6.96 ± 3.0 | 12.13 ± 4.6 | 28.9 ± 10.9 |
| p value | 0.7 | 0.22 | 0.02 *** | |
| Increase (%) | 34.90% | 72.90% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anter, A.; El-Ghany, M.A.; Abou El Dahab, M.; Mahana, N. Does Curcumin Have a Role in the Interaction between Gut Microbiota and Schistosoma mansoni in Mice? Pathogens 2020, 9, 767. https://doi.org/10.3390/pathogens9090767
Anter A, El-Ghany MA, Abou El Dahab M, Mahana N. Does Curcumin Have a Role in the Interaction between Gut Microbiota and Schistosoma mansoni in Mice? Pathogens. 2020; 9(9):767. https://doi.org/10.3390/pathogens9090767
Chicago/Turabian StyleAnter, Assmaa, Mohamed Abd El-Ghany, Marwa Abou El Dahab, and Noha Mahana. 2020. "Does Curcumin Have a Role in the Interaction between Gut Microbiota and Schistosoma mansoni in Mice?" Pathogens 9, no. 9: 767. https://doi.org/10.3390/pathogens9090767






