Foot Drop Stimulation via Piezoelectric Energy Harvester
Abstract
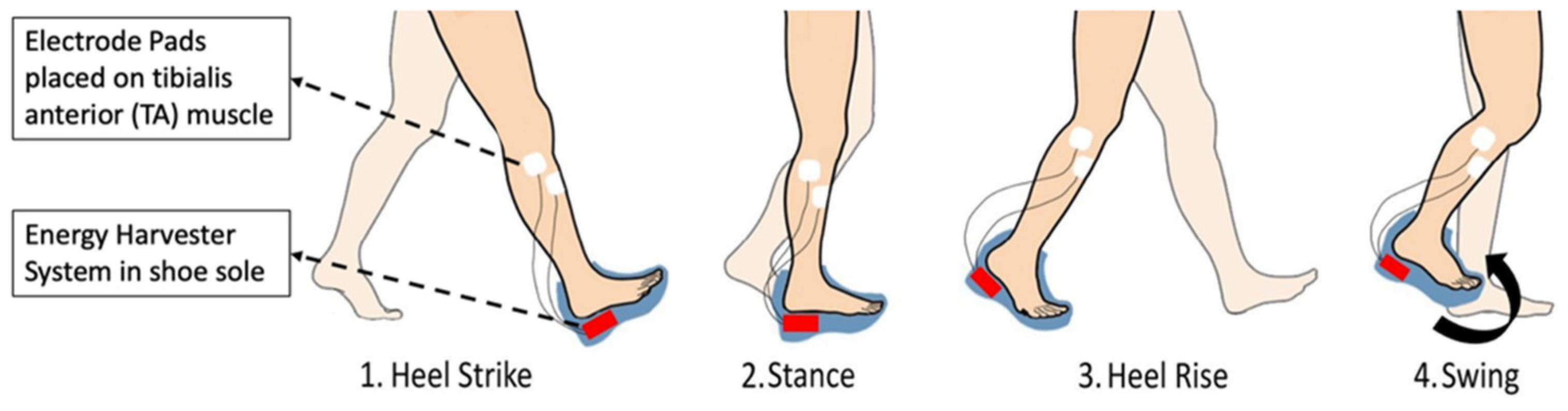
:1. Introduction
2. Materials and Methods
2.1. Power-Management Circuit (PMC)
2.2. FES System
2.2.1. Microprocessor Unit
2.2.2. Biphasic-Conversion Unit
2.2.3. DC–DC-Boost Converters
2.2.4. Output Driver
2.2.5. Biofeedback
2.3. Energy Harvester Assembly
3. Results
3.1. Experimental Results
Oscilloscope Readings
3.2. Analytical Analysis
3.3. Simulation Results
- (1)
- The piezoelectric sheet is fixed at both ends;
- (2)
- The surface is divided into sections representing the width of the crests/troughs;
- (3)
- Uniform loading is applied to all of those sections and the crest/trough geometry of the top and bottom plate, which results in a gradient of the input force is ignored;
- (4)
- The friction between the plates and the piezoelectric sheet is omitted.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.; Li, S.; Ahmed, R.U.; Yam, Y.M.; Thakur, S.; Wang, X.Y.; Tang, D.; Ng, S.; Zheng, Y.P. Development of a battery-free ultrasonically powered functional electrical stimulator for movement restoration after paralyzing spinal cord injury. J. Neuroeng. Rehabil. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carolus, A.E.; Becker, M.; Cuny, J.; Smektala, R.; Schmieder, K.; Brenke, C. The interdisciplinary management of foot drop. Dtsch. Ärztebl. Int. 2019, 116, 347. [Google Scholar] [CrossRef] [PubMed]
- Sabut, S.K.; Sikdar, C.; Kumar, R.; Mahadevappa, M. Functional electrical stimulation of dorsiflexor muscle: Effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation 2011, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Karniel, N.; Raveh, E.; Schwartz, I.; Portnoy, S. Functional electrical stimulation compared with ankle-foot orthosis in subacute post stroke patients with foot drop: A pilot study. Assist. Technol. Off. J. RESNA 2021, 33, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-B.; Jang, W.; Cho, J.Y.; Woo, S.B.; Jeon, D.H.; Ahn, J.H.; Do Hong, S.; Koo, H.Y.; Sung, T.H. Transparent and flexible piezoelectric sensor for detecting human movement with a boron nitride nanosheet (BNNS). Nano Energy 2018, 54, 91–98. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Pfenniger, A.; Stahel, A.; Stoeck, C.T.; Vandenberghe, S.; Koch, V.M.; Vogel, R. Energy Harvesting from the Beating Heart by a Mass Imbalance Oscillation Generator. Ann. Biomed. Eng. 2013, 41, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinchet, R.; Yoon, H.-J.; Ryu, H.; Kim, M.-K.; Choi, E.-K.; Kim, D.-S.; Kim, S.-W. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 2019, 365, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Xu, L.L.; Ryu, C.H.; Kumar, A.; Do Hong, S.; Jeon, D.H.; Cho, J.Y.; Ahn, J.H.; Joo, Y.H.; Jeong, I.W.; et al. Wearable Shoe-Mounted Piezoelectric Energy Harvester for a Self-Powered Wireless Communication System. Energies 2021, 15, 237. [Google Scholar] [CrossRef]
- Yin, Z.; Gao, S.; Jin, L.; Guo, S.; Wu, Q.; Li, Z. A shoe-mounted frequency up-converted piezoelectric energy harvester. Sens. Actuators A Phys. 2021, 318, 112530. [Google Scholar] [CrossRef]
- Fan, K.; Liu, Z.; Liu, H.; Wang, L.; Zhu, Y.; Yu, B. Scavenging energy from human walking through a shoe-mounted piezoelectric harvester. Appl. Phys. Lett. 2017, 110, 143902. [Google Scholar] [CrossRef]
- Narolia, T.; Gupta, V.K.; Parinov, I.A. Design and analysis of a shear mode piezoelectric energy harvester for rotational motion system. J. Adv. Dielectr. 2020, 10, 2050008. [Google Scholar] [CrossRef]
- Manan, A.; Rehman, M.U.; Ullah, A.; Ahmad, A.S.; Iqbal, Y.; Qazi, I.; Khan, M.A.; Shah, H.U.; Wazir, A.H. High energy storage density with ultra-high efficiency and fast charging–discharging capability of sodium bismuth niobate lead-free ceramics. J. Adv. Dielectr. 2021, 11, 2150018. [Google Scholar] [CrossRef]
- Chebanenko, V.A.; Zhilyaev, I.V.; Soloviev, A.N.; Cherpakov, A.V.; Parinov, I.A. Numerical optimization of the piezoelectric generators. J. Adv. Dielectr. 2020, 10, 2060016. [Google Scholar] [CrossRef]
- Shendkar, C.; Lenka, P.K.; Biswas, A.; Kumar, R.; Mahadevappa, M. Design and Development of A Low-Cost Biphasic Charge-Balanced Functional Electric Stimulator and Its Clinical Validation. Healthc. Technol. Lett. 2015, 2, 129–134. Available online: https://ietresearch.onlinelibrary.wiley.com/doi/10.1049/htl.2015.0001 (accessed on 6 December 2021). [CrossRef] [PubMed] [Green Version]
- Chen, C.F.; Chen, W.S.; Chou, L.W.; Chang, Y.J.; Chen, S.C.; Kuo, T.S.; Lai, J.S. Pulse Energy as A Reliable Reference for Twitch Forces Induced by Transcutaneous Neuromuscular Electrical Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 574–583. Available online: https://ieeexplore.ieee.org/document/6177269 (accessed on 23 January 2022). [CrossRef] [PubMed]
- Cheng, K.E.; Lu, Y.; Tong, K.Y.; Rad, A.B.; Chow, D.H.; Sutanto, D. Development of A Circuit for Functional Electrical Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D. Piezoelectric Energy Harvesting Power Supply. Analog Devices. 2010. Available online: https://www.analog.com/en/about-adi/news-room/press-releases/2010/piezoelectric-energy-harvesting-power-supply.html (accessed on 5 March 2022).
- DC1459B Evaluation Kit-Analog Devices. Analog.Com. Available online: https://www.analog.com/media/en/technical-documentation/user-guides/dc1459B.pdf (accessed on 7 March 2022).
- Falin, J. Coupled Inductors Broaden DC/DC Converter Usage. 2010. Available online: https://www.ti.com/lit/an/slyt380/slyt380.pdf (accessed on 12 March 2022).
- Zhao, J.; You, Z. A shoe-embedded piezoelectric energy harvester for wearable sensors. Sensors 2014, 14, 12497–12510. [Google Scholar] [CrossRef] [PubMed]
- Piezo Sheet PVDF Film with 100 nm Thick Aluminum Electrode. Piezoelectric & Pyroelectric PVDF & PVDF-TrFE, Resin, Film, Sensor, Transducer, and Test Instrument. (n.d.). Retrieved 24 April 2022. Available online: https://piezopvdf.com/Aluminium-piezo-sheet-pvdf-film-45um-120um/ (accessed on 28 March 2022).
- Chen, W.; Sonntag, C.; Boesten, F.; Oetomo, S.B.; Feijs, L. A design of power supply for neonatal monitoring with wearable sensors. J. Ambient. Intell. Smart Environ. 2009, 1, 185–196. [Google Scholar] [CrossRef]











| Material Property | Symbol | Value |
|---|---|---|
| PVDF layer thickness | h | 100 μm |
| Electrode (Al) thickness | he | 50 nm |
| Dielectric constant | ε | 12.5 |
| Piezoelectric constant Piezoelectric constant | d31 d33 | 31 pC/N 25 pC/N |
| Elastic modulus | Y | 2700 MPa |
| Design Parameter | Description | Value |
|---|---|---|
| l | PVDF-layer length | 100 mm |
| w | PVDF-layer width | 60 mm |
| As | PVDF-sheet surface area | 6.50 × 10−3 m2 |
| Acs L | PVDF-sheet cross-sectional area Chord length of crest/trough | 6.50 × 10−6 m2 12 mm |
| N | Number of PVDF layers | 6 |
| 2α | Contact angle of the plates | 20° |
| n | Number of crests/troughs | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soozandeh, P.; Poudel, G.; Sarkari, M.; Behdinan, K. Foot Drop Stimulation via Piezoelectric Energy Harvester. Actuators 2022, 11, 174. https://doi.org/10.3390/act11070174
Soozandeh P, Poudel G, Sarkari M, Behdinan K. Foot Drop Stimulation via Piezoelectric Energy Harvester. Actuators. 2022; 11(7):174. https://doi.org/10.3390/act11070174
Chicago/Turabian StyleSoozandeh, Parham, Ganga Poudel, Morteza Sarkari, and Kamran Behdinan. 2022. "Foot Drop Stimulation via Piezoelectric Energy Harvester" Actuators 11, no. 7: 174. https://doi.org/10.3390/act11070174
APA StyleSoozandeh, P., Poudel, G., Sarkari, M., & Behdinan, K. (2022). Foot Drop Stimulation via Piezoelectric Energy Harvester. Actuators, 11(7), 174. https://doi.org/10.3390/act11070174






