Lactobacillus rhamnosus Ameliorates Multi-Drug-Resistant Bacillus cereus-Induced Cell Damage through Inhibition of NLRP3 Inflammasomes and Apoptosis in Bovine Endometritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosecurity Statement
2.2. Clinical Isolates of B. cereus Culture and Sequencing
2.3. Determination of MIC
2.4. BC1908 De Novo Sequencing
2.5. Virulence Gene Testing
2.6. Bovine Endometrial Cell Culture
2.7. Lactobacillus rhamnosus GR-1 Culture
2.8. Lactate Dehydrogenase Assay
2.9. Western Blotting
2.10. Immunofluorescence
2.11. Data Analysis
3. Results
3.1. Cell Cytotoxicity of B. cereus
3.2. Protective Effect of LGR-1 on Cells Infected by B. cereus with Different MOIs
3.3. Antibiotic Sensitivity and Virulence Gene Tests of Bacillus cereus
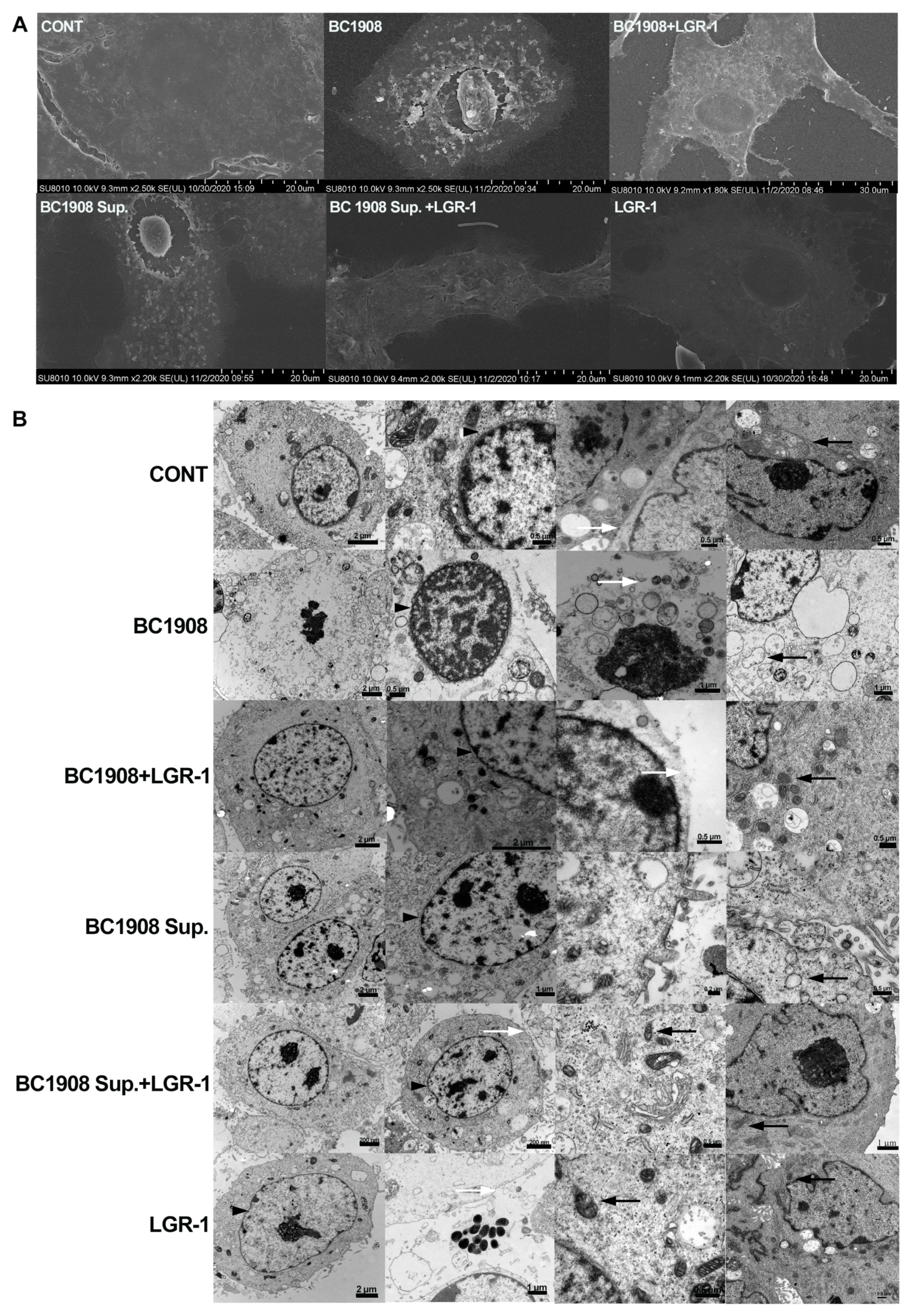
3.4. LGR-1 Ameliorates B. cereus-Induced Destruction of Endometrial Epithelial Cells
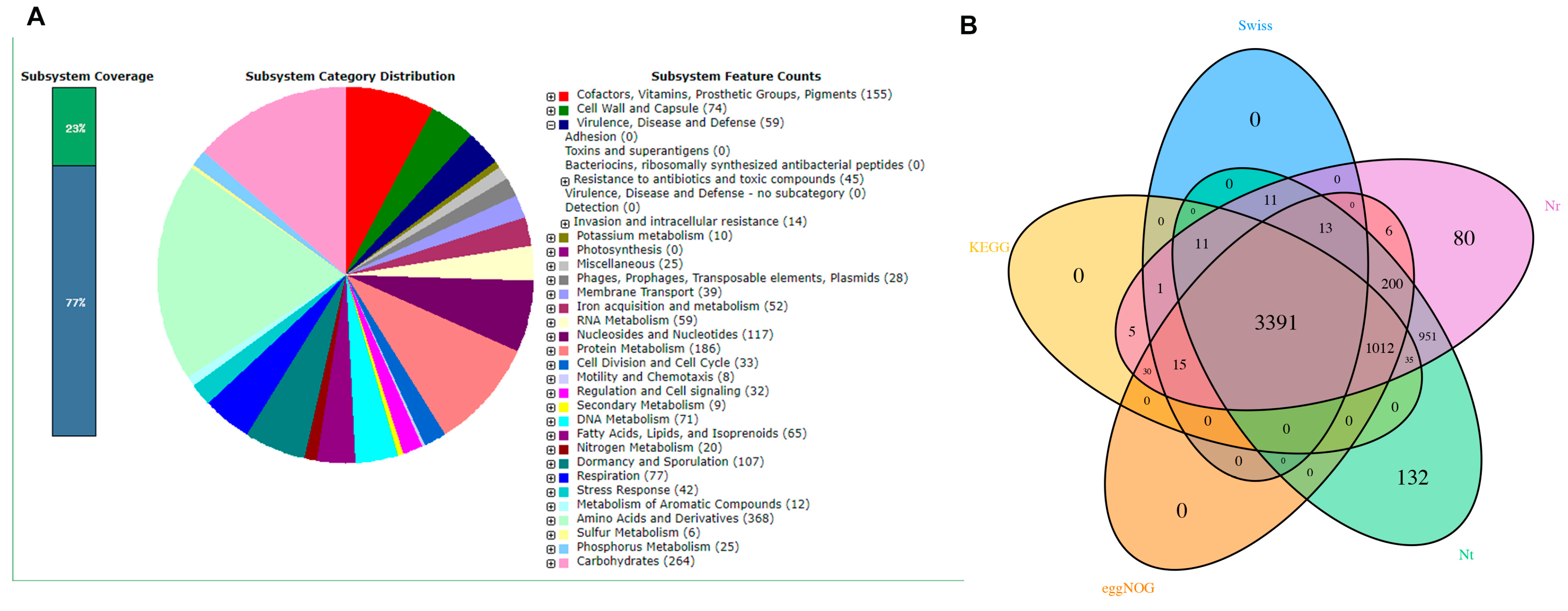
3.5. Genetic-Level Analysis of B. cereus through Second-Generation Sequencing
3.6. LGR-1 Alleviates the Inflammatory Response of BEECs Infected by B. cereus
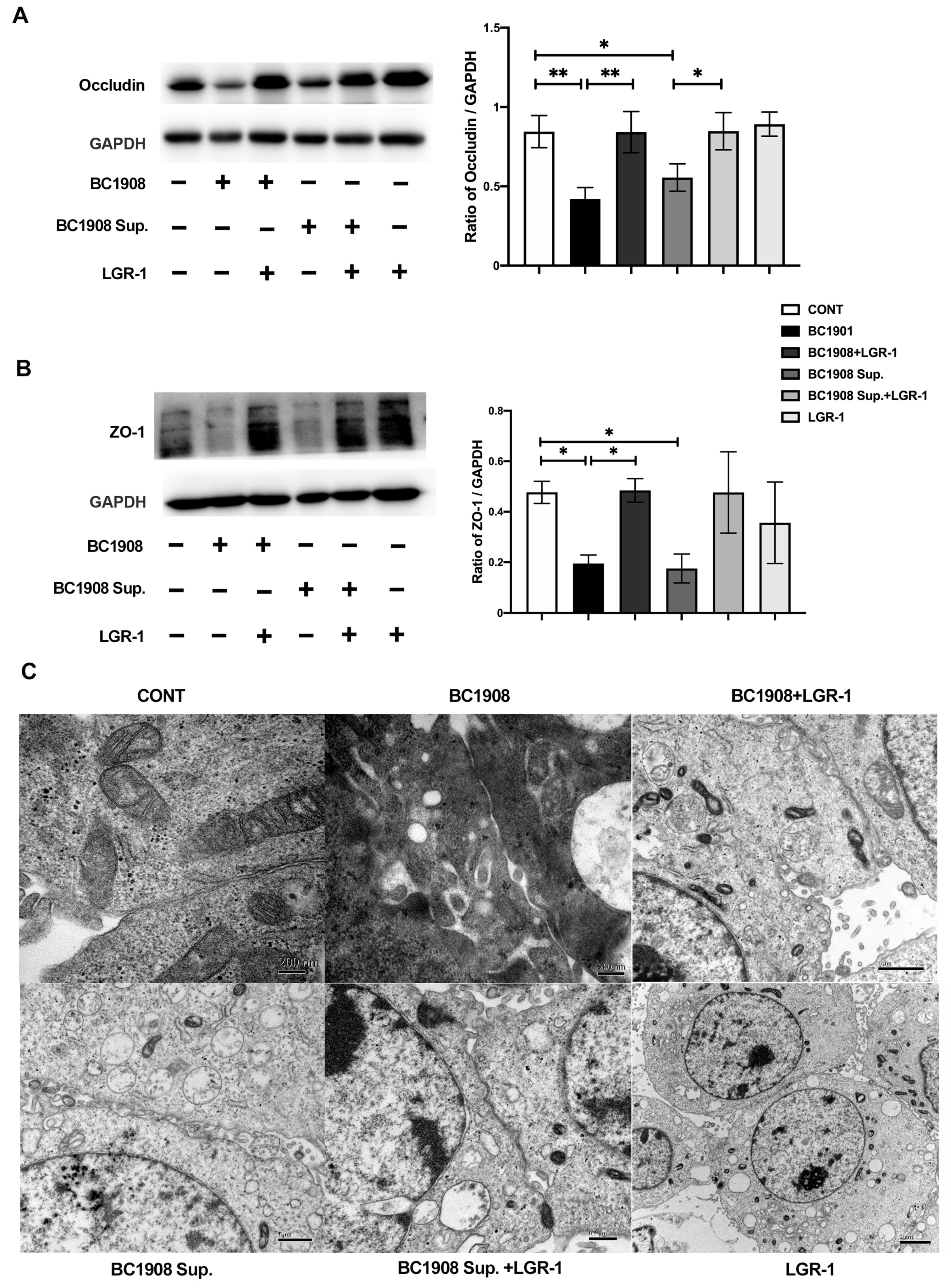
3.7. LGR-1 Promotes the Tight Junction of BEECs Infected by B. cereus
3.8. The Pathways of LGR-1 to Protect from Cell Apoptosis Caused by B. cereus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, X.; Liu, F.; Ding, S.; Shen, J.; Zhu, K. Sublethal Levels of Antibiotics Promote Bacterial Persistence in Epithelial Cells. Adv. Sci. 2020, 7, 1900840. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigourd, V.; Barnier, J.P.; Ferroni, A.; Nicloux, M.; Hachem, T.; Magny, J.F.; Lapillonne, A.; Frange, P.; Nassif, X.; Bille, E. Recent actuality about Bacillus cereus and human milk bank: A new sensitive method for microbiological analysis of pasteurized milk. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, E.E.; Hussien, H.; Hadad, G.A.E.; Mousa, W.S. Prevalence of Virulence Determinants among Bacillus cereus Isolated from Milk Products with Potential Public Health Concern. Pak. J. Biol. Sci. 2020, 23, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidic, J.; Chaix, C.; Manzano, M.; Heyndrickx, M. Food Sensing: Detection of Bacillus cereus Spores in Dairy Products. Biosensors 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedler, G.; Schneider, C.; Igbinosa, E.O.; Kabisch, J.; Brinks, E.; Becker, B.; Stoll, D.A.; Cho, G.S.; Huch, M.; Franz, C. Antibiotics resistance and toxin profiles of Bacillus cereus-group isolates from fresh vegetables from German retail markets. BMC Microbiol. 2019, 19, 250. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Feng, C.; Zhan, L.; Zhang, J.; Li, Y.; Yang, Y.; Chen, H.; Zhang, Z.; Zhang, Y.; et al. Quantitative Prevalence, Phenotypic and Genotypic Characteristics of Bacillus cereus Isolated from Retail Infant Foods in China. Foodborne Pathog. Dis. 2017, 14, 564–572. [Google Scholar] [CrossRef]
- Wang, M.L.; Liu, M.C.; Xu, J.; An, L.G.; Wang, J.F.; Zhu, Y.H. Uterine Microbiota of Dairy Cows With Clinical and Subclinical Endometritis. Front. Microbiol. 2018, 9, 2691. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madegowda, M.; Eswaramoorthy, S.; Burley, S.K.; Swaminathan, S. X-ray crystal structure of the B component of Hemolysin BL from Bacillus cereus. Proteins 2008, 71, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramarao, N.; Sanchis, V. The pore-forming haemolysins of Bacillus cereus: A review. Toxins 2013, 5, 1119–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins—The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Hu, Q.; Xu, F.; Ding, S.Y.; Zhu, K. Characterization of Bacillus cereus in Dairy Products in China. Toxins 2020, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Sorokin, A.; Anderson, I.; Galleron, N.; Candelon, B.; Kapatral, V.; Bhattacharyya, A.; Reznik, G.; Mikhailova, N.; Lapidus, A.; et al. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 2003, 423, 87–91. [Google Scholar] [CrossRef]
- Agerso, Y.; Jensen, L.B.; Givskov, M.; Roberts, M.C. The identification of a tetracycline resistance gene tet(M), on a Tn916-like transposon, in the Bacillus cereus group. FEMS Microbiol. Lett. 2002, 214, 251–256. [Google Scholar] [CrossRef]
- Brown, J.R.; Gentry, D.; Becker, J.A.; Ingraham, K.; Holmes, D.J.; Stanhope, M.J. Horizontal transfer of drug-resistant aminoacyl-transfer-RNA synthetases of anthrax and Gram-positive pathogens. EMBO Rep. 2003, 4, 692–698. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef]
- Torkar, K.G.; Bedenic, B. Antimicrobial susceptibility and characterization of metallo-beta-lactamases, extended-spectrum beta-lactamases, and carbapenemases of Bacillus cereus isolates. Microb. Pathog. 2018, 118, 140–145. [Google Scholar] [CrossRef]
- Ali, M.M.; Aburowes, A.H.; Albakush, A.M.; Rzeg, M.M.; Alrtail, A.; Ghenghesh, K.S. Identification of multidrug-resistant bacteria and Bacillus cereus from healthcare workers and environmental surfaces in a hospital. Libyan J. Med. 2014, 9, 25794. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhu, Y.H.; Xu, J.; Liu, X.; Duan, C.; Wang, M.J.; Wang, J.F. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Activation of NLRP3 and NLRC4 Inflammasomes with Differential Requirement for ASC. Front. Microbiol. 2018, 9, 1661. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liu, M.C.; Yang, J.; Wang, J.F.; Zhu, Y.H. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Inflammation and Cell Damage via Attenuation of ASC-Independent NLRP3 Inflammasome Activation. Appl. Environ. Microbiol. 2016, 82, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wu, Q.; Wang, M.; Fu, Y.; Wang, J. Lactobacillus rhamnosus GR-1 Limits Escherichia coli-Induced Inflammatory Responses via Attenuating MyD88-Dependent and MyD88-Independent Pathway Activation in Bovine Endometrial Epithelial Cells. Inflammation 2016, 39, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Genis, S.; Bach, A.; Aris, A. Effects of intravaginal lactic acid bacteria on bovine endometrium: Implications in uterine health. Vet. Microbiol. 2017, 204, 174–179. [Google Scholar] [CrossRef]
- Fortier, M.A.; Guilbault, L.A.; Grasso, F. Specific properties of epithelial and stromal cells from the endometrium of cows. J. Reprod. Fertil. 1988, 83, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza Santos, M.; Orth, K. Intracellular Vibrio parahaemolyticus escapes the vacuole and establishes a replicative niche in the cytosol of epithelial cells. mBio 2014, 5, e01506–e01514. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.L.; Sumpter, R., Jr.; Levine, B.; Hooper, L.V. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 2013, 13, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef]
- Fauvart, M.; De Groote, V.N.; Michiels, J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011, 60, 699–709. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Gerdes, K.; Lewis, K.; McKinney, J.D. A problem of persistence: Still more questions than answers? Nat. Rev. Microbiol. 2013, 11, 587–591. [Google Scholar] [CrossRef]
- Levin, B.R.; Rozen, D.E. Non-inherited antibiotic resistance. Nat. Rev. Microbiol. 2006, 4, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenyon, C.; Colebunders, R.; Crucitti, T. The global epidemiology of bacterial vaginosis: A systematic review. Am. J. Obstet. Gynecol. 2013, 209, 505–523. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.M.; Hyun, Y.J.; Myoung, K.S.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus johnsonii HY7042 ameliorates Gardnerella vaginalis-induced vaginosis by killing Gardnerella vaginalis and inhibiting NF-kappaB activation. Int. Immunopharmacol. 2011, 11, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.M.; Kim, K.A.; Myoung, K.S.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus helveticus HY7801 ameliorates vulvovaginal candidiasis in mice by inhibiting fungal growth and NF-kappaB activation. Int. Immunopharmacol. 2012, 14, 39–46. [Google Scholar] [CrossRef]
- Derikx, L.A.; Dieleman, L.A.; Hoentjen, F. Probiotics and prebiotics in ulcerative colitis. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 55–71. [Google Scholar] [CrossRef]
- Jang, S.E.; Han, M.J.; Kim, S.Y.; Kim, D.H. Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by polarizing M1 to M2-like macrophages. Int. Immunopharmacol. 2014, 21, 186–192. [Google Scholar] [CrossRef]
- Genis, S.; Bach, A.; Fabregas, F.; Aris, A. Potential of lactic acid bacteria at regulating Escherichia coli infection and inflammation of bovine endometrium. Theriogenology 2016, 85, 625–637. [Google Scholar] [CrossRef] [PubMed]
- You, G.E.; Jung, B.J.; Kim, H.R.; Kim, H.G.; Kim, T.R.; Chung, D.K. Lactobacillus sakei lipoteichoic acid inhibits MMP-1 induced by UVA in normal dermal fibroblasts of human. J. Microbiol. Biotechnol. 2013, 23, 1357–1364. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ding, S.; Shi, P.; Dietrich, R.; Martlbauer, E.; Zhu, K. Non-hemolytic enterotoxin of Bacillus cereus induces apoptosis in Vero cells. Cell. Microbiol. 2017, 19, e12684. [Google Scholar] [CrossRef]
- Bischofberger, M.; Iacovache, I.; van der Goot, F.G. Pathogenic pore-forming proteins: Function and host response. Cell Host Microbe 2012, 12, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Imre, G.; Heering, J.; Takeda, A.N.; Husmann, M.; Thiede, B.; zu Heringdorf, D.M.; Green, D.R.; van der Goot, F.G.; Sinha, B.; Dotsch, V.; et al. Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis. EMBO J. 2012, 31, 2615–2628. [Google Scholar] [CrossRef]
- Ratner, A.J.; Hippe, K.R.; Aguilar, J.L.; Bender, M.H.; Nelson, A.L.; Weiser, J.N. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J. Biol. Chem. 2006, 281, 12994–12998. [Google Scholar] [CrossRef] [Green Version]
- Verhagen, A.M.; Kratina, T.K.; Hawkins, C.J.; Silke, J.; Ekert, P.G.; Vaux, D.L. Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs. Cell Death Differ. 2007, 14, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Suen, D.F.; Norris, K.L.; Youle, R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008, 22, 1577–1590. [Google Scholar] [CrossRef] [Green Version]
- McArthur, K.; Chappaz, S.; Kile, B.T. Apoptosis in megakaryocytes and platelets: The life and death of a lineage. Blood 2018, 131, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1beta Activation. Cell Rep. 2018, 25, 2339–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, J.P.; Behnam, M.; Sutton, J.N.; Du, C.; Wang, X.; Hunt, D.F.; Weber, M.J.; Kulik, G. Smac is required for cytochrome c-induced apoptosis in prostate cancer LNCaP cells. Cancer Res. 2002, 62, 18–23. [Google Scholar] [PubMed]
- Petersen, S.L.; Wang, L.; Yalcin-Chin, A.; Li, L.; Peyton, M.; Minna, J.; Harran, P.; Wang, X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 2007, 12, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Mathur, A.; Feng, S.; Hayward, J.A.; Ngo, C.; Fox, D.; Atmosukarto, I.I.; Price, J.D.; Schauer, K.; Martlbauer, E.; Robertson, A.A.B.; et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat. Microbiol 2019, 4, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Liu, S.; Moayeri, M.; Leppla, S.H. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol. 2014, 22, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enosi Tuipulotu, D.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus cereus: Epidemiology, Virulence Factors, and Host-Pathogen Interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Primer Sequences (5′–3′) | Fragment Size (bp) | |
|---|---|---|---|
| NHEA | F | CGGGATCCACGAGTTGCTTCATTCCTGTAAGC | 1132 |
| R | CCCTCGAGTTAATGTACTTCAACGTTTGTAACGTAATCTTCAAAT | ||
| NHEB | F | CGGAATTCAATATTATGCCGGCTCATACGTATGCA | 1162 |
| R | CCCTCGAGTTATGCTTTTTTCGTATCTACTACTTTAATATCTTC | ||
| NHEC | F | CGGAATTCATGCCGGCTCATACGTAT | 1156 |
| R | CCCTCGAGTTATGCTTTTTTCGTATCTACTACTTTAATATCTTCA | ||
| HBLA | F | CGGGATCCGCAGTCATACCAATAGAAACTTTTGC | 1351 |
| R | CCCTCGAGTCAGTTCATTATATTTTGTACTTTGTCTTTATACAC | ||
| HBLB | F | CGGAATTCTCACCAGTAACAACTTTTGCAAGTGAA | 1072 |
| R | CCCTCGAGCTATTTTTGTGGAGTAACAGTTTCCACTTTT | ||
| HBLD | F | CGGGATCCGCATTTGCACAAGAAACGACCG | 1156 |
| R | CCCTCGAGCTACTCCTGTTTAAAAGCAATATCTTTTGAAATGAA | ||
| HlyIII | F | CGGAATTCGCAATTACACATGGTATCGGTG | 619 |
| R | CCCTCGAGTTATGCTGTAGGTAAGACATAAAAGAGTACA | ||
| inhA | F | CGGAATTCATGAGTGCTCCGTTAGCATATGCA | 2344 |
| R | CCCTCGAGTTAACGTTTAATCCAAACAGCGCCTGC | ||
| hlyA | F | CGGAATTCGCCATTATGGCCGGACT | 778 |
| R | CCCTCGAGTTATTCCCCTTTCCCTTTTTGTTTTAG | ||
| cytk | F | CGGAATTCCCTGCTACTTACGCTCAAAC | 949 |
| R | CCCTCGAGTTATTTTTTCTCTACTAATTTCTTATTCTTCCAATCTAG |
| Strain | Antibiotics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | KAN | AMP | STR | AZM | AMC | ENR | TET | GM | CIP | MEM | |
| BC1908 | R | R | R | S | R | R | S | R | R | R | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Wang, X.; Shan, Q.; Xu, L.; Li, Y.; Chu, B.; Yang, L.; Wang, J.; Zhu, Y. Lactobacillus rhamnosus Ameliorates Multi-Drug-Resistant Bacillus cereus-Induced Cell Damage through Inhibition of NLRP3 Inflammasomes and Apoptosis in Bovine Endometritis. Microorganisms 2022, 10, 137. https://doi.org/10.3390/microorganisms10010137
Liu N, Wang X, Shan Q, Xu L, Li Y, Chu B, Yang L, Wang J, Zhu Y. Lactobacillus rhamnosus Ameliorates Multi-Drug-Resistant Bacillus cereus-Induced Cell Damage through Inhibition of NLRP3 Inflammasomes and Apoptosis in Bovine Endometritis. Microorganisms. 2022; 10(1):137. https://doi.org/10.3390/microorganisms10010137
Chicago/Turabian StyleLiu, Ning, Xue Wang, Qiang Shan, Le Xu, Yanan Li, Bingxin Chu, Lan Yang, Jiufeng Wang, and Yaohong Zhu. 2022. "Lactobacillus rhamnosus Ameliorates Multi-Drug-Resistant Bacillus cereus-Induced Cell Damage through Inhibition of NLRP3 Inflammasomes and Apoptosis in Bovine Endometritis" Microorganisms 10, no. 1: 137. https://doi.org/10.3390/microorganisms10010137
APA StyleLiu, N., Wang, X., Shan, Q., Xu, L., Li, Y., Chu, B., Yang, L., Wang, J., & Zhu, Y. (2022). Lactobacillus rhamnosus Ameliorates Multi-Drug-Resistant Bacillus cereus-Induced Cell Damage through Inhibition of NLRP3 Inflammasomes and Apoptosis in Bovine Endometritis. Microorganisms, 10(1), 137. https://doi.org/10.3390/microorganisms10010137






