The Temporal Dynamics of Rumen Microbiota in Early Weaned Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animal Management, and Sampling
2.2. DNA Extraction and 16S rRNA Sequencing
2.3. Bioinformatics and Data Analysis
3. Results
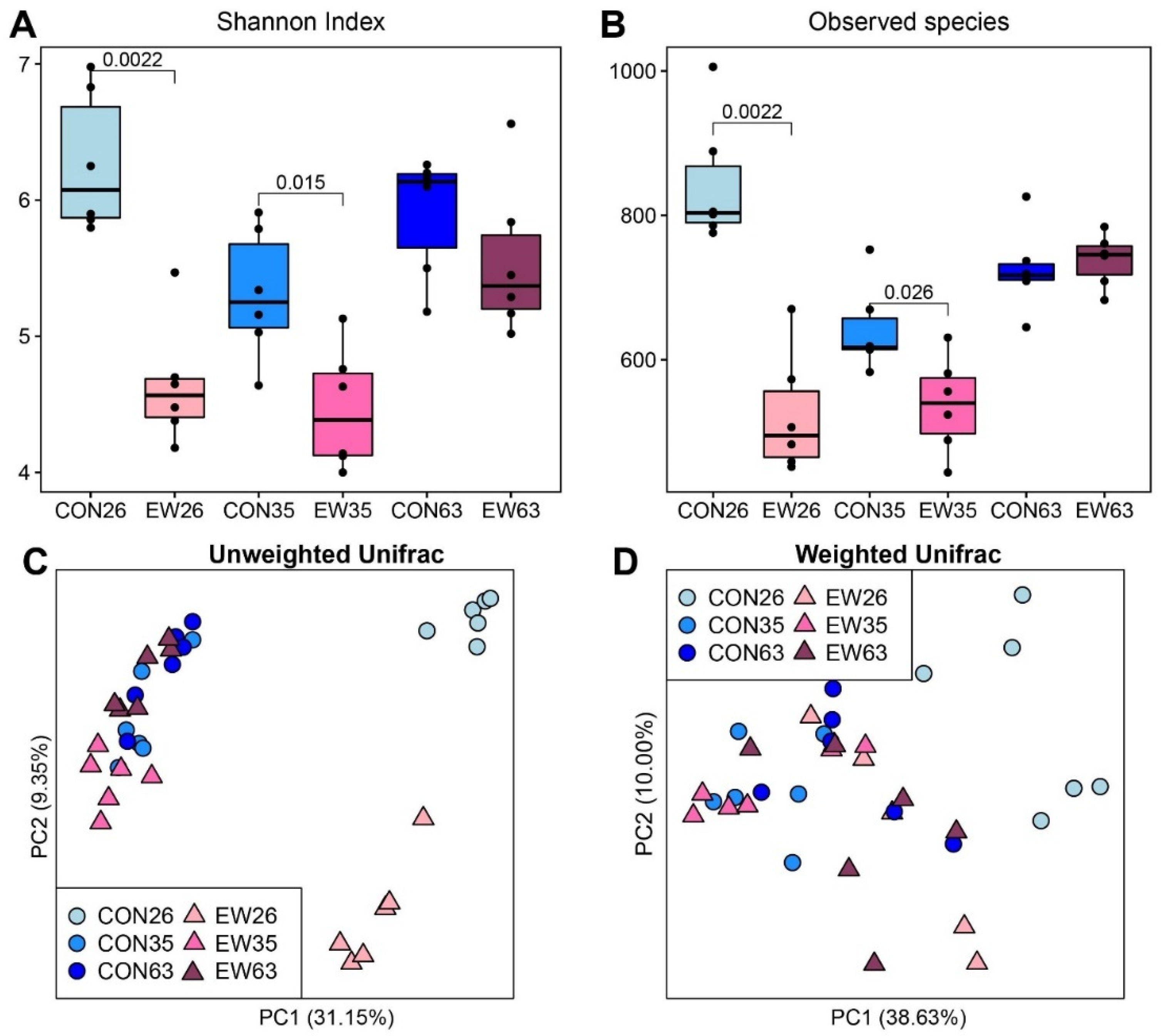
3.1. Diversity and Richness of Rumen Microbial Communities
3.2. Bacterial Composition at Different Taxonomical Levels
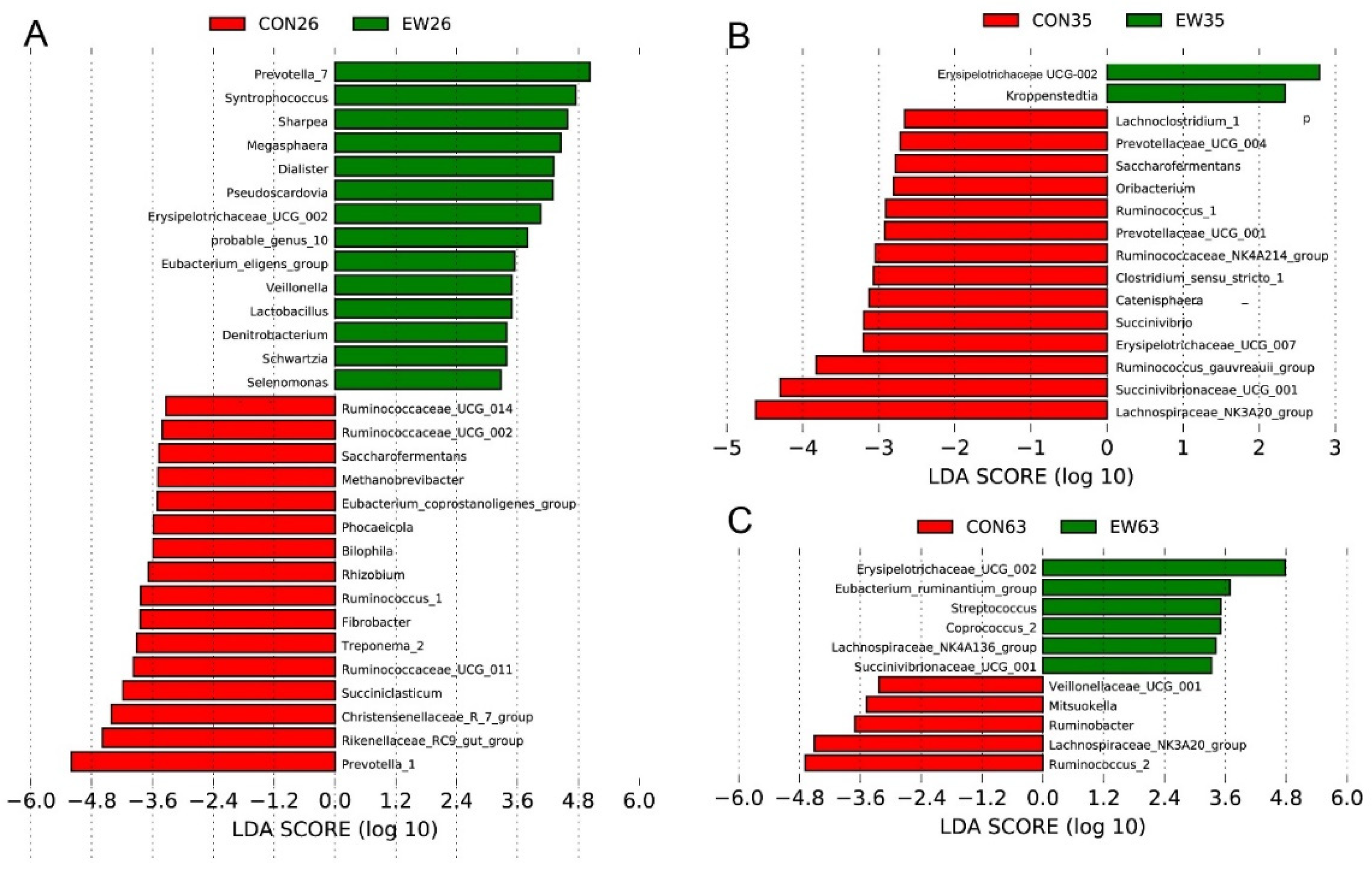
3.3. Bacterial Biomarkers for Early Weaning (EW) at Different Ages
3.4. Temporal Dynamics of Rumen Microbiota in CON and EW Groups
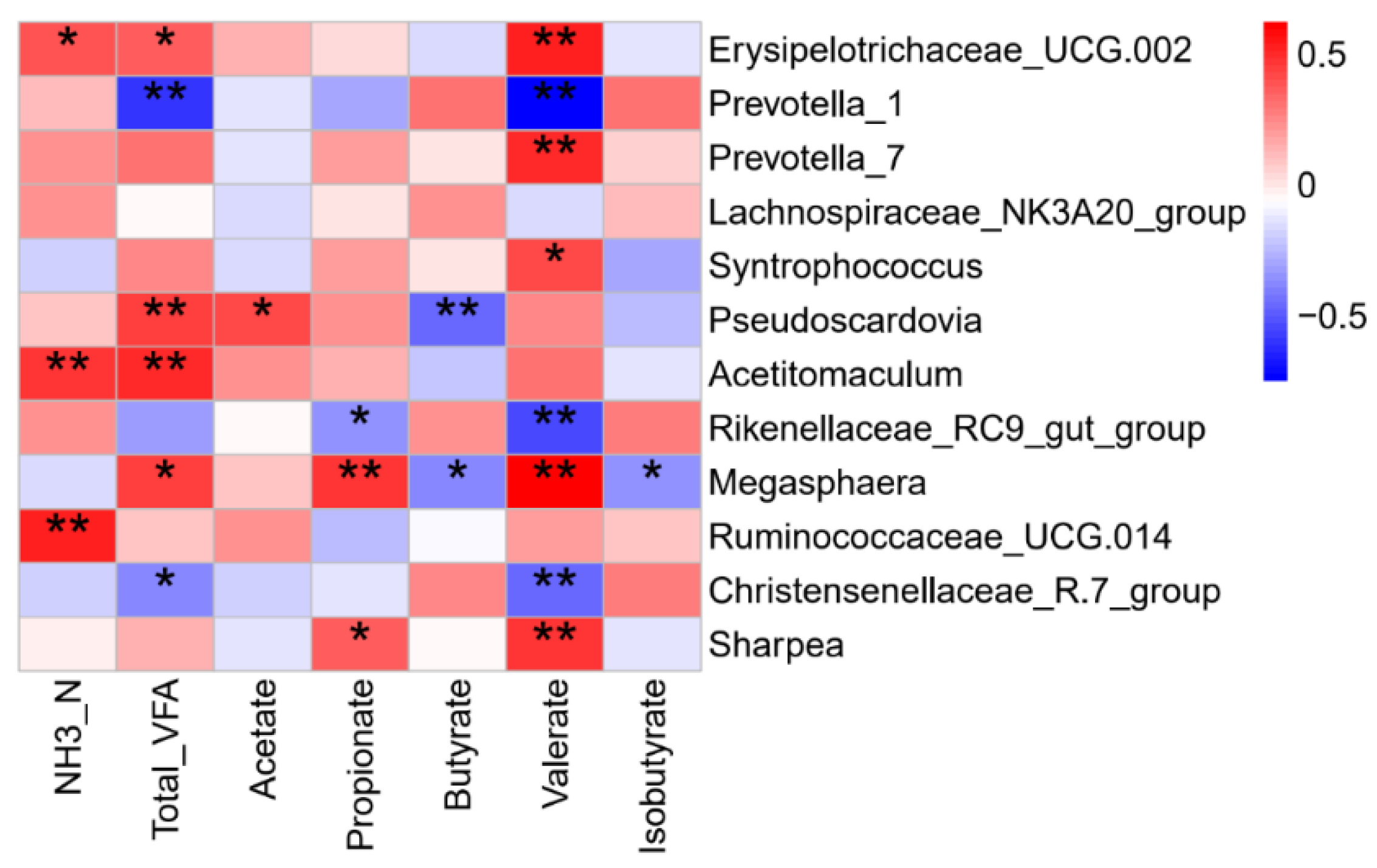
3.5. Rumen Bacteria Associated with Fermentation Parameters
4. Discussion
4.1. The Diversity and Richness of Rumen Microbiota Influenced by Early Weaning
4.2. The Compositions of Rumen Microbiota Are Associated with Early Weaning
4.3. The Temporal Dynamics of Rumen Microbiota after Early Weaning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carballo, O.C.; Khan, M.A.; Knol, F.W.; Lewis, S.J.; Stevens, D.R.; Laven, R.A.; McCoard, S.A. Impact of weaning age on rumen development in artificially reared lambs. J. Anim. Sci. 2019, 97, 3498–3510. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhang, Y.; Yun, Y.; Ji, W.; Jin, Z.; Wang, C.; Yu, Z. Weaning age affects the development of the ruminal bacterial and archaeal community in hu lambs during early life. Front. Microbiol. 2021, 12, 636865. [Google Scholar] [CrossRef] [PubMed]
- Klein-Jobstl, D.; Quijada, N.M.; Dzieciol, M.; Feldbacher, B.; Wagner, M.; Drillich, M.; Schmitz-Esser, S.; Mann, E. Microbiota of newborn calves and their mothers reveals possible transfer routes for newborn calves’ gastrointestinal microbiota. PLoS ONE 2019, 14, e0220554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, C.E.; Huffard, H.G.; Nin-Velez, A.I.; Duncan, J.; Teets, C.L.; Daniels, K.M.; Ealy, A.D.; James, R.E.; Knowlton, K.F.; Cockrum, R.R. Microbiomes of various maternal body systems are predictive of calf digestive bacterial ecology. Animals 2021, 11, 2210. [Google Scholar] [CrossRef]
- Arshad, M.A.; Hassan, F.U.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut microbiome colonization and development in neonatal ruminants: Strategies, prospects, and opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef]
- Bi, Y.; Cox, M.S.; Zhang, F.; Suen, G.; Zhang, N.; Tu, Y.; Diao, Q. Feeding modes shape the acquisition and structure of the initial gut microbiota in newborn lambs. Environ. Microbiol. 2019, 21, 2333–2346. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Chai, J.; Cui, K.; Bi, Y.; Diao, Q.; Huang, W.; Usdrowski, H.; Zhang, N. Longitudinal investigation of the gut microbiota in goat kids from birth to postweaning. Microorganisms 2020, 8, 1111. [Google Scholar] [CrossRef]
- Yang, B.; Le, J.; Wu, P.; Liu, J.; Guan, L.L.; Wang, J. Alfalfa intervention alters rumen microbial community development in hu lambs during early life. Front. Microbiol. 2018, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Chai, J.; Diao, Q.; Huang, W.; Zhuang, Y.; Zhang, N. The signature microbiota drive rumen function shifts in goat kids introduced to solid diet regimes. Microorganisms 2019, 7, 516. [Google Scholar] [CrossRef] [Green Version]
- Lv, F.; Wang, X.; Pang, X.; Liu, G. Effects of supplementary feeding on the rumen morphology and bacterial diversity in lambs. Peer.J. 2020, 8, e9353. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Wang, G.; Niu, X.; Wang, W.; Li, F.; Li, F.; Zhang, Z. The functional development of the rumen is influenced by weaning and associated with ruminal microbiota in lambs. Anim. Biotechnol. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.M.; Ma, T.; Wang, H.C.; Qi, M.L.; Tu, Y.; Diao, Q.Y.; Zhang, N.F. Effect of early weaning age on growth performance, nutrient digestibility, and serum parameters of lambs. Anim. Prod. Sci. 2017, 57, 110–115. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Niu, X.; Zhang, Z.; Li, F.; Li, F. The effects of milk replacer allowance and weaning age on the performance, nutrients digestibility, and ruminal microbiota communities of lambs. Anim. Feed. Sci. Technol. 2019, 257, 114263. [Google Scholar] [CrossRef]
- Wang, S.; Ma, T.; Zhao, G.; Zhang, N.; Tu, Y.; Li, F.; Cui, K.; Bi, Y.; Ding, H.; Diao, Q. Effect of age and weaning on growth performance, rumen fermentation, and serum parameters in lambs fed starter with limited ewe-lamb interaction. Animals 2019, 9, 825. [Google Scholar] [CrossRef] [Green Version]
- McCoard, S.A.; Cristobal-Carballo, O.; Knol, F.W.; Heiser, A.; Khan, M.A.; Hennes, N.; Johnstone, P.; Lewis, S.; Stevens, D.R. Impact of early weaning on small intestine, metabolic, immune and endocrine system development, growth and body composition in artificially reared lambs. J. Anim. Sci. 2020, 98, skz356. [Google Scholar] [CrossRef]
- Chai, J.; Diao, Q.; Wang, H.; Tu, Y.; Tao, X.; Zhang, N. Effects of weaning age on growth, nutrient digestibility and metabolism, and serum parameters in Hu lambs. Anim. Nutr. 2015, 1, 344–348. [Google Scholar] [CrossRef]
- Belanche, A.; Yanez-Ruiz, D.R.; Detheridge, A.P.; Griffith, G.W.; Kingston-Smith, A.H.; Newbold, C.J. Maternal versus artificial rearing shapes the rumen microbiome having minor long-term physiological implications. Environ. Microbiol. 2019, 21, 4360–4377. [Google Scholar] [CrossRef] [Green Version]
- Dill-McFarland, K.A.; Breaker, J.D.; Suen, G. Microbial succession in the gastrointestinal tract of dairy cows from 2 weeks to first lactation. Sci. Rep. 2017, 7, 40864. [Google Scholar] [CrossRef] [Green Version]
- Fomenky, B.E.; Chiquette, J.; Bissonnette, N.; Talbot, G.; Chouinard, P.Y.; Ibeagha-Awemu, E.M. Impact of Saccharomyces cerevisiae boulardii cncmi-1079 and Lactobacillus acidophilus Bt1386 on total Lactobacilli population in the gastrointestinal tract and colon histomorphology of holstein dairy calves. Anim. Feed. Sci. Technol. 2017, 234, 151–161. [Google Scholar] [CrossRef]
- Edgar, R.C. Uparse: Highly accurate otu sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16s rRNA-based studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sheng, H.F.; He, Y.; Wu, J.Y.; Jiang, Y.X.; Tam, N.F.; Zhou, H.W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, H.; Zhu, L.; Xu, Y.; Liu, N.; Sun, X.; Hu, L.; Huang, H.; Wei, K.; Zhu, R. Dynamic distribution of gut microbiota in goats at different ages and health states. Front. Microbiol. 2018, 9, 2509. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.; Lv, X.; Diao, Q.; Usdrowski, H.; Zhuang, Y.; Huang, W.; Cui, K.; Zhang, N. Solid diet manipulates rumen epithelial microbiota and its interactions with host transcriptomic in young ruminants. Environ. Microbiol. 2021, 23, 6557–6568. [Google Scholar] [CrossRef]
- Dill-McFarland, K.A.; Weimer, P.J.; Breaker, J.D.; Suen, G. Diet influences early microbiota development in dairy calves without long-term impacts on milk production. Appled Environ. Microbiol. 2019, 85, e02141-18. [Google Scholar] [CrossRef] [Green Version]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef]
- Myer, P.R.; Smith, T.P.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen microbiome from steers differing in feed efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar]
- Pan, X.; Xue, F.; Nan, X.; Tang, Z.; Wang, K.; Beckers, Y.; Jiang, L.; Xiong, B. Illumina sequencing approach to characterize thiamine metabolism related bacteria and the impacts of thiamine supplementation on ruminal microbiota in dairy cows fed high-grain diets. Front. Microbiol. 2017, 8, 1818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C.; Niu, X.; Zhang, Z.; Li, F.; Li, F. An intensive milk replacer feeding program benefits immune response and intestinal microbiota of lambs during weaning. BMC Vet. Res. 2018, 14, 366. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, D.M.; Weimer, P.J. Dominance of prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef]
- Belanche, A.; Doreau, M.; Edwards, J.E.; Moorby, J.M.; Pinloche, E.; Newbold, C.J. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 2012, 142, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Webb, M.; Ghimire, S.; Blair, A.; Olson, K.; Fenske, G.J.; Fonder, A.T.; Christopher-Hennings, J.; Brake, D.; Scaria, J. Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep. 2017, 7, 12257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, T.; Jiao, P.; AlZahal, O.; Xie, X.; Beauchemin, K.A.; Niu, D.; Yang, W. Fecal bacterial community of finishing beef steers fed ruminally protected and non-protected active dried yeast. J. Anim. Sci. 2020, 98, skaa058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, M.; Enjalbert, F.; Combes, S.; Cauquil, L.; Bouchez, O.; Monteils, V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J. Appl. Microbiol. 2014, 116, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Saito, T.; Ohshima, T.; Nakagawa, Y.; Arita, T.; Yashima, A.; Makino, T.; Konnai, R.; Gomi, K.; Arai, T.; et al. Terminal Rflp analysis to determine the oral microbiota with hyposalivation. Arch. Microbiol. 2014, 196, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Kamke, J.; Kittelmann, S.; Soni, P.; Li, Y.; Tavendale, M.; Ganesh, S.; Janssen, P.H.; Shi, W.; Froula, J.; Rubin, E.M.; et al. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome 2016, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Mackie, R.I.; Gilchrist, F.M. Changes in lactate-producing and lactate-utilizing bacteria in relation to pH in the rumen of sheep during stepwise adaptation to a high-concentrate diet. Appl. Environ. Microbiol. 1979, 38, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.; Gao, C.; Gao, Z.; Rahman, M.A.U.; He, Y.; Cao, B.; Su, H. Temporal dynamics in rumen bacterial community composition of finishing steers during an adaptation period of three months. Microorganisms 2019, 7, 410. [Google Scholar] [CrossRef] [Green Version]
- Zened, A.; Combes, S.; Cauquil, L.; Mariette, J.; Klopp, C.; Bouchez, O.; Troegeler-Meynadier, A.; Enjalbert, F. Microbial ecology of the rumen evaluated by 454 Gs Flx pyrosequencing is affected by starch and oil supplementation of diets. FEMS Microbiol. Ecol. 2013, 83, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Scheifinger, C.C.; Linehan, B.; Wolin, M.J. H2 production by selenomonas ruminantium in the absence and presence of methanogenic bacteria. Appl. Microbiol. 1975, 29, 480–483. [Google Scholar] [CrossRef]
- Weimer, P.J.; Stevenson, D.M.; Mantovani, H.C.; Man, S.L. Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents. J. Dairy Sci. 2010, 93, 5902–5912. [Google Scholar] [CrossRef] [PubMed]
- Meale, S.J.; Li, S.; Azevedo, P.; Derakhshani, H.; Plaizier, J.C.; Khafipour, E.; Steele, M.A. Development of ruminal and fecal microbiomes are affected by weaning but not weaning strategy in dairy calves. Front. Microbiol. 2016, 7, 582. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wu, S.; Liu, S.; Sun, L.; Feng, Y.; Cao, Y.; Chai, S.; Zhang, G.; Yao, J. From maternal grazing to barn feeding during pre-weaning period: Altered gastrointestinal microbiota contributes to change the development and function of the rumen and intestine of yak calves. Front. Microbiol. 2020, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Guo, C.; Gong, Y.; Sun, X.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. Rumen fermentation, digestive enzyme activity, and bacteria composition between pre-weaning and post-weaning dairy calves. Animals 2021, 11, 2527. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Wang, C.; Ding, L.; Zhao, F.; Wang, M.; Fu, J.; Wang, H. Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front. Microbiol. 2019, 10, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Bian, G.; Sun, D.; Zhu, W.; Mao, S. Starter feeding altered ruminal epithelial bacterial communities and some key immune-related genes’ expression before weaning in lambs. J. Anim. Sci. 2017, 95, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xie, F.; Sun, D.; Liu, J.; Zhu, W.; Mao, S. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model. Microbiome 2019, 7, 83. [Google Scholar] [CrossRef]
- Liu, J.; Bian, G.; Sun, D.; Zhu, W.; Mao, S. Starter feeding supplementation alters colonic mucosal bacterial communities and modulates mucosal immune homeostasis in newborn lambs. Front. Microbiol. 2017, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Gao, T.; Liu, Z.; Diao, X. Effects of dietary supplementation with high fiber (Stevia residue) on the fecal flora of pregnant sows. Animals 2020, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.; Han, J.; et al. Effects of Dietary energy levels on rumen fermentation, microbial diversity, and feed efficiency of yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Sartor, R.B. The role of diet on intestinal microbiota metabolism: Downstream impacts on host immune function and health, and therapeutic implications. J. Gastroenterol. 2014, 49, 785–798. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chai, J.; Zhao, G.; Zhang, N.; Cui, K.; Bi, Y.; Ma, T.; Tu, Y.; Diao, Q. The Temporal Dynamics of Rumen Microbiota in Early Weaned Lambs. Microorganisms 2022, 10, 144. https://doi.org/10.3390/microorganisms10010144
Wang S, Chai J, Zhao G, Zhang N, Cui K, Bi Y, Ma T, Tu Y, Diao Q. The Temporal Dynamics of Rumen Microbiota in Early Weaned Lambs. Microorganisms. 2022; 10(1):144. https://doi.org/10.3390/microorganisms10010144
Chicago/Turabian StyleWang, Shiqin, Jianmin Chai, Guohong Zhao, Naifeng Zhang, Kai Cui, Yanliang Bi, Tao Ma, Yan Tu, and Qiyu Diao. 2022. "The Temporal Dynamics of Rumen Microbiota in Early Weaned Lambs" Microorganisms 10, no. 1: 144. https://doi.org/10.3390/microorganisms10010144






