Human MAIT Cells Respond to Staphylococcus aureus with Enhanced Anti-Bacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Bacteria
2.3. In Vitro Infection Assay
2.4. Flow Cytometry
2.5. ELISA
2.6. Statistical Analysis
3. Results
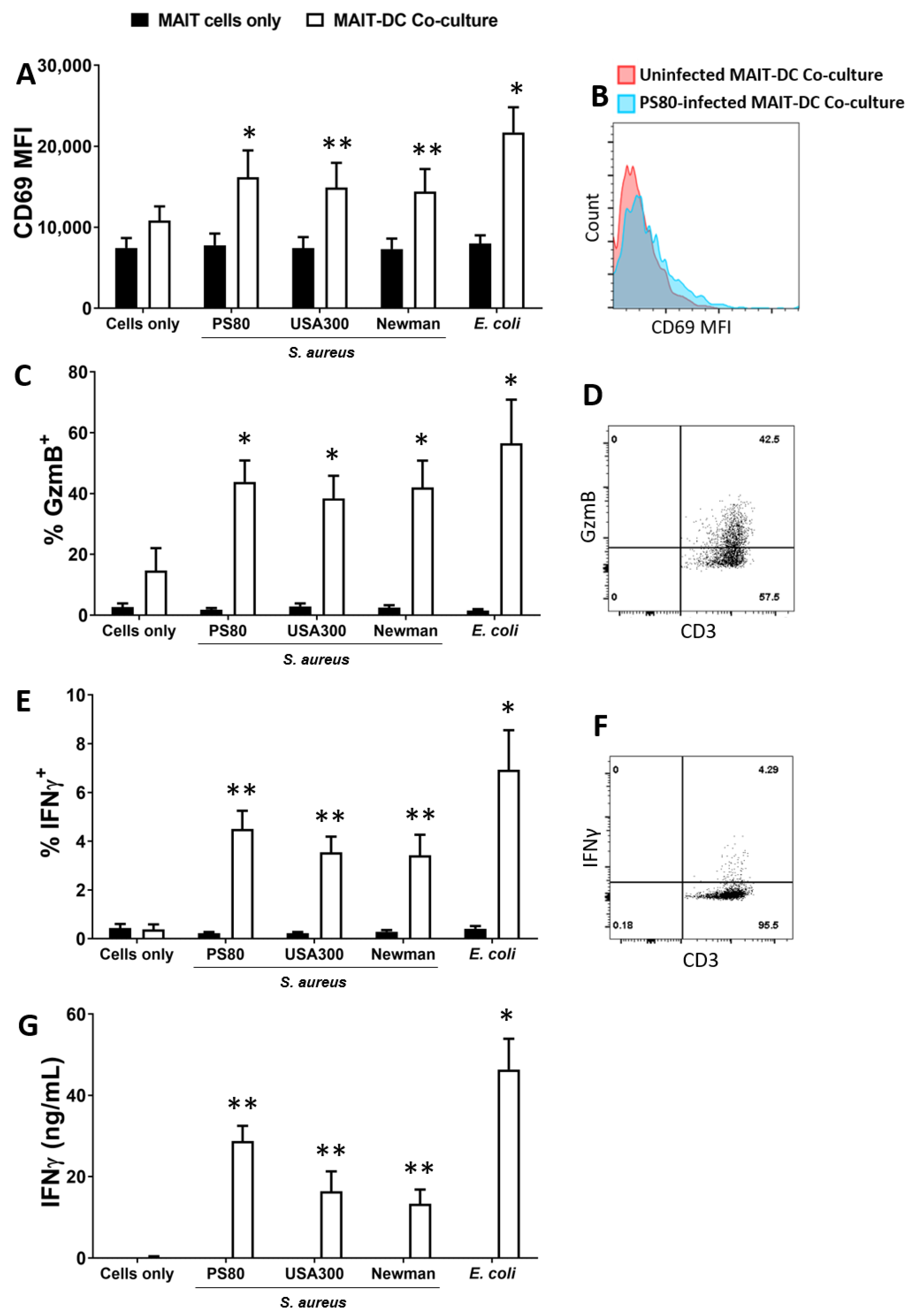
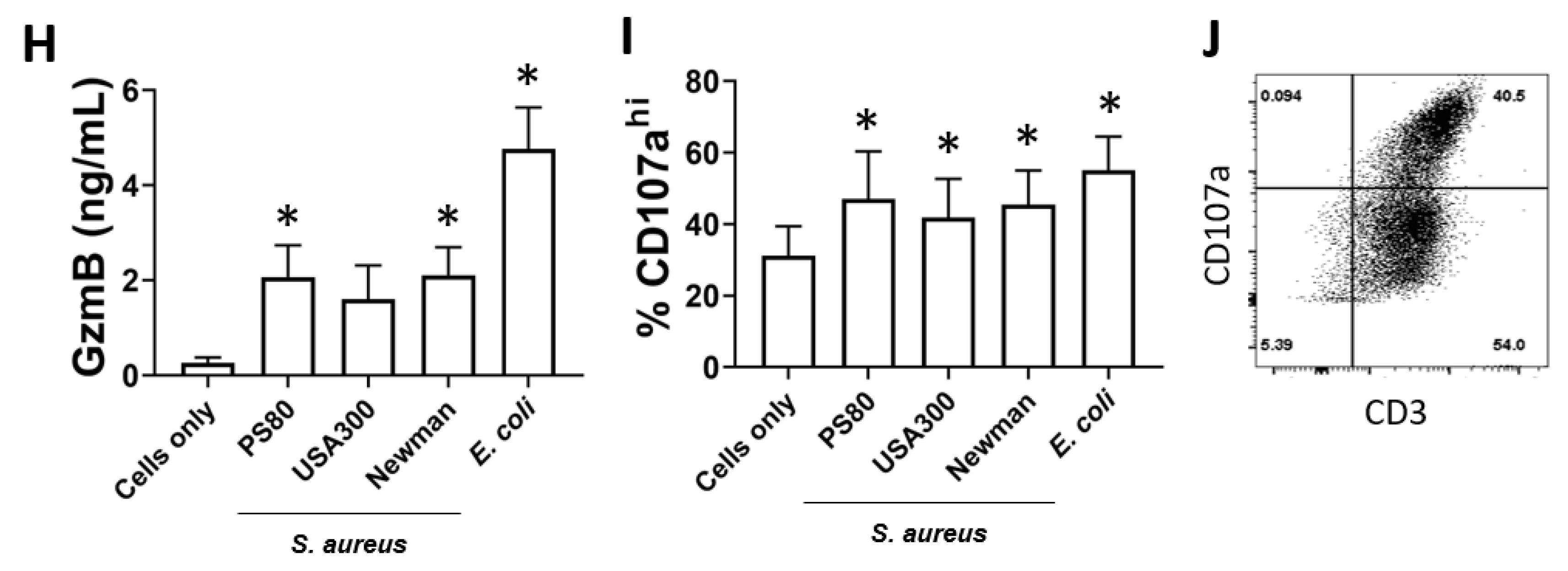
3.1. Human Blood-Derived MAIT Cells Are Activated in Co-Culture with S. aureus-Infected DCs
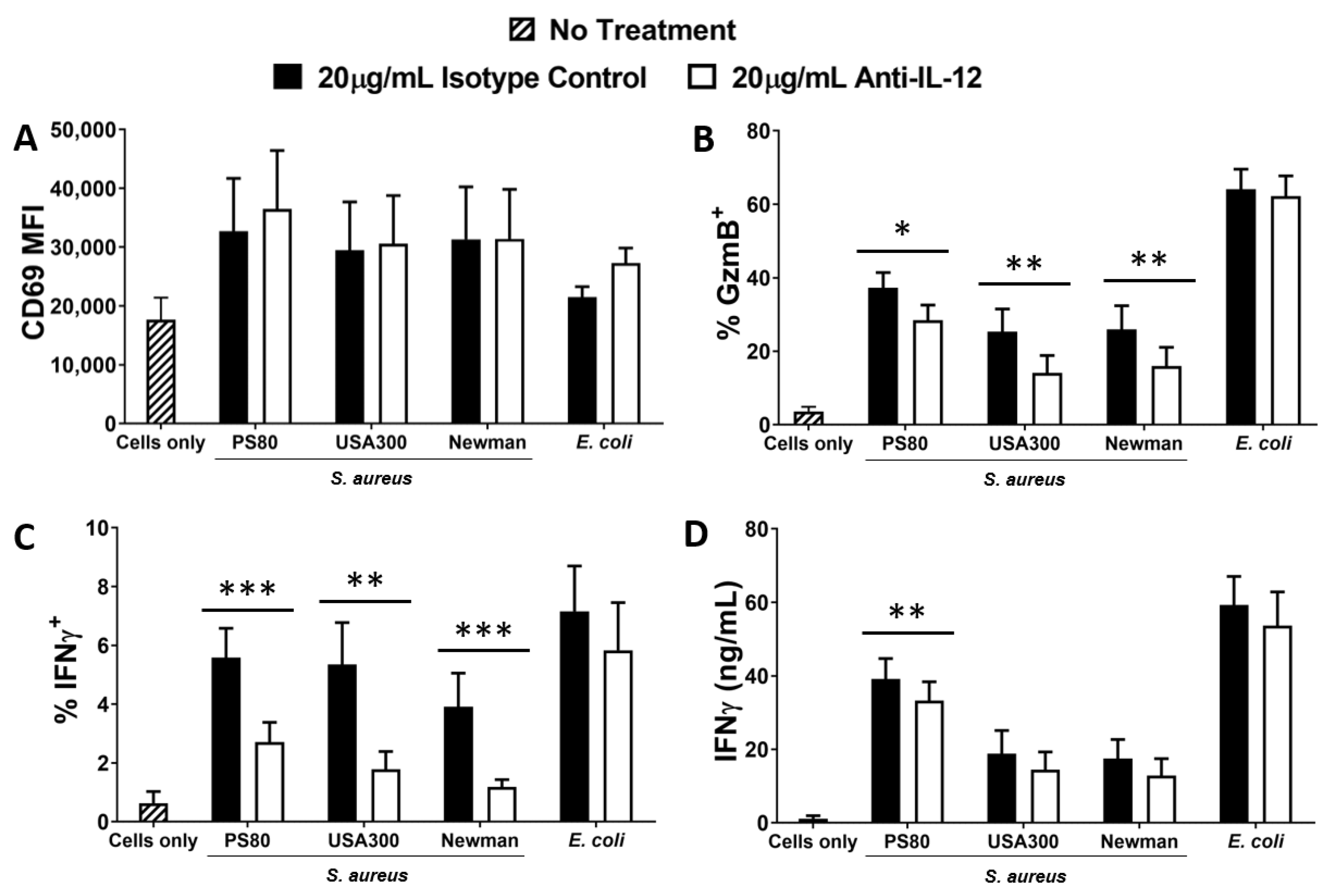
3.2. Activation of MAIT Cells by S. aureus-Infected DCs Is Enhanced by DC Production of IL-12
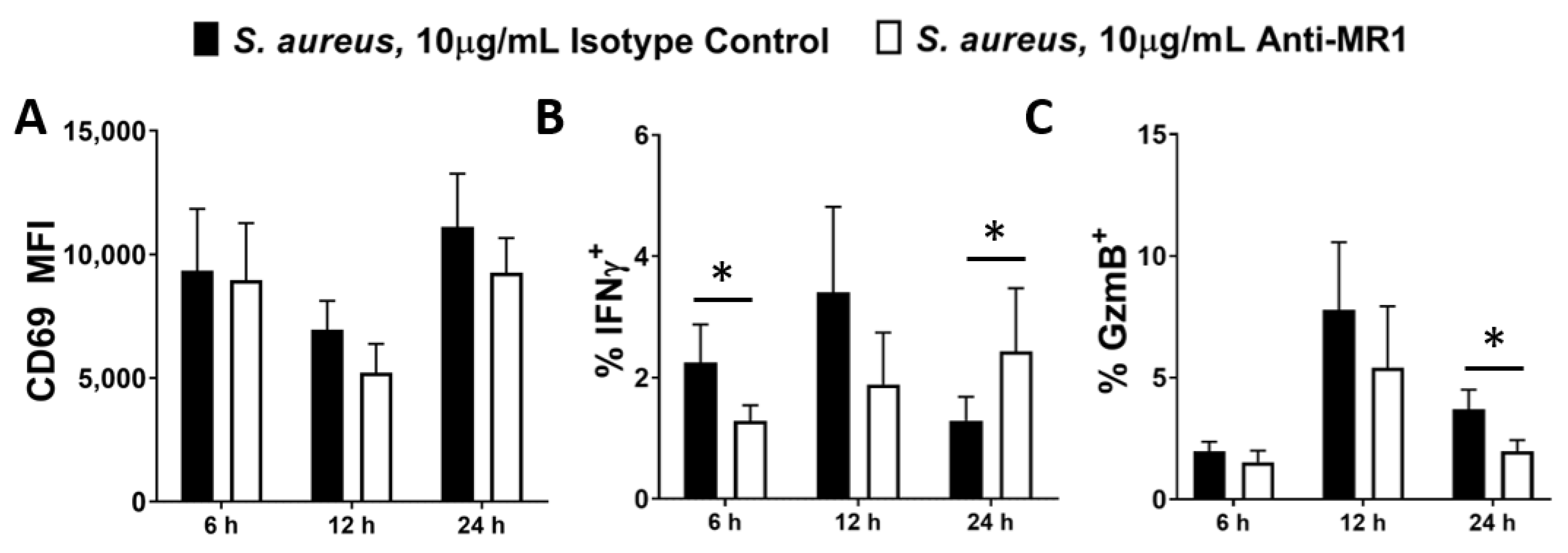
3.3. Activation of MAIT Cells by S. aureus-Infected DCs Is Dependent on Cell–Cell Contact
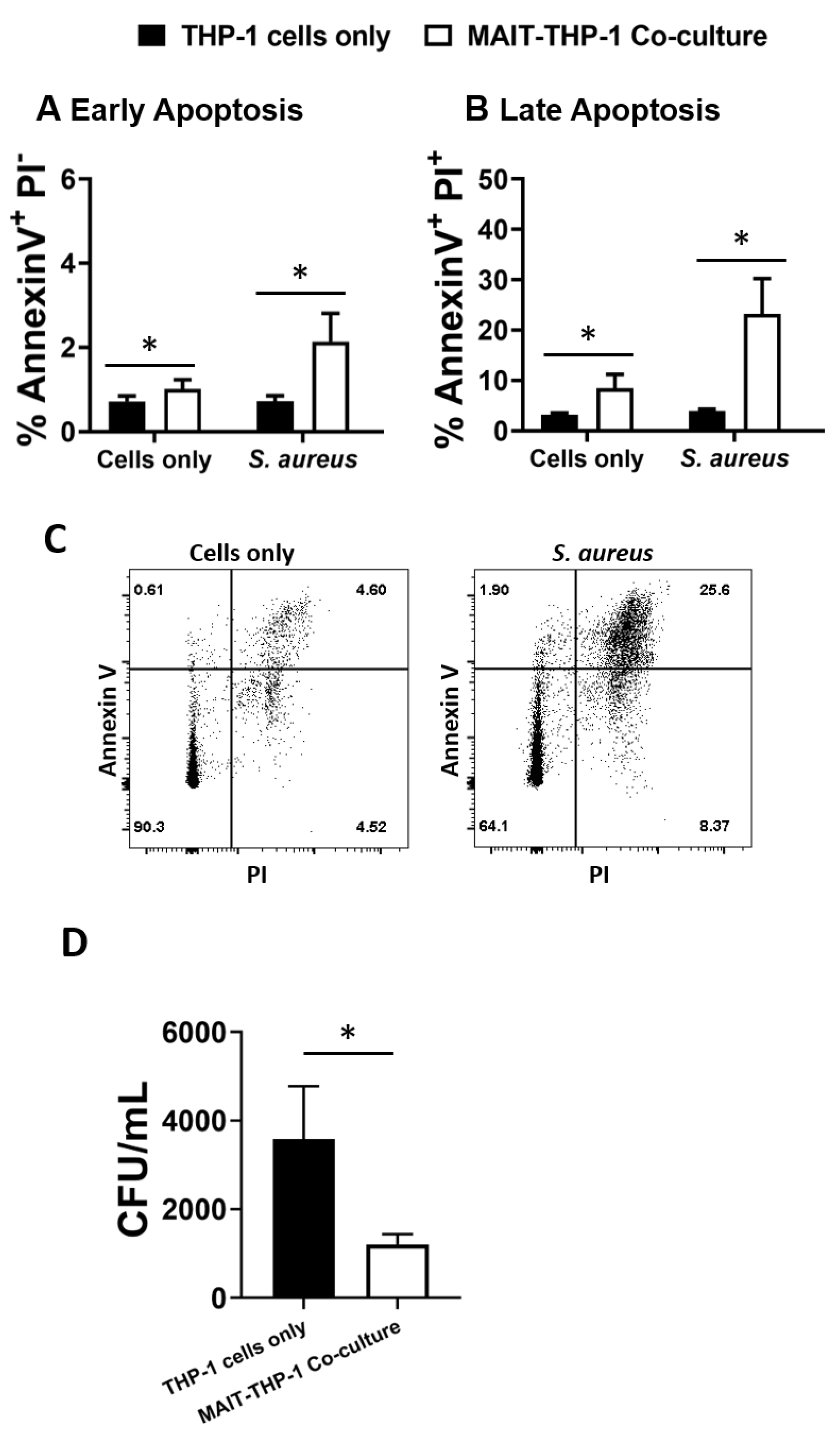
3.4. MAIT Cells Mediate Reduced Intracellular Survival of S. aureus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kern, W.; Rieg, S. Burden of bacterial bloodstream infection—A brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, H.; Thalme, A.; Weiland, O. Staphylococcus aureus bacteraemia and endocarditis—Epidemiology and outcome: A review. Infect. Dis. 2018, 50, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- McNeely, T.B.; Shah, N.A.; Fridman, A.; Joshi, A.; Hartzel, J.S.; Keshari, R.S.; Lupu, F.; DiNubile, M.J. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: An analysis of possible contributing host factors. Hum. Vaccines Immunother. 2014, 10, 3513–3516. [Google Scholar] [CrossRef] [Green Version]
- Fattom, A.; Matalon, A.; Buerkert, J.; Taylor, K.; Damaso, S.; Boutriau, D. Efficacy profile of a bivalent Staphylococcus aureusglycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum. Vaccines Immunother. 2015, 11, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, E.C.; McLoughlin, R.M. Considering the ‘Alternatives’ for Next-Generation Anti-Staphylococcus aureus Vaccine Development. Trends Mol. Med. 2019, 25, 171–184. [Google Scholar] [CrossRef]
- Scully, I.; Timofeyeva, Y.; Illenberger, A.; Lu, P.; Liberator, P.; Jansen, K.; Anderson, A. Performance of a Four-Antigen Staphylococcus aureus Vaccine in Preclinical Models of Invasive S. aureus Disease. Microorganisms 2021, 9, 177. [Google Scholar] [CrossRef]
- Redi, D.; Raffaelli, C.S.; Rossetti, B.; De Luca, A.; Montagnani, F. Staphylococcus aureus vaccine preclinical and clinical development: Current state of the art. New Microbiol. 2018, 41, 208–213. [Google Scholar]
- Armentrout, E.I.; Liu, G.Y.; Martins, G.A. T Cell Immunity and the Quest for Protective Vaccines against Staphylococcus aureus Infection. Microorganisms 2020, 8, 1936. [Google Scholar] [CrossRef] [PubMed]
- Wiese, L.; Mejer, N.; Schønheyder, H.; Westh, H.; Jensen, A.; Larsen, A.R.; Skov, R.; Benfield, T. A nationwide study of comorbidity and risk of reinfection after Staphylococcus aureus bacteraemia. J. Infect. 2013, 67, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Ross, T.; Gregson, D.B. Staphylococcus aureusBloodstream Infections: Risk Factors, Outcomes, and the Influence of Methicillin Resistance in Calgary, Canada, 2000–2006. J. Infect. Dis. 2008, 198, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Holland, S.; DeLeo, F.R.; Elloumi, H.Z.; Hsu, A.P.; Uzel, G.; Brodsky, N.; Freeman, A.F.; Demidowich, A.; Davis, J.; Turner, M.L.C.; et al. STAT3 Mutations in the Hyper-IgE Syndrome. N. Engl. J. Med. 2007, 357, 1608–1619. [Google Scholar] [CrossRef]
- Minegishi, Y.; Saito, M.; Nagasawa, M.; Takada, H.; Hara, T.; Tsuchiya, S.; Agematsu, K.; Yamada, M.; Kawamura, N.; Ariga, T.; et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 2009, 206, 1291–1301. [Google Scholar] [CrossRef]
- Decker, T.; Stockinger, S.; Karaghiosoff, M.; Müller, M.; Kovarik, P. IFNs and STATs in innate immunity to microorganisms. J. Clin. Investig. 2002, 109, 1271–1277. [Google Scholar] [CrossRef]
- Brown, A.F.; Murphy, A.; Lalor, S.; Leech, J.M.; O’Keeffe, K.M.; Mac Aogáin, M.; O’Halloran, D.P.; Lacey, K.; Tavakol, M.; Hearnden, C.H.; et al. Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection. PLoS Pathog. 2015, 11, e1005226. [Google Scholar] [CrossRef]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L.; et al. A Potential New Pathway for Staphylococcus aureus Dissemination: The Silent Survival of S. aureus Phagocytosed by Human Monocyte-Derived Macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef] [Green Version]
- Bröker, B.M.; Mrochen, D.; Péton, V. The T Cell Response to Staphylococcus aureus. Pathogens 2016, 5, 31. [Google Scholar] [CrossRef]
- Cooper, A.J.R.; Lalor, S.J.; McLoughlin, R.M. Activation of Human Vδ2+γδ T Cells by Staphylococcus aureus Promotes Enhanced Anti-Staphylococcal Adaptive Immunity. J. Immunol. 2020, 205, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Pietras, E.M.; Garcia, N.C.; Ramos, R.I.; Farzam, D.M.; Monroe, H.R.; Magorien, J.E.; Blauvelt, A.; Kolls, J.K.; Cheung, A.L.; et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Investig. 2010, 120, 1762–1773. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.; Liu, T.; Zhou, W.-Y.; Zhuang, Y.; Peng, L.-S.; Zhang, J.-Y.; Yin, Z.-N.; Mao, X.-H.; Guo, G.; Shi, Y.; et al. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 2012, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Maher, B.M.; Mulcahy, M.E.; Murphy, A.G.; Wilk, M.; O’Keeffe, K.M.; Geoghegan, J.A.; Lavelle, E.C.; McLoughlin, R.M. Nlrp-3-Driven Interleukin 17 Production by γδT Cells Controls Infection Outcomes during Staphylococcus aureus Surgical Site Infection. Infect. Immun. 2013, 81, 4478–4489. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.G.; O’Keeffe, K.M.; Lalor, S.; Maher, B.M.; Mills, K.; McLoughlin, R.M. Staphylococcus aureusInfection of Mice Expands a Population of Memory γδ T Cells That Are Protective against Subsequent Infection. J. Immunol. 2014, 192, 3697–3708. [Google Scholar] [CrossRef] [Green Version]
- Dillen, C.A.; Pinsker, B.L.; Marusina, A.I.; Merleev, A.A.; Farber, O.N.; Liu, H.; Archer, N.; Lee, D.B.; Wang, Y.; Ortines, R.V.; et al. Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J. Clin. Investig. 2018, 128, 1026–1042. [Google Scholar] [CrossRef]
- Amini, A.; Pang, D.; Hackstein, C.-P.; Klenerman, P. MAIT Cells in Barrier Tissues: Lessons from Immediate Neighbors. Front. Immunol. 2020, 11, 584521. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Koay, H.-F.; Enders, A.; Clanchy, R.; Eckle, S.; Meehan, B.; Chen, Z.; Whittle, B.; Liu, L.; Fairlie, D.; et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015, 212, 1095–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, E.; Treiner, E.; Duban, L.; Guerri, L.; Laude, H.; Toly, C.; Premel, V.; Devys, A.; Moura, I.C.; Tilloy, F.; et al. Stepwise Development of MAIT Cells in Mouse and Human. PLoS Biol. 2009, 7, e1000054. [Google Scholar] [CrossRef]
- Corbett, A.; Eckle, S.; Birkinshaw, R.; Liu, L.; Patel, O.; Mahony, J.; Chen, Z.; Reantragoon, R.; Meehan, B.; Cao, H.; et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509, 361–365. [Google Scholar] [CrossRef]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef]
- Salerno-Goncalves, R.; Rezwan, T.; Sztein, M.B. B Cells Modulate Mucosal Associated Invariant T Cell Immune Responses. Front. Immunol. 2014, 4, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgel, P.; Radosavljevic, M.; Macquin, C.; Bahram, S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol. Immunol. 2011, 48, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.-J.; Truscott, S.M.; Eickhoff, C.S.; Blazevic, A.; Hoft, D.F.; Hansen, T.H. Polyclonal MAIT cells have unique innate functions in bacterial infection. Infect. Immun. 2012, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bourhis, L.; Martin, E.; Péguillet, I.; Guihot, A.; Froux, N.; Coré, M.; Lévy, E.; Dusseaux, M.; Meyssonnier, V.; Premel, V.; et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010, 11, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Dsouza, C.; Lim, X.Y.; Kostenko, L.; Pediongco, T.J.; Eckle, S.B.G.; Meehan, B.S.; Shi, M.; Wang, N.; Li, S.; et al. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat. Commun. 2018, 9, 3350. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, S.; Labuz, D.; Anderson, C.P.; Araujo, C.V.; Blair, A.; Middleton, E.A.; Jensen, O.; Tran, A.; Mulvey, M.A.; Campbell, R.A.; et al. Mucosal-associated invariant T (MAIT) cells mediate protective host responses in sepsis. eLife 2020, 9, e55615. [Google Scholar] [CrossRef]
- Gold, M.C.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.E.; Vogt, T.M.; Delepine, J.; Winata, E.; Swarbrick, G.M.; Chua, W.-J.; Yu, Y.Y.L.; et al. Human Mucosal Associated Invariant T Cells Detect Bacterially Infected Cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef] [Green Version]
- Flament, H.; Rouland, M.; Beaudoin, L.; Toubal, A.; Bertrand, L.; Lebourgeois, S.; Gouda, Z.; Rousseau, C.; Soulard, P.; Hurtado-Nedelec, M.; et al. Outcome of SARS-CoV-2 infection linked to MAIT cell activation and cytotoxicity: Evidence for an IL-18 dependent mechanism. medRxiv 2020. [Google Scholar] [CrossRef]
- Grimaldi, D.; Le Bourhis, L.; Sauneuf, B.; Dechartres, A.; Rousseau, C.; Ouaaz, F.; Milder, M.; Louis, D.; Chiche, J.-D.; Mira, J.-P.; et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensiv. Care Med. 2014, 40, 192–201. [Google Scholar] [CrossRef]
- Sandberg, J.K.; Norrby-Teglund, A.; Leeansyah, E. Bacterial deception of MAIT cells in a cloud of superantigen and cytokines. PLoS Biol. 2017, 15, e2003167. [Google Scholar] [CrossRef] [Green Version]
- Shaler, C.R.; Choi, J.; Rudak, P.T.; Memarnejadian, A.; Szabo, P.A.; Tun-Abraham, M.E.; Rossjohn, J.; Corbett, A.; McCluskey, J.; McCormick, J.; et al. MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: Defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol. 2017, 15, e2001930. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Hrusch, C.L.; Jaffery, M.R.; David, M.Z.; Daum, R.S.; Hall, J.B.; Kress, J.P.; Sperling, A.I.; Verhoef, P.A. Distinct T-helper cell responses to Staphylococcus aureus bacteremia reflect immunologic comorbidities and correlate with mortality. Crit. Care 2018, 22, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; O’Brien, E.C.; O’Keeffe, K.M.; Vozza, E.G.; Leddy, N.; McLoughlin, R.M. Manipulation of Autophagy and Apoptosis Facilitates Intracellular Survival of Staphylococcus aureus in Human Neutrophils. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Gant, V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat. Rev. Genet. 2011, 9, 215–222. [Google Scholar] [CrossRef]
- Melo, A.; O’Brien, A.M.; Phelan, J.J.; Kennedy, S.; Wood, N.A.W.; Veerapen, N.; Besra, G.S.; Clarke, N.E.; Foley, E.K.; Ravi, A.; et al. Mucosal-Associated Invariant T Cells Display Diminished Effector Capacity in Oesophageal Adenocarcinoma. Front. Immunol. 2019, 10, 1580. [Google Scholar] [CrossRef] [PubMed]
- ECACC General Cell Collection: THP 1 (Product Information Page). Available online: https://www.phe-culturecollections.org.uk/products/celllines/generalcell/detail.jsp?refId=88081201&collection=ecacc_gc (accessed on 30 November 2021).
- Asheshov, E.H. The Genetics of Penicillinase Production in Staphylococcus aureus Strain PS 80. J. Gen. Microbiol. 1969, 59, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Mongodin, E.F.; et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367, 731–739. [Google Scholar] [CrossRef]
- Duthie, E.S.; Lorenz, L.L. Staphylococcal Coagulase: Mode of Action and Antigenicity. J. Gen. Microbiol. 1952, 6, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Forde, B.M.; BEN Zakour, N.; Stanton-Cook, M.; Phan, M.-D.; Totsika, M.; Peters, K.M.; Chan, K.G.; Schembri, M.; Upton, M.; Beatson, S.A. The Complete Genome Sequence of Escherichia coli EC958: A High Quality Reference Sequence for the Globally Disseminated Multidrug Resistant E. coli O25b:H4-ST131 Clone. PLoS ONE 2014, 9, e104400. [Google Scholar] [CrossRef] [Green Version]
- Schade, A.E.; Schieven, G.L.; Townsend, R.; Jankowska, A.M.; Susulic, V.; Zhang, R.; Szpurka, H.; Maciejewski, J.P. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 2008, 111, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfrey, D.I.; Koay, H.-F.; McCluskey, J.; Gherardin, N. The biology and functional importance of MAIT cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef]
- Turtle, C.J.; Delrow, J.; Joslyn, R.C.; Swanson, H.M.; Basom, R.; Tabellini, L.; Delaney, C.; Heimfeld, S.; Hansen, J.A.; Riddell, S.R. Innate signals overcome acquired TCR signaling pathway regulation and govern the fate of human CD161hi CD8α+ semi-invariant T cells. Blood 2011, 118, 2752–2762. [Google Scholar] [CrossRef] [Green Version]
- Ussher, J.E.; Bilton, M.; Attwod, E.; Shadwell, J.; Richardson, R.; de Lara, C.; Mettke, E.; Kurioka, A.; Hansen, T.H.; Klenerman, P.; et al. CD 161 ++ CD 8 + T cells, including the MAIT cell subset, are specifically activated by IL -12+ IL -18 in a TCR -independent manner. Eur. J. Immunol. 2014, 44, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgenburg, B.; Scherwitzl, I.; Hutchinson, E.C.; Leng, T.; Kurioka, A.; Kulicke, C.; De Lara, C.; Cole, S.; Vasanawathana, S.; Limpitikul, W.; et al. MAIT cells are activated during human viral infections. Nat. Commun. 2016, 7, 11653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, H.C.; van Wilgenburg, B.; Kurioka, A.; Parekh, K.; Stirling, K.; Roberts, S.; Dutton, E.E.; Hunter, S.; Geh, D.; Braitch, M.K.; et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J. Hepatol. 2016, 64, 1118–1127. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, R.; Schneider, M.; De La Harpe, S.M.; Harrop, T.W.; Hannaway, R.F.; Dearden, P.; Kirman, J.; Tyndall, J.D.; Vernall, A.J.; Ussher, J.E. TCR- or Cytokine-Activated CD8+ Mucosal-Associated Invariant T Cells Are Rapid Polyfunctional Effectors That Can Coordinate Immune Responses. Cell Rep. 2019, 28, 3061–3076.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinch, K.S.; Sandberg, A.; Baudoux, P.; Van Bambeke, F.; Tulkens, P.; Frimodt-Møller, N.; Høiby, N.; Kristensen, H.-H. Plectasin Shows Intracellular Activity against Staphylococcus aureus in Human THP-1 Monocytes and in a Mouse Peritonitis Model. Antimicrob. Agents Chemother. 2009, 53, 4801–4808. [Google Scholar] [CrossRef] [Green Version]
- Brinch, K.S.; Tulkens, P.M.; Van Bambeke, F.; Frimodt-Møller, N.; Høiby, N.; Kristensen, H.-H. Intracellular activity of the peptide antibiotic NZ2114: Studies with Staphylococcus aureus and human THP-1 monocytes, and comparison with daptomycin and vancomycin. J. Antimicrob. Chemother. 2010, 65, 1720–1724. [Google Scholar] [CrossRef] [Green Version]
- Musilova, J.; Mulcahy, M.E.; Kuijk, M.M.; McLoughlin, R.M.; Bowie, A.G. Toll-like receptor 2–dependent endosomal signaling by Staphylococcus aureus in monocytes induces type I interferon and promotes intracellular survival. J. Biol. Chem. 2019, 294, 17031–17042. [Google Scholar] [CrossRef] [Green Version]
- O’Keeffe, K.M.; Wilk, M.M.; Leech, J.M.; Murphy, A.G.; Laabei, M.; Monk, I.R.; Massey, R.C.; Lindsay, J.; Foster, T.J.; Geoghegan, J.A.; et al. Manipulation of Autophagy in Phagocytes Facilitates Staphylococcus aureus Bloodstream Infection. Infect. Immun. 2015, 83, 3445–3457. [Google Scholar] [CrossRef] [Green Version]
- Melzer, M.; Welch, C. Thirty-day mortality in UK patients with community-onset and hospital-acquired meticillin-susceptible Staphylococcus aureus bacteraemia. J. Hosp. Infect. 2013, 84, 143–150. [Google Scholar] [CrossRef]
- Turnidge, J.D.; Kotsanas, D.; Munckhof, W.; Roberts, S.; Bennett, C.M.; Nimmo, G.R.; Coombs, G.W.; Murray, R.J.; Howden, B.; Johnson, P.D.R.; et al. Staphylococcus aureus bacteraemia: A major cause of mortality in Australia and New Zealand. Med. J. Aust. 2009, 191, 368–373. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [Green Version]
- Meierovics, A.; Yankelevich, W.-J.; Cowley, S.C. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, E3119–E3128. [Google Scholar] [CrossRef] [Green Version]
- Meermeier, E.; Laugel, B.F.; Sewell, A.; Corbett, A.; Rossjohn, J.; McCluskey, J.; Harriff, M.J.; Franks, T.; Gold, M.C.; Lewinsohn, D.M. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat. Commun. 2016, 7, 12506. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.; Kalinichenko, A.; Colone, A.; Paleja, B.; Singhal, A.; Tschumi, A.; Lee, B.; Poidinger, M.; Zolezzi, F.; Quagliata, L.; et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat. Commun. 2014, 5, 3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, M.; Morgan, M.P.; Liuzzi, A.R.; Tyler, C.J.; Khan, M.W.A.; Szakmany, T.; Hall, J.E.; Moser, B.; Eberl, M. Microbe-Specific Unconventional T Cells Induce Human Neutrophil Differentiation into Antigen Cross-Presenting Cells. J. Immunol. 2014, 193, 3704–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.A.; Björkander, S.; Forsberg, M.M.; Qazi, K.R.; Celades, M.S.; Bittmann, J.; Eberl, M.; Sverremark-Ekström, E. Probiotic Lactobacilli Modulate Staphylococcus aureus-Induced Activation of Conventional and Unconventional T cells and NK Cells. Front. Immunol. 2016, 7, 273. [Google Scholar] [CrossRef] [Green Version]
- Tzianabos, A.O.; Wang, J.Y.; Lee, J. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. USA 2001, 98, 9365–9370. [Google Scholar] [CrossRef] [Green Version]
- Parker, D.; Ryan, C.L.; Alonzo, F., 3rd; Torres, V.J.; Planet, P.J.; Prince, A.S. CD4+ T cells promote the pathogenesis of Staphylococcus aureus pneumonia. J. Infect. Dis. 2015, 211, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Sattler, A.; Dang-Heine, C.; Reinke, P.; Babel, N. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur. J. Immunol. 2015, 45, 2286–2298. [Google Scholar] [CrossRef] [Green Version]
- Ussher, J.E.; Willberg, C.B.; Klenerman, P. MAIT cells and viruses. Immunol. Cell Biol. 2018, 96, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Simoni, Y.; Becht, E.; Loh, C.Y.; Li, N.; Lachance, D.; Koo, S.-L.; Lim, T.P.; Tan, E.K.W.; Mathew, R.; et al. Human Tumor-Infiltrating MAIT Cells Display Hallmarks of Bacterial Antigen Recognition in Colorectal Cancer. Cell Rep. Med. 2020, 1, 100039. [Google Scholar] [CrossRef]
- Jo, J.; Tan, A.T.; Ussher, J.; Sandalova, E.; Tang, X.-Z.; Tan-Garcia, A.; To, N.; Hong, M.; Chia, A.; Gill, U.S.; et al. Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver. PLoS Pathog. 2014, 10, e1004210. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Mann, P.A.; Xiao, L.; Gill, C.; Galgoci, A.M.; Howe, J.A.; Villafania, A.; Barbieri, C.M.; Malinverni, J.C.; Sher, X.; et al. Dual-Targeting Small-Molecule Inhibitors of the Staphylococcus aureus FMN Riboswitch Disrupt Riboflavin Homeostasis in an Infectious Setting. Cell Chem. Biol. 2017, 24, 576–588.e6. [Google Scholar] [CrossRef] [Green Version]
- Shafer, W.M.; Pohl, J.; Onunka, V.C.; Bangalore, N.; Travis, J. Human lysosomal cathepsin G and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J. Biol. Chem. 1991, 266, 112–116. [Google Scholar] [CrossRef]
- Spolski, R.; West, E.E.; Li, P.; Veenbergen, S.; Yung, S.; Kazemian, M.; Oh, J.; Yu, Z.-X.; Freeman, A.F.; Holland, S.M.; et al. IL-21/type I interferon interplay regulates neutrophil-dependent innate immune responses to Staphylococcus aureus. eLife 2019, 8, e45501. [Google Scholar] [CrossRef] [PubMed]
- Le Bourhis, L.; Dusseaux, M.; Bohineust, A.; Bessoles, S.; Martin, E.; Premel, V.; Coré, M.; Sleurs, D.; Serriari, N.-E.; Treiner, E.; et al. MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells. PLoS Pathog. 2013, 9, e1003681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeansyah, E.; Svärd, J.; Dias, J.; Buggert, M.; Nyström, J.; Quigley, M.F.; Moll, M.; Sönnerborg, A.; Nowak, P.; Sandberg, J.K. Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection. PLoS Pathog. 2015, 11, e1005072. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Sobkowiak, M.J.; Sandberg, J.K.; Leeansyah, E. Human MAIT-cell responses toEscherichia coli: Activation, cytokine production, proliferation, and cytotoxicity. J. Leukoc. Biol. 2016, 100, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, J.S.; Salerno-Goncalves, R.; Blanchard, T.G.; Patil, S.A.; Kader, H.; Safta, A.M.; Morningstar, L.M.; Czinn, S.J.; Greenwald, B.D.; Sztein, M.B. Mucosal-Associated Invariant T Cells in the Human Gastric Mucosa and Blood: Role in Helicobacter pylori Infection. Front. Immunol. 2015, 6, 466. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Wong, E.B.; Napier, R.J.; Bishai, W.R.; Ndung’U, T.; Kasprowicz, V.O.; Lewinsohn, D.A.; Lewinsohn, D.; Gold, M.C. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunol. 2015, 145, 443–453. [Google Scholar] [CrossRef]
- Wallington, J.C.; Williams, A.P.; Staples, K.J.; Wilkinson, T.M. IL-12 and IL-7 synergize to control mucosal-associated invariant T-cell cytotoxic responses to bacterial infection. J. Allergy Clin. Immunol. 2018, 141, 2182–2195.e6. [Google Scholar] [CrossRef] [Green Version]
- Medema, J.P.; Schuurhuis, D.H.; Rea, D.; Van Tongeren, J.; De Jong, J.; Bres, S.A.; Laban, S.; Toes, R.; Toebes, M.; Schumacher, T.; et al. Expression of the Serpin Serine Protease Inhibitor 6 Protects Dendritic Cells from Cytotoxic T Lymphocyte–Induced Apoptosis. J. Exp. Med. 2001, 194, 657–668. [Google Scholar] [CrossRef]
- Wakao, H.; Sugimoto, C.; Kimura, S.; Wakao, R. Mucosal-Associated Invariant T Cells in Regenerative Medicine. Front. Immunol. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Downey, A.M.; Kapłonek, P.; Seeberger, P.H. MAIT cells as attractive vaccine targets. FEBS Lett. 2019, 593, 1627–1640. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Kjer-Nielsen, L.; Shi, M.; D’Souza, C.; Pediongco, T.J.; Cao, H.; Kostenko, L.; Lim, X.Y.; Eckle, S.B.G.; Meehan, B.S.; et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Howson, L.J.; Napolitani, G.; Shepherd, D.; Ghadbane, H.; Kurupati, P.; Preciado-Llanes, L.; Rei, M.; Dobinson, H.; Gibani, M.; Teng, K.W.W.; et al. MAIT cell clonal expansion and TCR repertoire shaping in human volunteers challenged with Salmonella Paratyphi A. Nat. Commun. 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provine, N.M.; Amini, A.; Garner, L.C.; Spencer, A.J.; Dold, C.; Hutchings, C.; Reyes, L.S.; FitzPatrick, M.E.B.; Chinnakannan, S.; Oguti, B.; et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science 2021, 371, 521–526. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, A.J.R.; Clegg, J.; Cassidy, F.C.; Hogan, A.E.; McLoughlin, R.M. Human MAIT Cells Respond to Staphylococcus aureus with Enhanced Anti-Bacterial Activity. Microorganisms 2022, 10, 148. https://doi.org/10.3390/microorganisms10010148
Cooper AJR, Clegg J, Cassidy FC, Hogan AE, McLoughlin RM. Human MAIT Cells Respond to Staphylococcus aureus with Enhanced Anti-Bacterial Activity. Microorganisms. 2022; 10(1):148. https://doi.org/10.3390/microorganisms10010148
Chicago/Turabian StyleCooper, Andrew J. R., Jonah Clegg, Féaron C. Cassidy, Andrew E. Hogan, and Rachel M. McLoughlin. 2022. "Human MAIT Cells Respond to Staphylococcus aureus with Enhanced Anti-Bacterial Activity" Microorganisms 10, no. 1: 148. https://doi.org/10.3390/microorganisms10010148
APA StyleCooper, A. J. R., Clegg, J., Cassidy, F. C., Hogan, A. E., & McLoughlin, R. M. (2022). Human MAIT Cells Respond to Staphylococcus aureus with Enhanced Anti-Bacterial Activity. Microorganisms, 10(1), 148. https://doi.org/10.3390/microorganisms10010148






