Boswellia serrata Extract as an Antibiofilm Agent against Candida spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antimicrobials
2.2. Microorganisms
2.3. Cultivation of Microorganisms
2.4. Assay of Minimum Inhibitory Concentrations (MIC) of Antimicrobials for Suspension Yeast Populations Using a Bioscreen C Microculture Device
2.5. Biofilm Formation
2.6. Quantification of Biofilm by Crystal Violet (CV) Staining and MTT
2.7. Determining the Minimum Concentration of Biofilm Inhibitors (MBIC)
2.8. Statistical Analysis
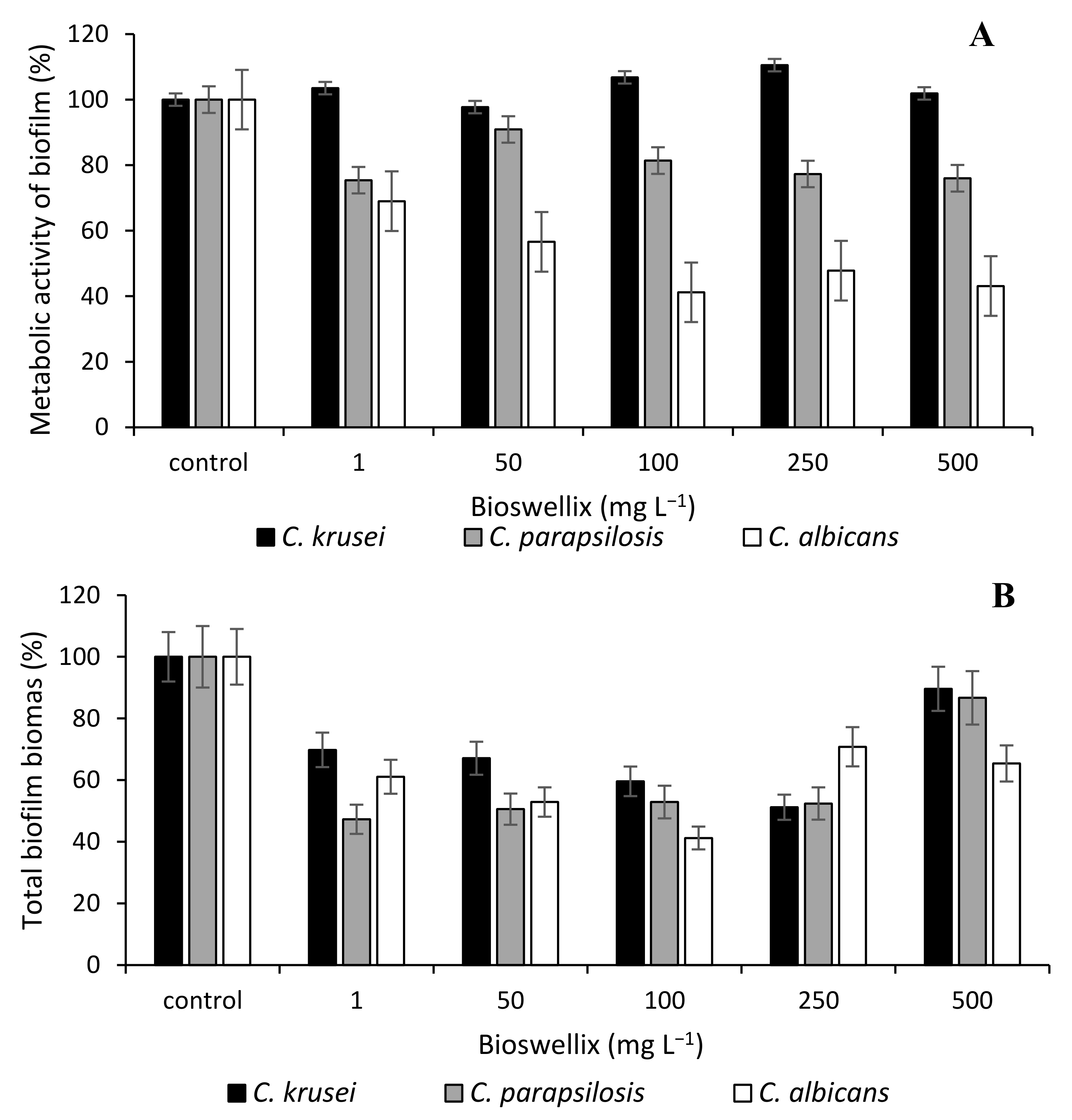
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.-E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Kašparová, P.; Maťátková, O.; Čejková, A. Can the advantages of the genus Candida exceed the strong pathogenesis of some of its species? Chem. Lett. 2019, 113, 415–421. [Google Scholar]
- Schindler, J. The Universe; Vesmír, s.r.o.: Prague, Czech Republic, 2001. [Google Scholar]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Melicharčíková, V. Sterilization and Disinfection in Healthcare; GRADA Publishing: Prague, Czech Republic, 1998. [Google Scholar]
- Patocka, J. Biologically active pentacyclic triterpenes and their current medicine signification. J. Appl. Biomed. 2003, 1, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Kolouchová, I.; Melzoch, K.; Šmidrkal, J.; Filip, V. The content of resveratrol in vegetables and fruit. Chem. Lett. 2005, 99, 492–495. [Google Scholar]
- Yauan, G.; Wahlqvist, M.; He, G.; Yang, M.; Li, D. Natural products and anti inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143. [Google Scholar]
- Etzel, R. Use of Incense in the Treatment of Alzheimer’s. Disease Patent US720975A, 24 February 1998. [Google Scholar]
- Tsukada, T.; Nakashima, K.; Shirakawa, S. Arachidonate 5 lipoxygenase inhibitors show potent antiproliferative effects on human leukemia cell lines. Biochem. Biophys. Res. Commun. 1986, 140, 832–836. [Google Scholar] [CrossRef]
- Huang, M.T.; Badmaev, V.; Ding, Y.; Liu, Y.; Xie, J.G.; Ho, C.T. Anti tumor and anti carcinogenic activities of triterpenoid, beta boswellic acid. Biofactors 2000, 13, 225–230. [Google Scholar] [CrossRef]
- Gupta, I.; Gupta, V.; Parihar, A.; Gupta, S.; Lüdtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia serrata gum resin in patients with bronchial asthma. Results of a double blind, placebo controlled, 6 week clinical study. Eur. J. Med. Res. 1998, 3, 511–514. [Google Scholar]
- Krieglstein, C.F.; Anthoni, C.; Rijcken, E.J.; Laukötter, M.; Spiegel, H.U.; Boden, S.E.; Schweizer, S.; Safayhi, H.; Senninger, N.; Schürmann, G. Acetyl 11 keto beta boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int. J. Colorectal Dis. 2001, 16, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ravanan, P.; Singh, S.K.; Rao, G.S.; Kondaiah, P. Growth inhibitory, apoptotic and anti inflammatory activities displayed by a novel modified triterpenoid, cyano enone of methyl boswellates. J. Biosci. 2011, 36, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Flavin, D.F. A lipoxygenase inhibitor in breast cancer brain metastases. J. Neurooncol. 2007, 82, 91–93. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Safayhi, H.; Rall, B.; Sailer, E.R. Ammon, H.P. Inhibition by boswellic acids of human leukocyte elastase. J. Pharm. Exp. Ther. 1997, 281, 460–463. [Google Scholar]
- Ammon, H.P.; Mack, T.; Singh, G.B.; Safayhi, H. Inhibition of leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of Boswellia Serrata. Planta Med. 1991, 57, 203–207. [Google Scholar] [CrossRef]
- Camarda, L.; Dayton, T.; Di Stefano, V.; Pitonzo, R.; Schillaci, D. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp. (Burseraceae). Ann. Chim. 2007, 97, 837–844. [Google Scholar] [CrossRef]
- Schillaci, D.; Arizza, V.; Dayton, T.; Camarda, L.; Di Stefano, V. In vitro anti- biofilm activity of Boswellia spp. oleogum resin essential oils. Lett. Appl. Microbiol. 2008, 47, 433–438. [Google Scholar] [CrossRef]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S.; Shah, B.A.; Taneja, S.C. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Ammon, H.P.T. Boswellic Acids in chronic inflammatory diseases. Planta Med. 2006, 72, 1100–1116. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011, 19, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Trofa, D.; Gacser, A.; Nosanchuk, J.D. Candida parapsilosis, an Emerging Fungal Pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef] [Green Version]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, R.G. Role of Antifungal Combinations in difficult to treat Candida infections. J. Fungi 2021, 7, 731. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hagihara, M.; Shibata, Y.; Asai, N.; Yamagishi, Y.; Iwamoto, T.; Mikamo, H. Comparison of mortality between echinocandins and polyenes for an initial treatment of candidemia: A systematic review and meta-analysis. J. Infect. Chemother. 2021, 27, 1562–1570. [Google Scholar] [CrossRef]
- Baillie, G.S.; Douglas, L.J. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 2000, 46, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [Green Version]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef]
- Singh, B.; Upreti, D.; Singh, B.; Pandey, G.; Verma, S.; Roy, S.; Naqvi, A.; Rawat, A. Quercetin sensitizes fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob. Agents Chemother. 2015, 59, 2153–2168. [Google Scholar] [CrossRef] [Green Version]
- Andrews, H.P. Determination of minimum inhibitory concentrations. J. Antimicrob Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yan, Z.; Xu, J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 2003, 149, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Riss, T.; Moravec, R.; Niles, A.; Benink, H.; Worzella, T.; Minor, L. Cell Viability Assays. Assay Guidance Manual [Internet]; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Paldrychová, M.; Kolouchová, I.; Vaňková, E.; Maťátková, O.; Šmidrkal, J.; Krmela, A.; Schulzová, V.; Hajšlová, J.; Masák, J. Effect of resveratrol and regrapex-R-forte on Trichosporon cutaneum biofilm. Folia Microbiol. 2019, 64, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Stanbury, R.M.; Graham, E.M. Systemic corticosteroid therapy side effects and their management. Br. J. Ophthalmol. 1998, 82, 704–708. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, A.C. Immunosuppressive medications. Clin. J. Am. Soc. Nephrol. 2016, 11, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, S.; Shirzad, H.; Rafieian-Kopaei, M. Recent findings in molecular basis of inflammation and anti-inflammatory plants. Curr. Pharm. Des. 2018, 24, 1551–1562. [Google Scholar] [CrossRef]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef] [Green Version]
- Krcmery, V.C. Antifungal Chemotherapeutics. Med. Princ. Pract. 2005, 14, 125–135. [Google Scholar] [CrossRef]
- Krausová, L.; Grim, J.; Pávek, P. Azolová antimykotika: Mechanizmy lékových interakcí. Klin. Farmakol. Farm. 2009, 23, 86–89. [Google Scholar]
- Afrin, S.R.; Islam, M.R.; Proma, N.M.; Shorna, M.K.; Akbar, S.; Hossain, M.K. Quantitative screening of phytochemicals and pharmacological attributions of the leaves and stem barks of Macropanax dispermus (Araliaceae) in treating the inflammation and arthritis. J. Herbmed. Pharmacol. 2020, 10, 75–83. [Google Scholar] [CrossRef]
- Al Qaraghuli, M.M.; Alzahrani, A.R.; Niwasabutra, K.; Obeid, M.A.; Ferro, V.A. Where traditional drug discovery meets modern technology in the quest for new drugs. Ann. Pharmacol. Pharm. 2017, 2, 1–5. [Google Scholar]
- Mukherjee, P.K. Evidence-Based Validation of Herbal Medicine; Elsevier: Boston, MA, USA, 2015. [Google Scholar]
- Hamza, M.; Nadir, M.; Mehmood, N.; Farooq, A. In vitro effectiveness of triterpenoids and their synergistic effect with antibiotics against Staphylococcus aureus strains. Indian J. Pharmacol. 2016, 48, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G. An update on pharmacological potential of boswellic acids against chronic diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Pan, X.; Wong, H.; Wagner, C.; Lahey, L.; Robinson, W.; Sokolove, J. Oral and topical boswellic acid attenuates mouse osteoarthritis. Osteoarth. Cartil. 2017, 22, 128–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsha, K.; Shreya, K. Topical Nanoemmigel Formulation of Boswellia serrata. Indian Pharm. Sci. 2018, 80, 261–267. [Google Scholar] [CrossRef]
- Bertocchi, M.; Isani, G.; Medici, F.; Andreani, G.; Tubon Usca, I.; Roncada, P.; Forni, M.; Bernardini, C. Anti-inflammatory activity of Boswellia serrata extracts: An in vitro study on porcine aortic endothelial cells. Oxidative Med. Cell Longev. 2018, 2018, 2504305. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Singh, S.; Saksena, A.K.; Pal, R.; Jaiswal, R.; Kumar, R. Effect of Boswellia serrata extract on acute inflammatory parameters and tumor necrosis factor-a in complete Freund’s adjuvant-induced animal model of rheumatoid arthritis. Int. J. Appl. Basic Med. Res. 2019, 9, 100–106. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Fazeli, M.; Mehri, S.; Taherianfard, M.; Hosseinzadeh, H. Boswellic acid improves cognitive function in a rat model through its antioxidant activity:-Neuroprotective effect of Boswellic acid. J. Pharmacopunct. 2017, 20, 10–17. [Google Scholar]
- Ismail, S.M.; Rao, S.; Bhaskar, M. Evaluation of antiinflammatory activity of Boswellia serrata on carrageenan induced paw edema in albino Wistar rats. Int. J. Res. Med. Sci. 2016, 4, 2980–2986. [Google Scholar] [CrossRef]
- Lv, M.; Shao, S.; Zhang, Q.; Zhuang, X.; Qiao, T. Acetyl-11-Keto-b-Boswellic Acid Exerts the Anti-Cancer Effects via Cell Cycle Arrest, Apoptosis Induction and Autophagy Suppression in Non-Small Cell Lung Cancer Cells. OncoTargets Ther. 2020, 13, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Büchele, B.; Simmet, T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 795, 355–362. [Google Scholar] [CrossRef]
- Karpinski, T.M.; Ozarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. plant preparations and compounds with activities against biofilms formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Rajendran, R.; Syed, N.; Ramanathan, K.S. Anti-candidal and anti-virulence efficiency of selected seaweeds againstazole resistance Candida albicans. Biocatal. Agric. Biotechnol. 2019, 20, 101195. [Google Scholar] [CrossRef]
- Rajkowska, K.; Nowicka-Krawczyk, P.; Kunicka-Styczynska, A. Efect of clove and thyme essential oils on Candida biofilm formation and the oil distribution in yeast cells. Molecules 2019, 24, 1954. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Manoharlal, R.; Negi, A.S.; Prasad, R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 2010, 10, 570–578. [Google Scholar] [CrossRef]
- Choonharuangdej, S.; Srithavaj, T.; Thummawanit, S. Fungicidal and inhibitory efficacy of cinnamon and lemongrass essential oils on Candida albicans biofilm established on acrylic resin: An in vitro study. J. Prosthet. Dent. 2021, 125, 707.e1–707.e6. [Google Scholar] [CrossRef]
- Freires, I.A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.; de Alencar, S.M.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.; Rosalen, P.L. Coriandrum sativum L. (Coriander) Essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e099086. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, D.S.; Tonial, F.; Lopez, P.V.; Sales Maia, B.H.; Santos, G.D.; Ribas, M.O.; Glienke, C.; Vicente, V.A. Antiadherent activity of Schinus terebinthifolius and Croton urucurana extracts on in vitro biofilm formation of Candida albicans and Streptococcus mutans. Arch. Oral. Biol. 2014, 59, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Zago, P.M.W.; Dos Santos Castelo Branco, S.J.; de Albuquerque Bogea Fecury, L.; Carvalho, L.T.; Rocha, C.Q.; Madeira, P.L.B.; de Sousa, E.M.; de Siqueira, F.S.F.; Paschoal, M.A.B.; Diniz, R.S.; et al. Anti-biofilm action of Chenopodium ambrosioides extract, cytotoxic potential and effects on acrylic denture surface. Front. Microbiol. 2019, 10, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira, P.L.B.; Carvalho, L.T.; Paschoal, M.; de Sousa, E.M.; Moffa, E.; da Silva, M.A.S.; Tavarez, R.R.; Gonçalves, L. In vitro effects of lemongrass extract on Candida albicans biofilms, human cells viability, and denture surface. Front. Cell Infect. Microbiol. 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quatrin, P.M.; Verdi, C.M.; de Souza, M.E.; de Godoi, S.N.; Klein, B.; Gundel, A.; Wagner, R.; Vaucher, R.A.; Ourique, A.; Santos, R.C. Antimicrobial and antibiofilm activities of nanoemulsions containing Eucalyptus globulus oil against Pseudomonas aeruginosa and Candida spp. Microb. Pathog. 2017, 112, 230–242. [Google Scholar] [CrossRef]
- Salete, M.F.B.; Galvo, L.C.C.; Goes, V.F.F.; Sartoratto, A.; Figueira, G.; Rehder, V.L.; Alencar, S.M.; Duarte, R.M.; Rosalen, P.L.; Duarte, M.C. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement. Altern. Med. 2014, 14, 451. [Google Scholar]
- Sun, F.-J.; Li, M.; Gu, L.; Wang, M.-L.; Yang, M.-H. Recent progress on anti-Candida natural products. Chin. J. Nat. Med. 2021, 19, 561–579. [Google Scholar] [CrossRef]
- Weckesser, S.; Engel, K.; Simon-Haarhaus, B.; Wittmer, A.; Pelz, K.; Schempp, C.M. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine 2017, 14, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, S.; Hakemi-Vala, M.; Gholami, S. In vitro antibacterial effect of hydroalcoholic extract of Lawsonia inermis, Malva sylvestris, and Boswellia serrata on Aggregatibacter actinomycetemcomitans. Adv. Biomed. Res. 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Palanivel, S.; Ghosh, S. Synergistic antimicrobial activity of Boswellia serrata Roxb. ex Colebr. (Burseraceae) essential oil with various azoles against pathogens associated with skin, scalp and nail infections. Lett. Appl. Microbiol. 2016, 63, 495–501. [Google Scholar] [CrossRef]
- Maiolo, E.M.; Tafin, U.F.; Borens, O.; Trampuz, A. Activities of fluconazole, caspofungin, anidulafungin, and amphotericin b on planktonic and biofilm Candida species determined by microcalorimetry. Antimicrob. Agents Chemother. 2014, 58, 2709–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinne, D.; Watelle, M.; Nolard, N. In vitro activities of voriconazole, fluconazole, itraconazole and amphotericin B against non Candida albicans yeast isolates. Rev. Iberoam. Micol. 2005, 22, 24–28. [Google Scholar] [CrossRef]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.S.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; de Castro, R.D. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch. Oral. Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef]
- Butassi, E.; Svetaz, L.; Carpinella, M.C.; Efferth, T.; Zacchino, S. Fungal biofilms as a valuable target for the discovery of natural products that cope with the resistance of medically important fungi—Latest findings. Antibiotics 2021, 10, 1053. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchic, N.I.; Tabassum, N.; Jo, D.-M.; Khan, M.M.; Kim, Y.-M. Suppression of hyphal formation and virulence of Candida albicans by natural and synthetic compounds. Biofouling 2021, 37, 626–655. [Google Scholar] [CrossRef]
- Jenks, J.D.; Cornely, O.A.; Chen, S.C.A.; Thompson III, G.R.; Hoenigl, M. Breakthrough invasive fungal infections: Who is at risk? Mycoses 2020, 63, 1021–1032. [Google Scholar] [CrossRef]
- Katragkou, A.; Roilides, E.; Walsh, T.J. Role of echinocandins in fungal biofilm–related disease: Vascular catheter–related infections, immunomodulation, and mucosal surfaces. Clin. Infect. Dis. 2015, 61, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [Green Version]
- Borjian Boroujeni, Z.; Shamsaei, S.; Yarahmadi, M.; Getso, M.I.; Salimi Khorashad, A.; Haghighi, L.; Raissi, V.; Zareei, M.; Saleh Mohammadzade, A.; Moqarabzadeh, V.; et al. Distribution of invasive fungal infections: Molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: A 3-year experience with 490 patients under intensive care. Microb. Pathog. 2021, 152, 104616. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Walsh, T.J. Drug development challenges and strategies to address emerging and resistant fungal pathogens. Expert Rev. Anti Infect Ther. 2017, 15, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Milho, C.; Liberal, A.; Silva, J.; Fonseca, C.; Barbosa, A.; Ferreira, I.C.F.R.; Alves, M.J.; Barros, L. Antibiofilm Potential of Medicinal Plants against Candida spp. Oral Biofilms: A Review. Antibiotics 2021, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Khan, M.S.A. Phytomedicine from Middle Eastern countries: An alternative remedy to modern medicine against Candida spp. infection. Evid.-Based Complement. Altern. Med. 2021, 2021, 6694876. [Google Scholar] [CrossRef]




| MIC50 (mg L−1) | Microorganism | ||
|---|---|---|---|
| C. parapsilosis CCM 8260 | C. krusei CCM 8271 | C. albicans ATCC 2091 | |
| Bioswellix (EtOH) | 200 | 280 | 250 |
| Bioswellix (DMSO) | 80 | 120 | 20 |
| Bioswellix (medium) | 500 † | 500 † | 500 † |
| Fluconazole | 20 | 35 | 50 |
| MBIC50 (mg L−1) | Microorganism | ||
|---|---|---|---|
| C. parapsilosis CCM 8260 | C. krusei CCM 8271 | C. albicans ATCC 2091 | |
| Bioswellix (DMSO) | 500 † | 500 † | 100 |
| Fluconazole | 75 | 100 † | 100 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaroš, P.; Vrublevskaya, M.; Lokočová, K.; Michailidu, J.; Kolouchová, I.; Demnerová, K. Boswellia serrata Extract as an Antibiofilm Agent against Candida spp. Microorganisms 2022, 10, 171. https://doi.org/10.3390/microorganisms10010171
Jaroš P, Vrublevskaya M, Lokočová K, Michailidu J, Kolouchová I, Demnerová K. Boswellia serrata Extract as an Antibiofilm Agent against Candida spp. Microorganisms. 2022; 10(1):171. https://doi.org/10.3390/microorganisms10010171
Chicago/Turabian StyleJaroš, Petr, Maria Vrublevskaya, Kristýna Lokočová, Jana Michailidu, Irena Kolouchová, and Kateřina Demnerová. 2022. "Boswellia serrata Extract as an Antibiofilm Agent against Candida spp." Microorganisms 10, no. 1: 171. https://doi.org/10.3390/microorganisms10010171
APA StyleJaroš, P., Vrublevskaya, M., Lokočová, K., Michailidu, J., Kolouchová, I., & Demnerová, K. (2022). Boswellia serrata Extract as an Antibiofilm Agent against Candida spp. Microorganisms, 10(1), 171. https://doi.org/10.3390/microorganisms10010171






