The Darkest Place Is under the Candlestick-Healthy Urogenital Tract as a Source of Worldwide Disseminated Extraintestinal Pathogenic Escherichia coli Lineages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Description
2.2. Identification of E. coli Isolates
2.3. Characterization of E. coli Population
2.4. Whole Genome Sequencing
2.5. Comparative Genomics of B2 E. coli Strains
2.6. Single Nucleotide Polymorphisms Analysis
2.7. Accessory Genome and Ordination-Based Analysis
2.8. Statistical Analysis
3. Results
3.1. Frequency and Diversity of E. coli in Urinary and Vaginal Microbiome
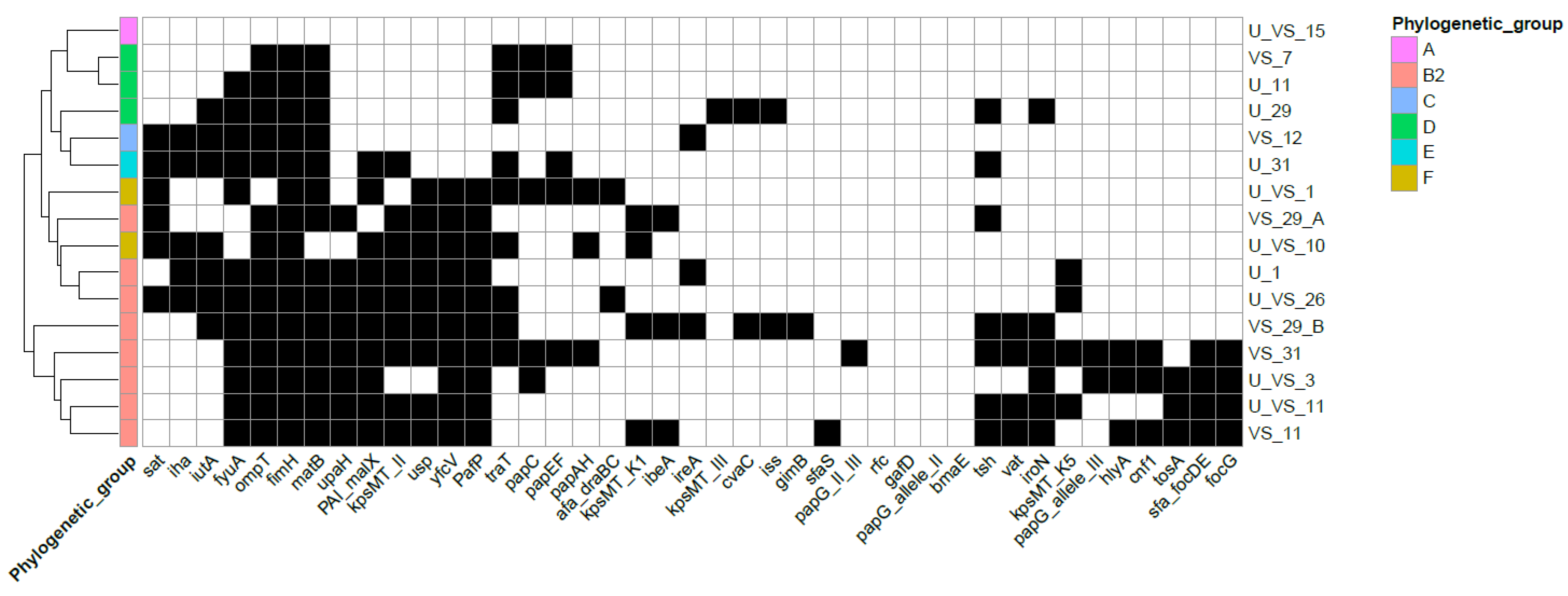
3.2. Virulence Profile Characterization of Urogenital E. coli
3.3. Genomic Background and Antimicrobial Resistance of B2 E. coli Strains
3.4. Phylogenomics of Urogenital ST131 E. coli from Healthy Urinary Microbiome
3.5. Phylogenomic Analysis of Other B2 E. coli Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negus, M.; Phillips, C.; Hindley, R. Recurrent Urinary Tract Infections: A Critical Review of the Currently Available Treatment Options. Obstet. Gynaecol. 2020, 22, 115–121. [Google Scholar] [CrossRef]
- Öztürk, R.; Murt, A. Epidemiology of Urological Infections: A Global Burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.; Mobley, H.L.T. Virulence and Fitness Determinants of Uropathogenic Escherichia coli. In Urinary Tract Infections: Molecular Pathogenesis and Clinical Management, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 235–261. [Google Scholar] [CrossRef] [Green Version]
- Foxman, B. The Epidemiology of Urinary Tract Infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [Green Version]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Tamadonfar, K.O.; Omattage, N.S.; Spaulding, C.N.; Hultgren, S.J. Reaching the End of the Line: Urinary Tract Infections. Microbiol. Spectr. 2019, 7, 3. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Proposal for a New Inclusive Designation for Extraintestinal Pathogenic Isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef] [Green Version]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef]
- Brzuszkiewicz, E.; Brüggemann, H.; Liesegang, H.; Emmerth, M.; Olschläger, T.; Nagy, G.; Albermann, K.; Wagner, C.; Buchrieser, C.; Emody, L.; et al. How to Become a Uropathogen: Comparative Genomic Analysis of Extraintestinal Pathogenic Escherichia coli Strains. Proc. Natl. Acad. Sci. USA 2006, 103, 12879–12884. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Pitout, J.D.D.; Peirano, G.; DeVinney, R.; Noguchi, T.; Yamamoto, M.; Gomi, R.; Matsuda, T.; Nakano, S.; Nagao, M.; et al. Rapid Identification of Different Escherichia coli Sequence Type 131 Clades. Antimicrob. Agents Chemother. 2017, 61, e00179-17. [Google Scholar] [CrossRef] [Green Version]
- Stoesser, N.; Sheppard, A.E.; Pankhurst, L.; De Maio, N.; Moore, C.E.; Sebra, R.; Turner, P.; Anson, L.W.; Kasarskis, A.; Batty, E.M.; et al. Evolutionary History of the Global Emergence of the Escherichia coli Epidemic Clone ST131. MBio 2016, 7, e02162. [Google Scholar] [CrossRef] [Green Version]
- Ciesielczuk, H.; Jenkins, C.; Chattaway, M.; Doumith, M.; Hope, R.; Woodford, N.; Wareham, D.W. Trends in ExPEC Serogroups in the UK and Their Significance. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1661–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallonen, T.; Brodrick, H.J.; Harris, S.R.; Corander, J.; Brown, N.M.; Martin, V.; Peacock, S.J.; Parkhill, J. Systematic Longitudinal Survey of Invasive Escherichia coli in England Demonstrates a Stable Population Structure Only Transiently Disturbed by the Emergence of ST131. Genome Res. 2017, 27, 1437–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fibke, C.D.; Croxen, M.A.; Geum, H.M.; Glass, M.; Wong, E.; Avery, B.P.; Daignault, D.; Mulvey, M.R.; Reid-Smith, R.J.; Parmley, E.J.; et al. Genomic Epidemiology of Major Extraintestinal Pathogenic Escherichia coli Lineages Causing Urinary Tract Infections in Young Women Across Canada. Open Forum Infect. Dis. 2019, 6, ofz431. [Google Scholar] [CrossRef] [PubMed]
- Flament-Simon, S.-C.; Nicolas-Chanoine, M.-H.; García, V.; Duprilot, M.; Mayer, N.; Alonso, M.P.; García-Meniño, I.; Blanco, J.E.; Blanco, M.; Blanco, J. Clonal Structure, Virulence Factor-Encoding Genes and Antibiotic Resistance of Escherichia coli, Causing Urinary Tract Infections and Other Extraintestinal Infections in Humans in Spain and France during 2016. Antibiotics 2020, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Davis, G.; Clabots, C.; Johnston, B.D.; Porter, S.; DebRoy, C.; Pomputius, W.; Ender, P.T.; Cooperstock, M.; Slater, B.S.; et al. Household Clustering of Escherichia coli Sequence Type 131 Clinical and Fecal Isolates According to Whole Genome Sequence Analysis. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2016; Volume 3. [Google Scholar]
- Banerjee, R.; Johnson, J.R. A New Clone Sweeps Clean: The Enigmatic Emergence of Escherichia coli Sequence Type 131. Antimicrob. Agents Chemother. 2014, 58, 4997–5004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, A.L.; Gilbert, N.M. Roles of the Vagina and the Vaginal Microbiota in Urinary Tract Infection: Evidence from Clinical Correlations and Experimental Models. GMS Infect. Dis. 2020, 8, Doc02. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, V.P.; Gilbert, N.M.; Lebratti, T.; Agarwal, K.; Foster, L.; Shin, H.; Lewis, A.L. Low-Dose Inoculation of Escherichia coli Achieves Robust Vaginal Colonization and Results in Ascending Infection Accompanied by Severe Uterine Inflammation in Mice. PLoS ONE 2019, 14, e0219941. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Russo, T.A. Molecular Epidemiology of Extraintestinal Pathogenic Escherichia coli. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Brannon, J.R.; Dunigan, T.L.; Beebout, C.J.; Ross, T.; Wiebe, M.A.; Reynolds, W.S.; Hadjifrangiskou, M. Invasion of Vaginal Epithelial Cells by Uropathogenic Escherichia coli. Nat. Commun. 2020, 11, 2803. [Google Scholar] [CrossRef]
- Siddiqui, H.; Nederbragt, A.J.; Lagesen, K.; Jeansson, S.L.; Jakobsen, K.S. Assessing Diversity of the Female Urine Microbiota by High Throughput Sequencing of 16S RDNA Amplicons. BMC Microbiol. 2011, 11, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; Fitzgerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; Nisco, N.J.D. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. MBio 2020, 11, e00218-20. [Google Scholar] [CrossRef]
- Jones-Freeman, B.; Chonwerawong, M.; Marcelino, V.R.; Deshpande, A.V.; Forster, S.C.; Starkey, M.R. The Microbiome and Host Mucosal Interactions in Urinary Tract Diseases. Mucosal Immunol. 2021, 14, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Van Kuiken, M.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of Female Bladder Bacteria Reveals an Interconnected Urogenital Microbiota. Nat. Commun. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Coorevits, L.; Heytens, S.; Boelens, J.; Claeys, G. The Resident Microflora of Voided Midstream Urine of Healthy Controls: Standard versus Expanded Urine Culture Protocols. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 635–639. [Google Scholar] [CrossRef]
- Ksiezarek, M.; Ugarcina-Perovic, S.; Rocha, J.; Grosso, F.; Peixe, L. Long-Term Stability of the Urogenital Microbiota of Asymptomatic European Women. BMC Microbiol. 2021, 21, 64. [Google Scholar] [CrossRef]
- Garretto, A.; Miller-Ensminger, T.; Ene, A.; Merchant, Z.; Shah, A.; Gerodias, A.; Biancofiori, A.; Canchola, S.; Canchola, S.; Castillo, E.; et al. Genomic Survey of E. coli From the Bladders of Women With and Without Lower Urinary Tract Symptoms. Front. Microbiol. 2020, 11, 2094. [Google Scholar] [CrossRef]
- Wang, R.F.; Cao, W.W.; Cerniglia, C.E. PCR Detection and Quantitation of Predominant Anaerobic Bacteria in Human and Animal Fecal Samples. Appl. Environ. Microbiol. 1996, 62, 1242–1247. [Google Scholar] [CrossRef] [Green Version]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli Phylo-Typing Method Revisited: Improvement of Specificity and Detection of New Phylo-Groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ksiezarek, M.; Novais, Â.; Felga, H.; Mendes, F.; Escobar, M.; Peixe, L. Phylogenomic Analysis of a Highly Virulent Escherichia coli ST83 Lineage with Potential Animal-Human Transmission. Microb. Pathog. 2021, 155, 104920. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Murray, A.C.; Gajewski, A.; Sullivan, M.; Snippes, P.; Kuskowski, M.A.; Smith, K.E. Isolation and Molecular Characterization of Nalidixic Acid-Resistant Extraintestinal Pathogenic Escherichia coli from Retail Chicken Products. Antimicrob. Agents Chemother. 2003, 47, 2161–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautom, R.K. Rapid Pulsed-Field Gel Electrophoresis Protocol for Typing of Escherichia coli O157:H7 and Other Gram-Negative Organisms in 1 Day. J. Clin. Microbiol. 1997, 35, 2977–2980. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Jolley, K.A.; Maiden, M.C. BIGSdb: Scalable Analysis of Bacterial Genome Variation at the Population Level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [Green Version]
- Croucher, N.J.; Page, A.J.; Connor, T.R.; Delaney, A.J.; Keane, J.A.; Bentley, S.D.; Parkhill, J.; Harris, S.R. Rapid Phylogenetic Analysis of Large Samples of Recombinant Bacterial Whole Genome Sequences Using Gubbins. Nucleic Acids Res. 2015, 43, e15. [Google Scholar] [CrossRef] [Green Version]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid Large-Scale Prokaryote Pan Genome Analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 10 April 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 April 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Salinas, A.M.; Osorio, V.G.; Pacha-Herrera, D.; Vivanco, J.S.; Trueba, A.F.; Machado, A. Vaginal Microbiota Evaluation and Prevalence of Key Pathogens in Ecuadorian Women: An Epidemiologic Analysis. Sci. Rep. 2020, 10, 18358. [Google Scholar] [CrossRef]
- Johnson, J.R.; Stell, A.L. Extended Virulence Genotypes of Escherichia coli Strains from Patients with Urosepsis in Relation to Phylogeny and Host Compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Ulett, G.C.; Totsika, M.; Phan, M.-D.; Schembri, M.A. Role of Capsule and O Antigen in the Virulence of Uropathogenic Escherichia coli. PLoS ONE 2014, 9, e94786. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, X.; Skogerbø, G.; Zhang, P.; Chen, R.; He, S.; Huang, D.-W. The Human Microbiome: A Hot Spot of Microbial Horizontal Gene Transfer. Genomics 2012, 100, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Langille, M.G.I.; Meehan, C.J.; Beiko, R.G. Human Microbiome: A Genetic Bazaar for Microbes? Curr. Biol. 2012, 22, R20–R22. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.L.; Dynesen, P.; Larsen, P.; Frimodt-Møller, N. Faecal Escherichia coli from Patients with E. coli Urinary Tract Infection and Healthy Controls Who Have Never Had a Urinary Tract Infection. J. Med. Microbiol. 2014, 63, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E.; Andreu, A.; Perez, T.; Sabate, M.; Johnson, J.R.; Prats, G. Relationship between Escherichia coli Strains Causing Urinary Tract Infection in Women and the Dominant Faecal Flora of the Same Hosts. Epidemiol. Infect. 2006, 134, 1015–1023. [Google Scholar] [CrossRef]
- Petty, N.K.; Ben Zakour, N.L.; Stanton-Cook, M.; Skippington, E.; Totsika, M.; Forde, B.M.; Phan, M.-D.; Gomes Moriel, D.; Peters, K.M.; Davies, M.; et al. Global Dissemination of a Multidrug Resistant Escherichia coli Clone. Proc. Natl. Acad. Sci. USA 2014, 111, 5694–5699. [Google Scholar] [CrossRef] [Green Version]
- Peirano, G.; van der Bij, A.K.; Freeman, J.L.; Poirel, L.; Nordmann, P.; Costello, M.; Tchesnokova, V.L.; Pitout, J.D.D. Characteristics of Escherichia coli Sequence Type 131 Isolates That Produce Extended-Spectrum β-Lactamases: Global Distribution of the H30-Rx Sublineage. Antimicrob. Agents Chemother. 2014, 58, 3762–3767. [Google Scholar] [CrossRef] [Green Version]
- Finn, T.J.; Scriver, L.; Lam, L.; Duong, M.; Peirano, G.; Lynch, T.; Dong, T.; Pitout, J.D.D.; DeVinney, R. A Comprehensive Account of Escherichia coli Sequence Type 131 in Wastewater Reveals an Abundance of Fluoroquinolone-Resistant Clade A Strains. Appl. Environ. Microbiol. 2020, 86, e01913-19. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Stegger, M.; Aziz, M.; Johnson, T.J.; Waits, K.; Nordstrom, L.; Gauld, L.; Weaver, B.; Rolland, D.; Statham, S.; et al. Escherichia coli ST131-H22 as a Foodborne Uropathogen. MBio 2018, 9, e00470-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenza, G.; Werner, M.; Eisenberger, D.; Nickel, S.; Lehner-Reindl, V.; Höller, C.; Bogdan, C. First Report of the New Emerging Global Clone ST1193 among Clinical Isolates of Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli from Germany. J. Glob. Antimicrob. Resist. 2019, 17, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, J.; Yao, K.; Gao, W.; Wang, Y. Molecular Characteristics of the New Emerging Global Clone ST1193 among Clinical Isolates of Escherichia coli from Neonatal Invasive Infections in China. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W. Extraintestinal Foodborne Pathogens. Ann. Rev. Food Sci. Technol. 2020, 11, 275–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manges, A.R.; Johnson, J.R. Reservoirs of Extraintestinal Pathogenic Escherichia coli. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Blas, J.F.; Ovejero, C.M.; Abadia-Patiño, L.; Gonzalez-Zorn, B. Coexistence of Mcr-1 and BlaNDM-1 in Escherichia coli from Venezuela. Antimicrob. Agents Chemother. 2016, 60, 6356–6358. [Google Scholar] [CrossRef] [Green Version]
- Mbelle, N.M.; Feldman, C.; Osei Sekyere, J.; Maningi, N.E.; Modipane, L.; Essack, S.Y. The Resistome, Mobilome, Virulome and Phylogenomics of Multidrug-Resistant Escherichia coli Clinical Isolates from Pretoria, South Africa. Sci. Rep. 2019, 9, 16457. [Google Scholar] [CrossRef]
- Bozcal, E.; Eldem, V.; Aydemir, S.; Skurnik, M. The Relationship between Phylogenetic Classification, Virulence and Antibiotic Resistance of Extraintestinal Pathogenic Escherichia coli in İzmir Province, Turkey. PeerJ 2018, 6, e5470. [Google Scholar] [CrossRef] [Green Version]
- Alghoribi, M.F.; Gibreel, T.M.; Farnham, G.; Al Johani, S.M.; Balkhy, H.H.; Upton, M. Antibiotic-Resistant ST38, ST131 and ST405 Strains Are the Leading Uropathogenic Escherichia coli Clones in Riyadh, Saudi Arabia. J. Antimicrob. Chemother. 2015, 70, 2757–2762. [Google Scholar] [CrossRef] [Green Version]
- Yasugi, M.; Hatoya, S.; Motooka, D.; Matsumoto, Y.; Shimamura, S.; Tani, H.; Furuya, M.; Mie, K.; Miyake, M.; Nakamura, S.; et al. Whole-Genome Analyses of Extended-Spectrum or AmpC β-Lactamase-Producing Escherichia coli Isolates from Companion Dogs in Japan. PLoS ONE 2021, 16, e0246482. [Google Scholar]
- Alonso, C.A.; González-Barrio, D.; Ruiz-Fons, F.; Ruiz-Ripa, L.; Torres, C. High Frequency of B2 Phylogroup among Non-Clonally Related Fecal Escherichia coli Isolates from Wild Boars, Including the Lineage ST131. FEMS Microbiol. Ecol. 2017, 93, fix016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster-Nyarko, E.; Alikhan, N.-F.; Ravi, A.; Thilliez, G.; Thomson, N.M.; Baker, D.; Kay, G.; Cramer, J.D.; O’Grady, J.; Antonio, M.; et al. Genomic Diversity of Escherichia coli Isolates from Non-Human Primates in the Gambia. Microb. Genom. 2020, 6, mgen000428. [Google Scholar] [CrossRef]
- Hertz, F.B.; Nielsen, J.B.; Schønning, K.; Littauer, P.; Knudsen, J.D.; Løbner-Olesen, A.; Frimodt-Møller, N. Population Structure of Drug-Susceptible, -Resistant and ESBL-Producing Escherichia coli from Community-Acquired Urinary Tract Infections. BMC Microbiol. 2016, 16, 63. [Google Scholar]
- Banerjee, R.; Johnston, B.; Lohse, C.; Chattopadhyay, S.; Tchesnokova, V.; Sokurenko, E.V.; Johnson, J.R. The Clonal Distribution and Diversity of Extraintestinal Escherichia coli Isolates Vary According to Patient Characteristics. Antimicrob. Agents Chemother. 2013, 57, 5912–5917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, R.; Friedman, C.R.; Rubin, J.; Suh, J.; Thys, E.; McDermott, P.; Hung-Fan, M.; Riley, L.W. A Population-Based Surveillance Study of Shared Genotypes of Escherichia coli Isolates from Retail Meat and Suspected Cases of Urinary Tract Infections. Msphere 2018, 3, e00179-18. [Google Scholar] [CrossRef] [Green Version]
- Mbanga, J.; Amoako, D.G.; Abia, A.L.K.; Allam, M.; Ismail, A.; Essack, S.Y. Genomic Insights of Multidrug-Resistant Escherichia coli From Wastewater Sources and Their Association With Clinical Pathogens in South Africa. Front. Vet. Sci. 2021, 8, 636715. [Google Scholar] [CrossRef]
- Price, L.B.; Hungate, B.A.; Koch, B.J.; Davis, G.S.; Liu, C.M. Colonizing Opportunistic Pathogens (COPs): The Beasts in All of Us. PLoS Pathog. 2017, 13, e1006369. [Google Scholar] [CrossRef] [Green Version]




| Donor | Origin | Strains | Phylogenetic Group | ExPEC | PFGE | WGS | MLST | Serotype |
|---|---|---|---|---|---|---|---|---|
| 1 | U | c1Ub_48 | F | + | EC1 | − | − | − |
| VS | c1VSb_14 | F | + | EC1 | − | − | − | |
| U | c1Ub_56 | B2 | + | − | + | ST452 | O81:H27 | |
| 3 | U | c3Ub_1 | B2 | + | EC2 | + | ST681 | O8:H10 |
| VS | c3VSb_22 | B2 | + | EC2 | − | − | − | |
| 7 | VS | c7VSa_62 | D | − | − | − | − | − |
| 10 | U | c10Ua_105 | F | + | EC3 | − | − | − |
| VS | c10VSa_39 | F | + | EC3 | − | − | − | |
| 11 * | U | c11Ua_88 | D | − | − | − | − | − |
| U | c11Ub_17 | B2 | + | EC4 | + | ST1154 (ST73 complex) | O2:H1 | |
| VS | c11VSb_7 | B2 | + | EC4 | − | − | − | |
| VS | c11VSb_12 | B2 | + | EC5 | + | ST998 | O2:H6 | |
| 12 | VS | c12VSb_42 | C | − | − | − | − | − |
| 15 | U | c15Ub_26 | A | − | EC6 | − | − | − |
| VS | c15VSb_20 | A | − | EC6 | − | − | − | |
| 26 | U | c26Ub_7 | B2 | + | EC7 | + | ST131 | O25:H4 |
| VS | c26VSb_8 | B2 | + | EC7 | − | − | − | |
| 29 * | U | c29Ub_57 | D | − | − | − | − | − |
| VS | c29VSb_15 | B2 | + | EC8 | + | ST140 (ST95 complex) | O2:H5 | |
| VS | c29VSa_23 | B2 | − | EC9 | + | ST569 | O46:H31 | |
| 31 | U | c31Ua_56 | E | − | − | − | − | − |
| VS | c31VSa_9 | B2 | + | − | + | ST127 | O6:H31 |
| Total (n = 22) | Urinary Isolates (n = 10) | Vaginal Isolates (n = 12) | |
|---|---|---|---|
| Phylogenetic group | |||
| A | 2 (9) | 1 (10) | 1 (8) |
| B2 | 11 (50) | 4 (40) | 7 (58) |
| C | 1 (5) | 0 | 1 (8) |
| D | 3 (14) | 2 (20) | 1 (8) |
| E | 1 (5) | 1 (10) | 0 |
| F | 4 (18) | 2 (20) | 2 (17) |
| Adhesins | |||
| fimH | 20 (91) | 9 (90) | 11 (92) |
| papAH | 3 (14) | 2 (20) | 1 (8) |
| papC | 7 (32) | 3 (30) | 4 (33) |
| papEF | 7 (32) | 3 (30) | 4 (33) |
| papG II, III | 1 (5) | 0 | 1 (8) |
| papG allele III | 3 (14) | 1 (10) | 2 (17) |
| sfa/focD | 6 (27) | 2 (20) | 4 (33) |
| sfaS | 1 (5) | 0 | 1 (8) |
| focG | 6 (27) | 2 (20) | 4 (33) |
| afa/draBC | 3 (14) | 2 (20) | 1 (8) |
| iha | 7 (32) | 4 (40) | 3 (25) |
| matB | 18 (82) | 8 (80) | 10 (83) |
| yfcV | 15 (68) | 6 (60) | 9 (75) |
| Toxins | |||
| hlyA | 3 (14) | 1 (10) | 2 (17) |
| cnf1 | 3 (14) | 1 (10) | 2 (17) |
| sat | 9 (41) | 4 (40) | 5 (42) |
| tsh | 8 (36) | 3 (30) | 5 (42) |
| vat | 5 (23) | 1 (10) | 4 (33) |
| tosA | 4 (18) | 2 (20) | 2 (17) |
| Siderophores | |||
| fyuA | 14 (64) | 8 (80) | 6 (50) |
| iutA | 8 (36) | 5 (50) | 3 (25) |
| iroN | 8 (36) | 3 (30) | 5 (42) |
| ireA | 3 (14) | 1 (10) | 2 (17) |
| Capsule | |||
| kpsMT II | 12 (55) | 5 (50) | 7 (58) |
| kpsMT III | 2 (9) | 1 (10) | 1 (8) |
| kpsMT K1 | 5 (23) | 1 (10) | 4 (33) |
| kpsMT K5 | 6 (27) | 3 (30) | 3 (25) |
| Protectins | |||
| cvaC | 2 (9) | 1 (10) | 1 (8) |
| traT | 12 (55) | 6 (60) | 6 (50) |
| iss | 2 (9) | 1 (10) | 1 (8) |
| Invasins | |||
| ibeA | 3 (14) | 0 | 3 (25) |
| gimB | 1 (5) | 0 | 1 (8) |
| Miscellaneous | |||
| usp | 13 (59) | 5 (50) | 8 (67) |
| ompT | 18 (82) | 8 (80) | 10 (83) |
| PAI (malX) | 12 (55) | 7 (70) | 5 (42) |
| pafP | 15 (68) | 6 (60) | 9 (75) |
| upaH | 11 (50) | 4 (40) | 7 (58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ksiezarek, M.; Novais, Â.; Peixe, L. The Darkest Place Is under the Candlestick-Healthy Urogenital Tract as a Source of Worldwide Disseminated Extraintestinal Pathogenic Escherichia coli Lineages. Microorganisms 2022, 10, 27. https://doi.org/10.3390/microorganisms10010027
Ksiezarek M, Novais Â, Peixe L. The Darkest Place Is under the Candlestick-Healthy Urogenital Tract as a Source of Worldwide Disseminated Extraintestinal Pathogenic Escherichia coli Lineages. Microorganisms. 2022; 10(1):27. https://doi.org/10.3390/microorganisms10010027
Chicago/Turabian StyleKsiezarek, Magdalena, Ângela Novais, and Luísa Peixe. 2022. "The Darkest Place Is under the Candlestick-Healthy Urogenital Tract as a Source of Worldwide Disseminated Extraintestinal Pathogenic Escherichia coli Lineages" Microorganisms 10, no. 1: 27. https://doi.org/10.3390/microorganisms10010027
APA StyleKsiezarek, M., Novais, Â., & Peixe, L. (2022). The Darkest Place Is under the Candlestick-Healthy Urogenital Tract as a Source of Worldwide Disseminated Extraintestinal Pathogenic Escherichia coli Lineages. Microorganisms, 10(1), 27. https://doi.org/10.3390/microorganisms10010027







