Gut Microbiome Profiles in Colonizations with the Enteric Protozoa Blastocystis in Korean Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Fecal Samples and Blastocystis Screening
2.2. Metagenomic Analysis
2.3. Statistical Analysis
3. Results
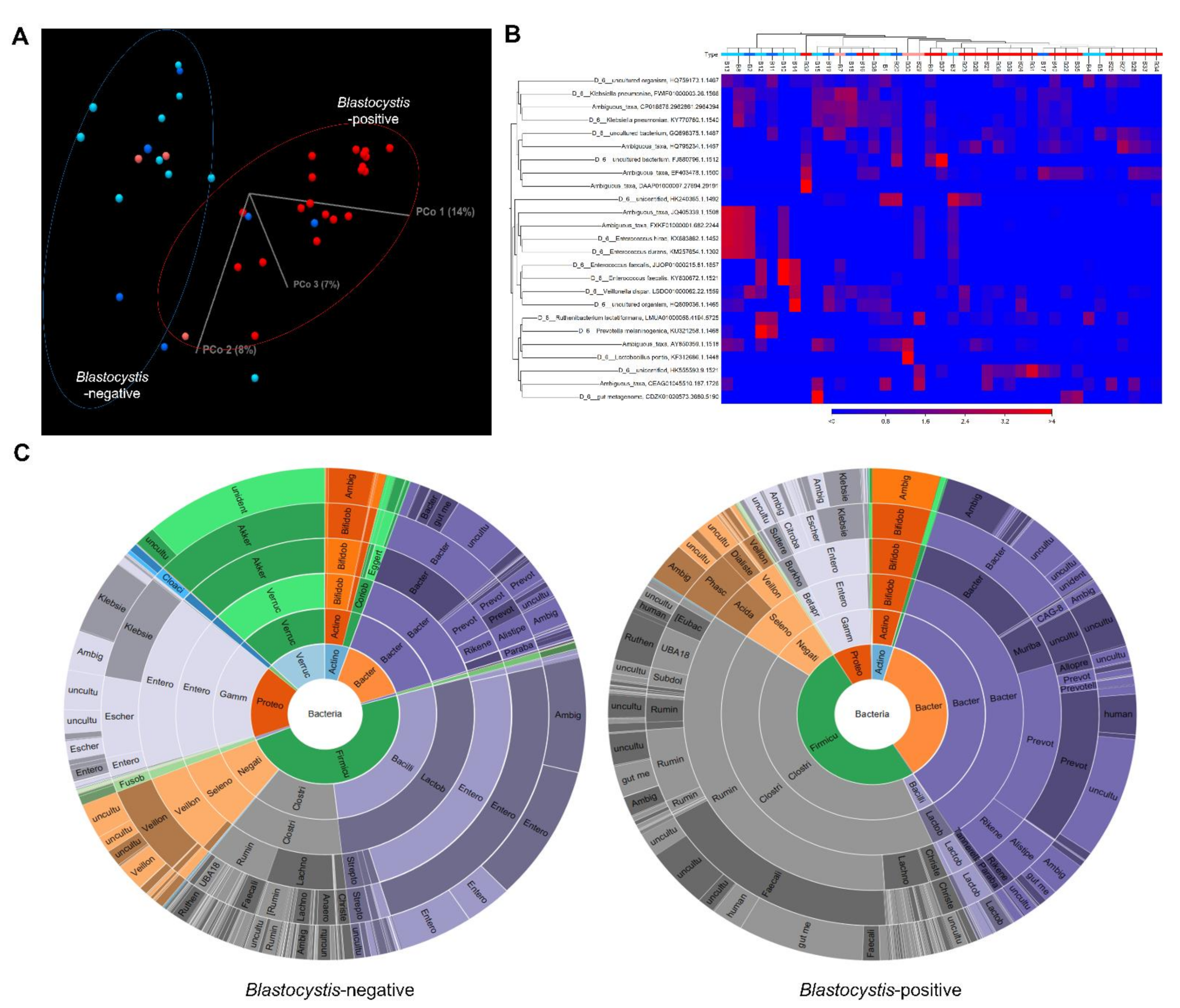
3.1. Blastocystis Colonization Is Associated with Increased Diversity of Gut Bacterial Microbiota
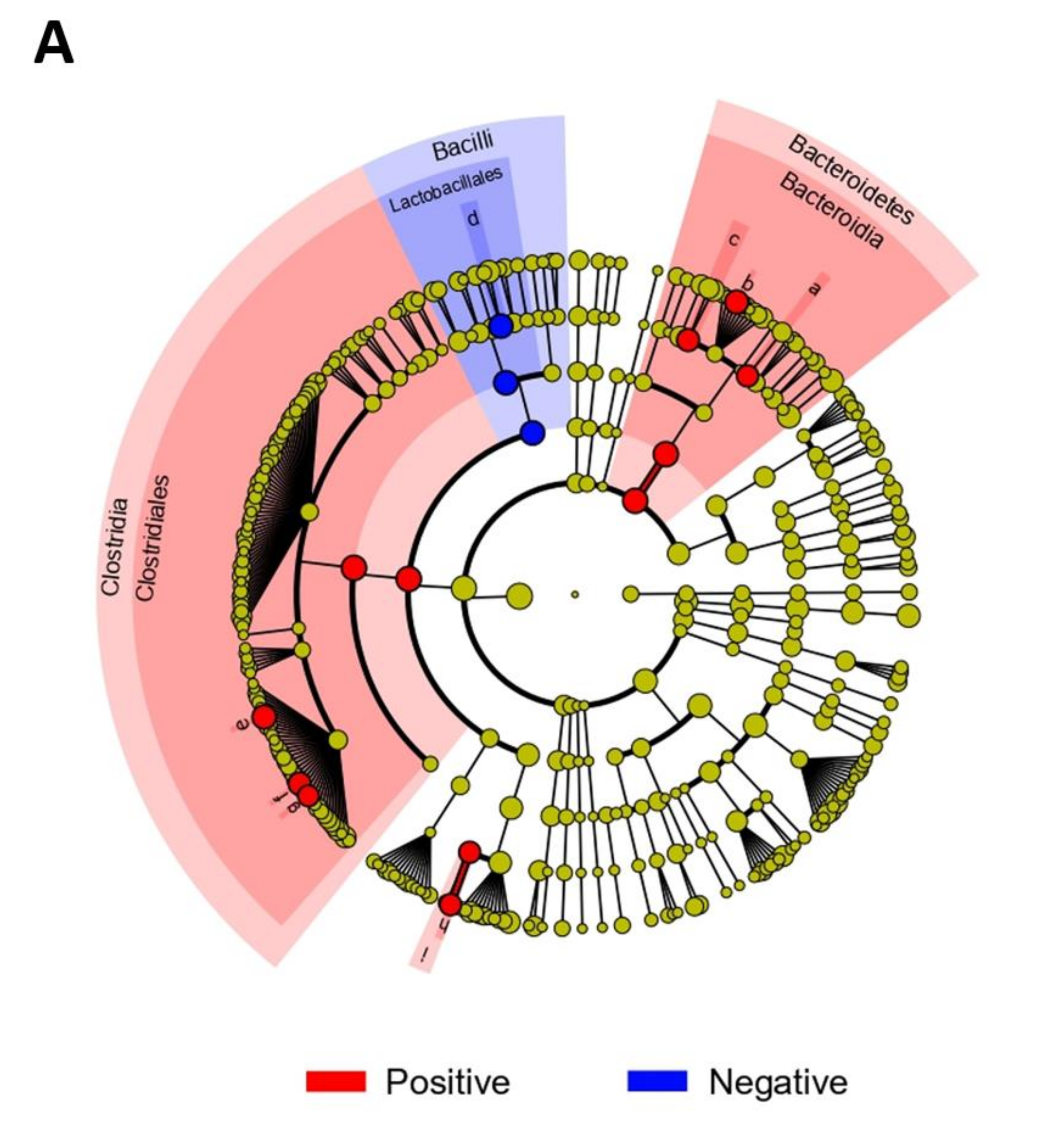
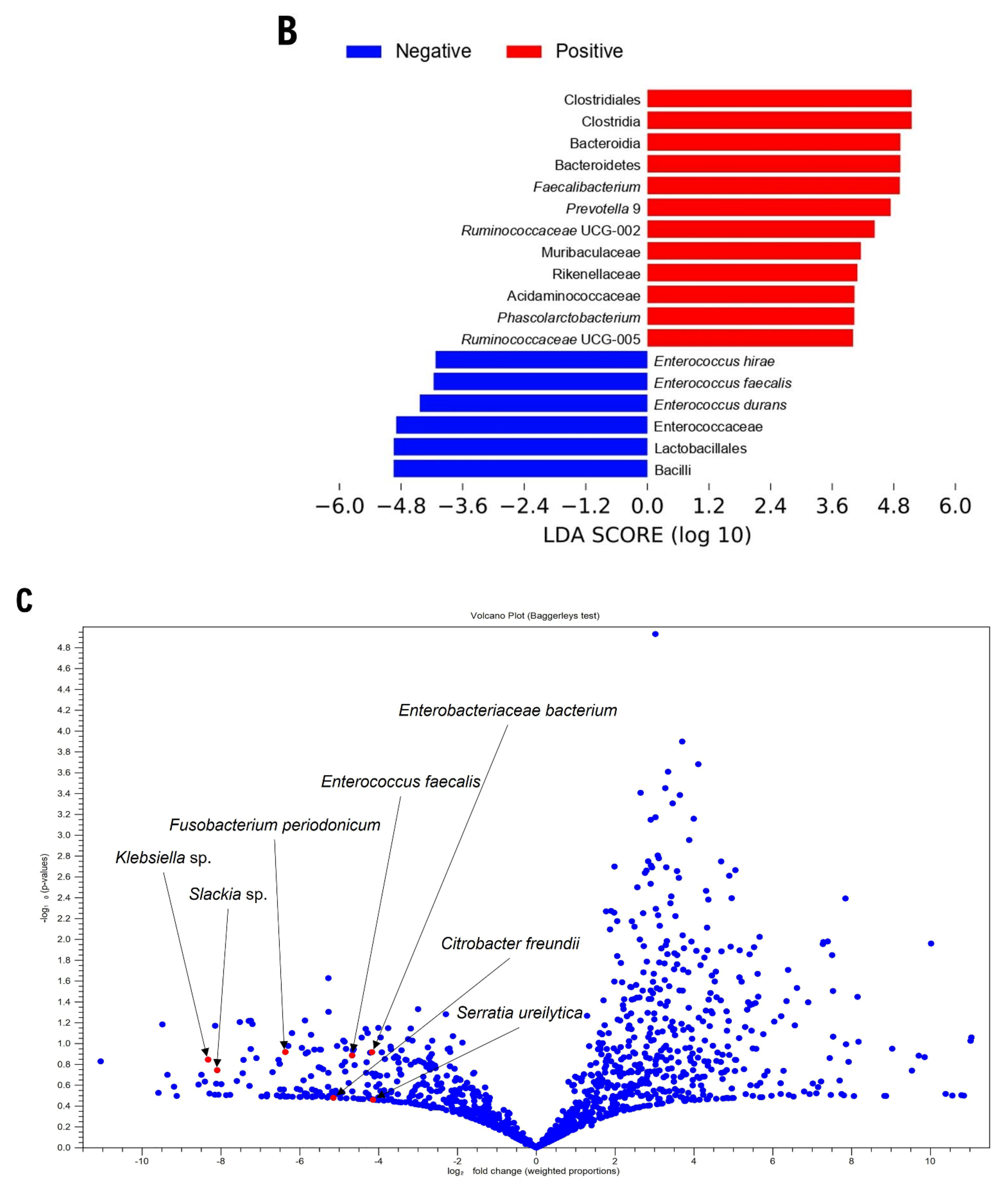
3.2. Gut Microbiome Composition According to Blastocystis Colonization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verweij, J.J.; Stensvold, C.R. Molecular Testing for Clinical Diagnosis and Epidemiological Investigations of Intestinal Parasitic Infections. Clin. Microbiol. Rev. 2014, 27, 371–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stensvold, C.R.; van der Giezen, M. Associations between Gut Microbiota and Common Luminal Intestinal Parasites. Trends Parasitol. 2018, 34, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lukeš, J.; Stensvold, C.R.; Jirků-Pomajbíková, K.; Parfrey, L.W. Are Human Intestinal Eukaryotes Beneficial or Commensals? PLoS Pathog. 2015, 11, e1005039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audebert, C.; Even, G.; Cian, A.; Loywick, A.; Merlin, S.; Viscogliosi, E.; Chabe, M.; Blastocystis Investigation Group. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci. Rep. 2016, 6, 25255. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D.; Stensvold, C.R.; Rajilic-Stojanovic, M.; Heilig, H.G.; De Vos, W.M.; O’Toole, P.W.; Cotter, P. The microbial eukaryoteBlastocystisis a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beghini, F.; Pasolli, E.; Truong, T.D.; Putignani, L.; Cacciò, S.M.; Segata, N. Large-scale comparative metagenomics of Blastocystis, a common member of the human gut microbiome. ISME J. 2017, 11, 2848–2863. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Won, E.J.; Kim, S.H.; Shin, J.H.; Chai, J.-Y. Molecular Detection and Subtyping of Human Blastocystis and the Clinical Implications: Comparisons between Diarrheal and Non-diarrheal Groups in Korean Populations. Korean J. Parasitol. 2020, 58, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Autier, B.; Gangneux, J.-P.; Robert-Gangneux, F. Evaluation of the AllplexTM Gastrointestinal Panel—Parasite Assay for Protozoa Detection in Stool Samples: A Retrospective and Prospective Study. Microorganisms 2020, 8, 569. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.O.; Bonde, I.; Nielsen, H.B.; Stensvold, C.R. A retrospective metagenomics approach to studyingBlastocystis. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieves-Ramírez, M.E.; Partida-Rodríguez, O.; Laforest-Lapointe, I.; Reynolds, L.A.; Brown, E.M.; Valdez-Salazar, A.; Morán-Silva, P.; Rojas-Velázquez, L.; Morien, E.; Parfrey, L.W.; et al. Asymptomatic Intestinal Colonization with Protist Blastocystis Is Strongly Associated with Distinct Microbiome Ecological Patterns. mSystems 2018, 3, e00007-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, M.; Chen, W.; Ma, J.; Peng, Y.; Zhang, M.; Wang, C.; Yan, G.; Lu, G. Altered Gut Microbiota Taxonomic Compositions of Patients With Sepsis in a Pediatric Intensive Care Unit. Front. Pediatr. 2021, 9, 645060. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilic-Stojanovic, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Tito, R.Y.; Chaffron, S.; Caenepeel, C.; Lima-Mendez, G.; Wang, J.; Vieira-Silva, S.; Falony, G.; Hildebrand, F.; Darzi, Y.; Rymenans, L.; et al. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut 2019, 68, 1180–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Lee, Y.J.; Kim, T.-J.; Won, E.J. Gut Microbiome Profiles in Colonizations with the Enteric Protozoa Blastocystis in Korean Populations. Microorganisms 2022, 10, 34. https://doi.org/10.3390/microorganisms10010034
Kim M-J, Lee YJ, Kim T-J, Won EJ. Gut Microbiome Profiles in Colonizations with the Enteric Protozoa Blastocystis in Korean Populations. Microorganisms. 2022; 10(1):34. https://doi.org/10.3390/microorganisms10010034
Chicago/Turabian StyleKim, Moon-Ju, Yu Jeong Lee, Tae-Jong Kim, and Eun Jeong Won. 2022. "Gut Microbiome Profiles in Colonizations with the Enteric Protozoa Blastocystis in Korean Populations" Microorganisms 10, no. 1: 34. https://doi.org/10.3390/microorganisms10010034





