Propionibacterium freudenreichii Inhibits RANKL-Induced Osteoclast Differentiation and Ameliorates Rheumatoid Arthritis in Collagen-Induced Arthritis Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Heat-Killed Propionibacterium freudenreichii MJ2 (hkMJ2)
2.3. Cell Culture and Cytotoxicity Assay
2.4. Tartrate-Resistant Acid Phosphatase (TRAP) Staining and Activity Assay
2.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.6. Western Blotting
2.7. Animal Model of Collagen-Induced Arthritis (CIA) and Scoring of Severity
2.8. Micro-Computed Tomography (CT)
2.9. Histological Analysis
2.10. Measurement of Cytokines and Collagen-Specific IgG Levels
2.11. Statistical Analysis
3. Results
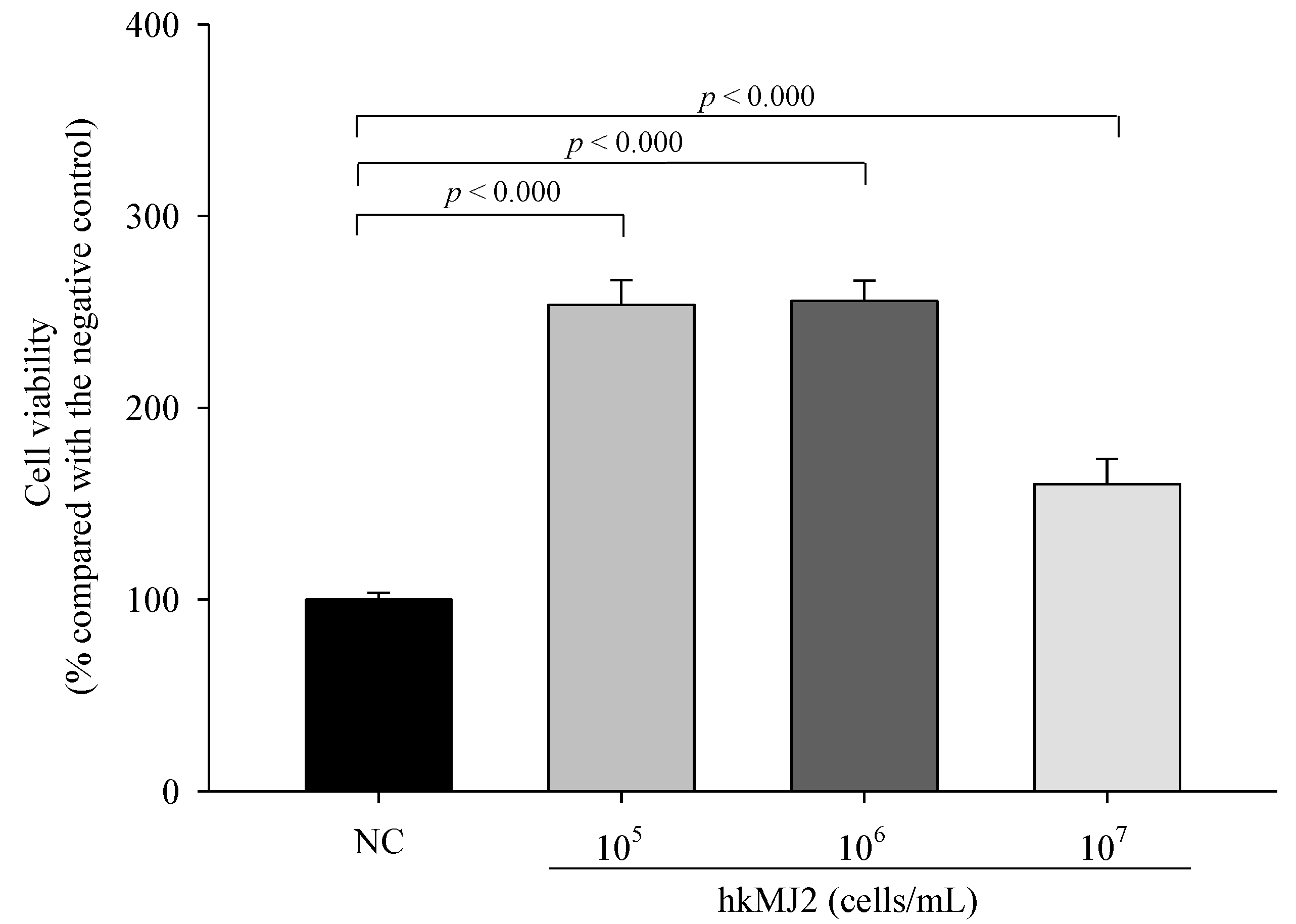
3.1. Cytotoxicity of hkMJ2 in RAW 264.7 Cells
3.2. HkMJ2 Inhibits Osteoclastogenesis and TRAP Activity in Raw 264.7 Cells
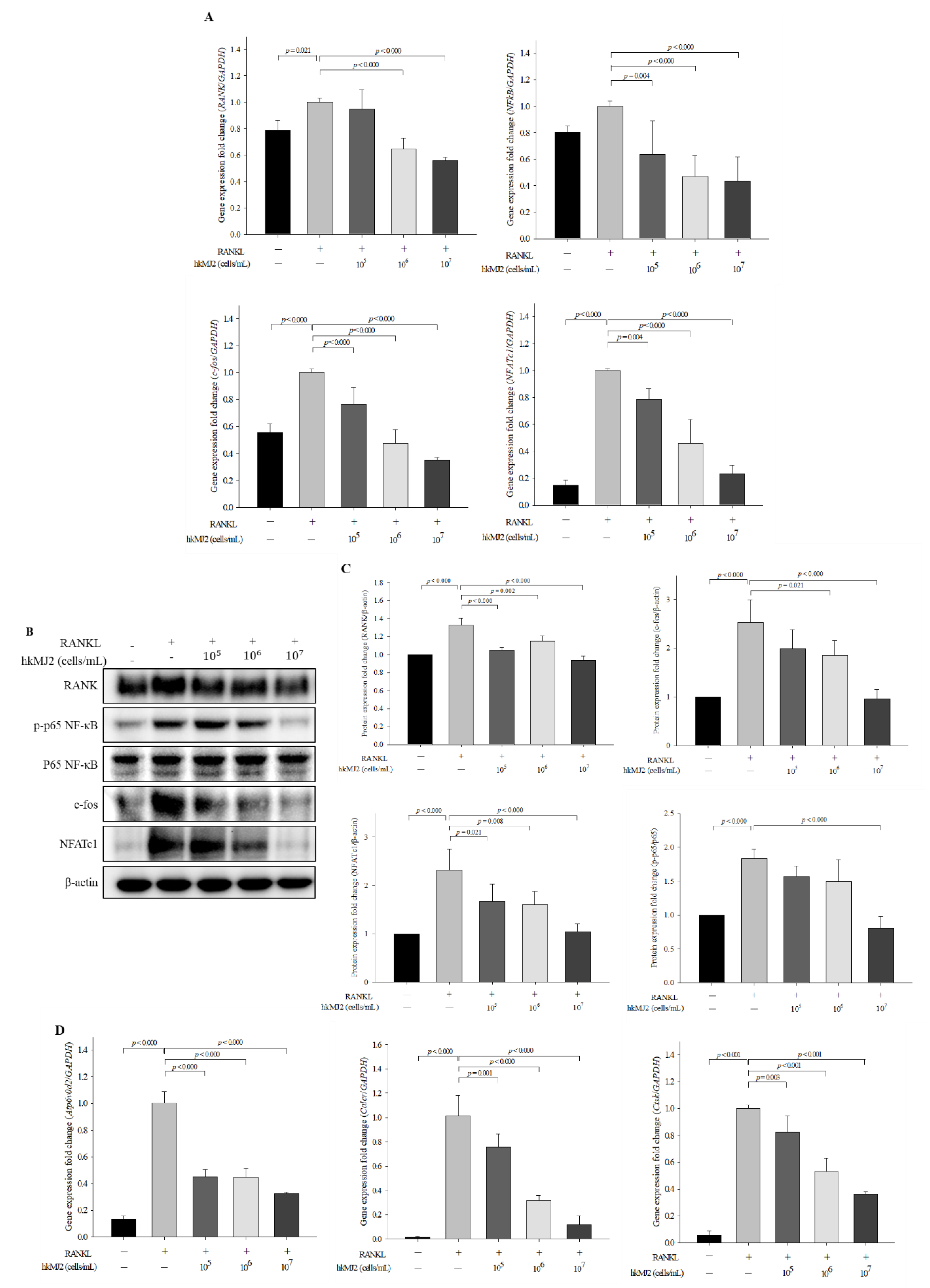
3.3. HkMJ2 Decreases the Expression Levels of Osteoclast-Related Genes and Proteins
3.4. MJ2 Attenuates Collagen-Induced Arthritis (CIA)-Associated Symptoms
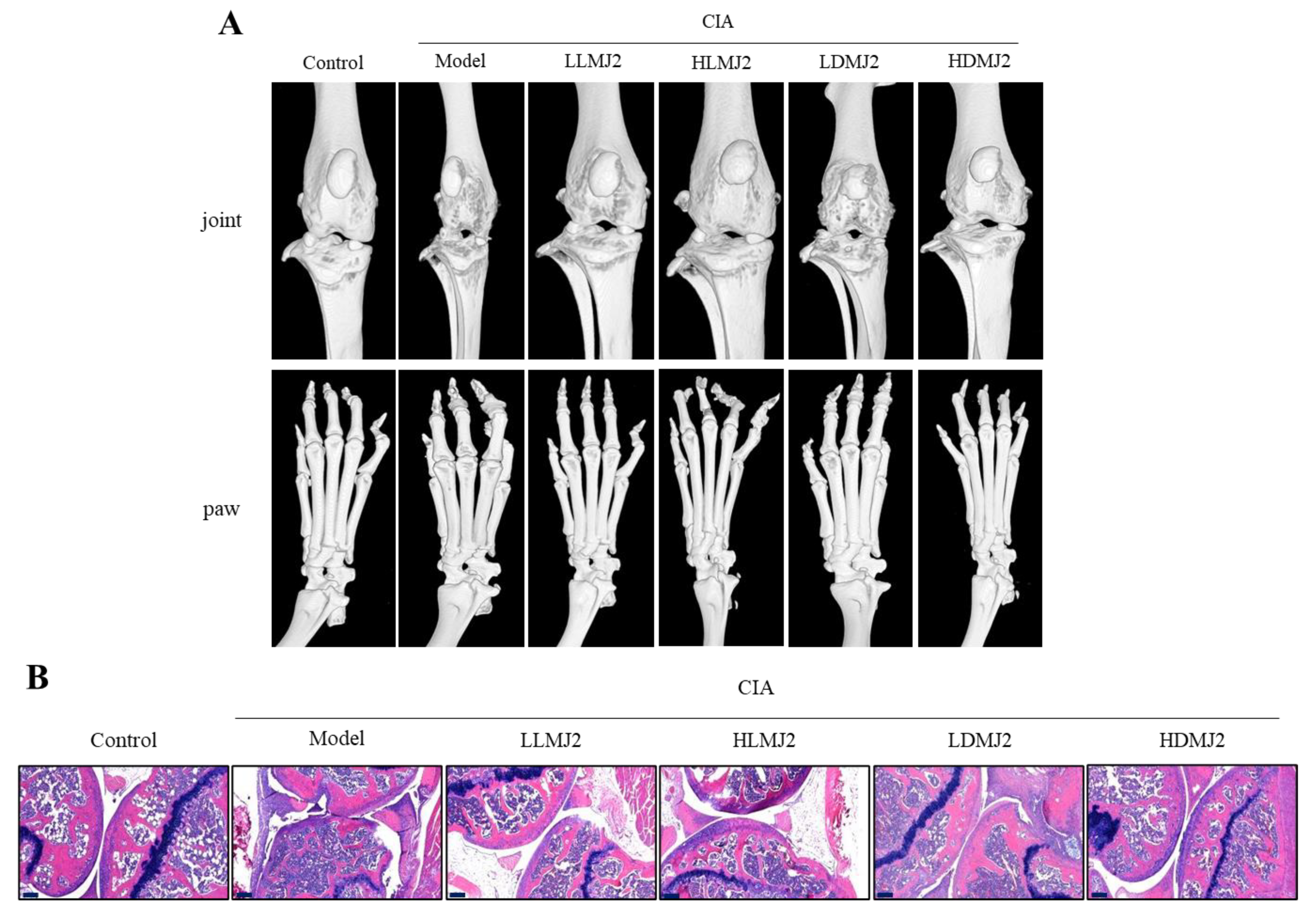
3.5. MJ2 Inhibits the Bone Erosion in CIA Mice
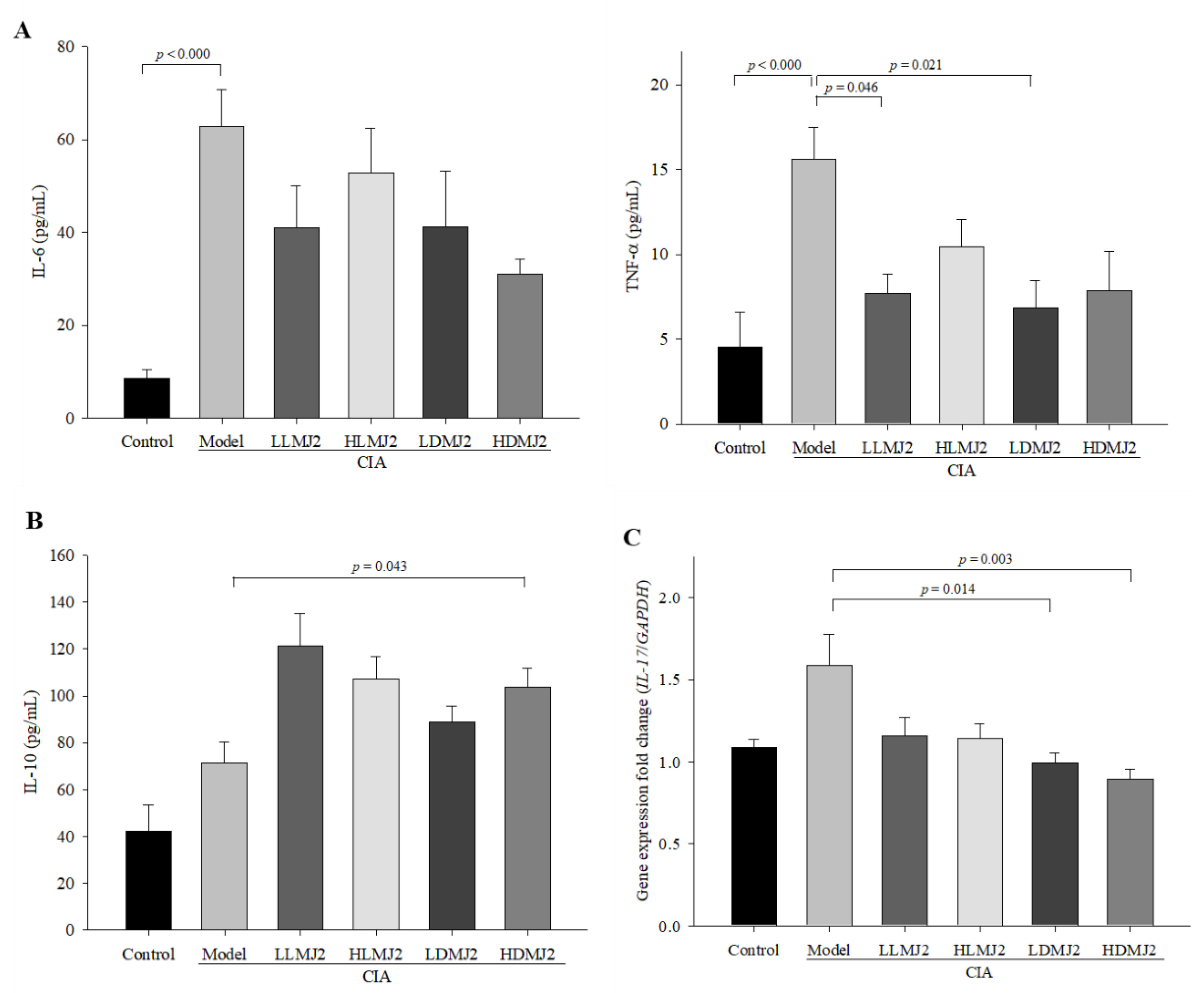
3.6. MJ2 Decreases the Levels of Proinflammatory Cytokines and Increases the Level of IL-10 in CIA Mice
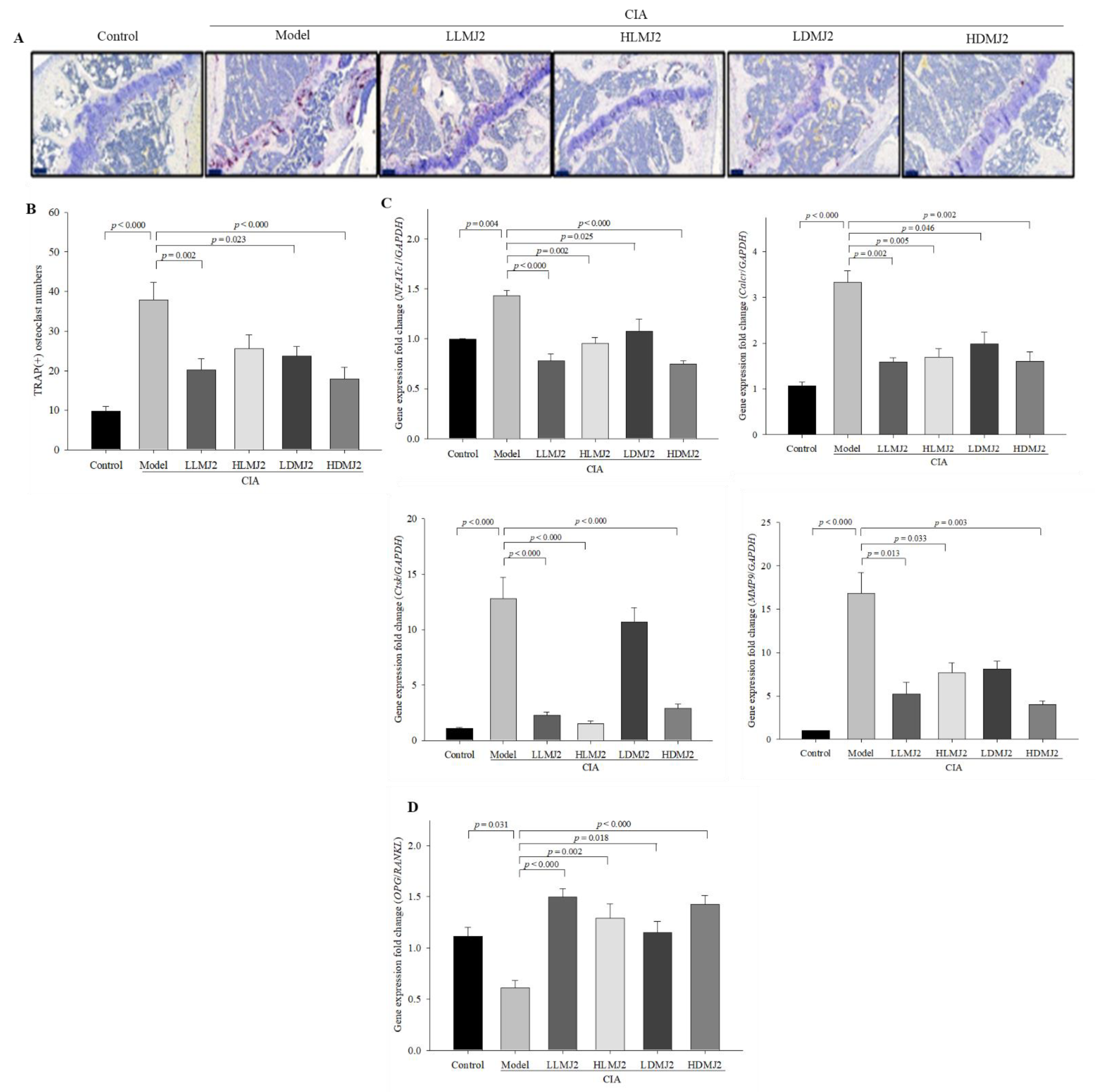
3.7. MJ2 Inhibits the Osteoclast Differentiation in CIA Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef]
- Kareem, R.; Botleroo, R.A.; Bhandari, R.; Ogeyingbo, O.D.; Ahmed, R.; Gyawali, M.; Venkatesan, N.; Elhaikh, A.O. The Impact of Rheumatoid Arthritis on Bone Loss: Links to Osteoporosis and Osteopenia. Cureus 2021, 13, e17519. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl. S3), S2. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, O.; Bansal, P.; Goyal, A.; Lappin, S.L. Disease modifying anti-rheumatic drugs (DMARD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507863/ (accessed on 6 July 2021).
- Schuna, A.A.; Megeff, C. New drugs for the treatment of rheumatoid arthritis. Am. J. Health Syst. Pharm. 2000, 57, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S. Emerging anti-osteoclast therapy for rheumatoid arthritis. J. Orthop. Sci. 2018, 23, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takayanagi, H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 2006, 18, 419–426. [Google Scholar] [CrossRef]
- Haynes, D.; Crotti, T.; Loric, M.; Bain, G.; Atkins, G.; Findlay, D. Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology 2001, 40, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; de Vos, P.; Ren, Y. Overexpression of osteoprotegerin promotes preosteoblast differentiation to mature osteoblasts. Angle Orthod. 2011, 81, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S. RANKL is a therapeutic target of bone destruction in rheumatoid arthritis. F1000Research 2019, 8, F1000 Faculty Rev-533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, Y.G.; Ritchlin, C.T. Denosumab: Targeting the RANKL pathway to treat rheumatoid arthritis. Expert Opin. Biol. Ther. 2017, 17, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Romas, E.; Sims, N.A.; Hards, D.K.; Lindsay, M.; Quinn, J.W.; Ryan, P.F.; Dunstan, C.R.; Martin, T.J.; Gillespie, M.T. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am. J. Pathol. 2002, 161, 1419–1427. [Google Scholar] [CrossRef] [Green Version]
- Téletchéa, S.; Stresing, V.; Hervouet, S.; Baud’Huin, M.; Heymann, M.F.; Bertho, G.; Charrier, C.; Ando, K.; Heymann, D. Novel RANK antagonists for the treatment of bone-resorptive disease: Theoretical predictions and experimental validation. J. Bone Miner. Res. 2014, 29, 1466–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwase, M.; Kim, K.J.; Kobayashi, Y.; Itoh, M.; Itoh, T. A novel bisphosphonate inhibits inflammatory bone resorption in a rat osteolysis model with continuous infusion of polyethylene particles. J. Orthop. Res. 2002, 20, 499–505. [Google Scholar] [CrossRef]
- Herrak, P.; Görtz, B.; Hayer, S.; Redlich, K.; Reiter, E.; Gasser, J.; Bergmeister, H.; Kollias, G.; Smolen, J.S.; Schett, G. Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor–mediated arthritis. Arthritis Rheum. 2004, 50, 2327–2337. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hashempour-Baltork, F.; Sheikh, M.; Eskandarzadeh, S.; Tarlak, F.; Tripathi, A.D.; Khosravi-Darani, K.; Sadanov, A. The Effect of Probiotics on Various Diseases and their Therapeutic Role: An Update Review. J. Pure Appl. Microbiol. 2021, 15, 1042–1059. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Oliviero, F.; Spinella, P. Benefits of Probiotics in Rheumatic Diseases. Front. Nutr. 2020, 7, 157. [Google Scholar] [CrossRef]
- Devi, S.M.; Kurrey, N.K.; Halami, P.M. In vitro anti-inflammatory activity among probiotic Lactobacillus species isolated from fermented foods. J. Funct. Foods 2018, 47, 19–27. [Google Scholar] [CrossRef]
- Riedel, C.U.; Foata, F.; Philippe, D.; Adolfsson, O.; Eikmanns, B.J.; Blum, S. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-κB activation. World J. Gastroenterol. 2006, 12, 3729–3735. [Google Scholar] [CrossRef]
- Jhun, J.; Min, H.K.; Ryu, J.; Lee, S.-Y.; Ryu, J.-G.; Choi, J.W.; Na, H.S.; Lee, S.Y.; Jung, Y.; Park, S.-J. Lactobacillus sakei suppresses collagen-induced arthritis and modulates the differentiation of T helper 17 cells and regulatory B cells. J. Transl. Med. 2020, 18, 317. [Google Scholar] [CrossRef]
- Kim, J.-E.; Chae, C.S.; Kim, G.-C.; Hwang, W.; Hwang, J.-S.; Hwang, S.-M.; Kim, Y.; Ahn, Y.-T.; Park, S.-G.; Jun, C.-D. Lactobacillus helveticus suppresses experimental rheumatoid arthritis by reducing inflammatory T cell responses. J. Funct. Foods 2015, 13, 350–362. [Google Scholar] [CrossRef]
- Fan, Z.; Ross, R.P.; Stanton, C.; Hou, B.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus casei CCFM1074 Alleviates Collagen-Induced Arthritis in Rats via Balancing Treg/Th17 and Modulating the Metabolites and Gut Microbiota. Front. Immunol. 2021, 12, 680073. [Google Scholar] [CrossRef]
- Zárate, G. Dairy Propionibacteria: Less conventional probiotics to improve the human and animal health. In Probiotic in Animals; InTech: London, UK, 2012; pp. 153–202. [Google Scholar]
- Le Maréchal, C.; Peton, V.; Plé, C.; Vroland, C.; Jardin, J.; Briard-Bion, V.; Durant, G.; Chuat, V.; Loux, V.; Foligné, B. Surface proteins of Propionibacterium freudenreichii are involved in its anti-inflammatory properties. J. Proteom. 2015, 113, 447–461. [Google Scholar] [CrossRef]
- Yeom, J.; Ma, S.; Lim, Y.-H. Probiotic Propionibacterium freudenreichii MJ2 Enhances Osteoblast Differentiation and Mineralization by Increasing the OPG/RANKL Ratio. Microorganisms 2021, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.T.; Lee, S.H.; Jang, H.D.; Kim, Y.H. Anti-osteoporotic and antioxidant activities by rhizomes of Kaempferia parviflora Wall. ex Baker. Nat. Prod. Sci. 2016, 22, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yamashita, M.; Matsumoto, K.; Endo, T.; Ukibe, K.; Hosoya, T.; Matsubara, Y.; Nakagawa, H.; Sakai, F.; Miyazaki, T. Preventive effect of Lactobacillus helveticus SBT2171 on collagen-induced arthritis in mice. Front. Microbiol. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Collin-Osdoby, P.; Osdoby, P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Biol. 2012, 816, 187–202. [Google Scholar]
- Ljusberg, J.; Wang, Y.; Lång, P.; Norgård, M.; Dodds, R.; Hultenby, K.; Ek-Rylander, B.; Andersson, G. Proteolytic Excision of a Repressive Loop Domain in Tartrate-resistant Acid Phosphatase by Cathepsin K in Osteoclasts. J. Biol. Chem. 2005, 280, 28370–28381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuboi, H.; Matsui, Y.; Hayashida, K.; Yamane, S.; Maeda-Tanimura, M.; Nampei, A.; Hashimoto, J.; Suzuki, R.; Yoshikawa, H.; Ochi, T. Tartrate resistant acid phosphatase (TRAP) positive cells in rheumatoid synovium may induce the destruction of articular cartilage. Ann. Rheum. Dis. 2003, 62, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissers, Y.M.; Snel, J.; Zuurendonk, P.F.; Smit, B.A.; Wichers, H.J.; Savelkoul, H.F. Differential effects of Lactobacillus acidophilus and Lactobacillus plantarum strains on cytokine induction in human peripheral blood mononuclear cells. FEMS Immunol. Med. Microbiol. 2010, 59, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Azaïs-Braesco, V.; Bresson, J.; Guarner, F.; Corthier, G. Not all lactic acid bacteria are probiotics,… but some are. Br. J. Nutr. 2010, 103, 1079–1081. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Yang, B.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. The prophylactic effects of different Lactobacilli on collagen-induced arthritis in rats. Food Funct. 2020, 11, 3681–3694. [Google Scholar] [CrossRef]
- Pan, H.; Guo, R.; Ju, Y.; Wang, Q.; Zhu, J.; Xie, Y.; Zheng, Y.; Li, T.; Liu, Z.; Lu, L.; et al. A single bacterium restores the microbiome dysbiosis to protect bones from destruction in a rat model of rheumatoid arthritis. Microbiome 2019, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Zaiss, M.M.; Joyce Wu, H.-J.; Mauro, D.; Schett, G.; Ciccia, F. The gut-joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237. [Google Scholar] [CrossRef]
- Dürholz, K.; Hofmann, J.; Iljazovic, A.; Häger, J.; Lucas, S.; Sarter, K.; Strowig, T.; Bang, H.; Rech, J.; Schett, G.; et al. Dietary Short-Term Fiber Interventions in Arthritis Patients Increase Systemic SCFA Levels and Regulate Inflammation. Nutrients 2020, 12, 3207. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Yeom, J.; Lim, Y.-H. Dairy Propionibacterium freudenreichii ameliorates acute colitis by stimulating MUC2 expression in intestinal goblet cell in a DSS-induced colitis rat model. Sci. Rep. 2020, 10, 5523. [Google Scholar] [CrossRef]
- Burmester, G.R.; Feist, E.; Dörner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Koperski, K.; Schumacher, M.; Gelinsky, M. Relevance of osteoclast-specific enzyme activities in cell-based in vitro resorption assays. Eur. Cells Mater. 2017, 33, 28–42. [Google Scholar] [CrossRef]
- Quach, D.; Parameswaran, N.; McCabe, L.; Britton, R.A. Characterizing how probiotic Lactobacillus reuteri 6475 and lactobacillic acid mediate suppression of osteoclast differentiation. Bone Rep. 2019, 11, 100227. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Dar, H.Y.; Bhardwaj, A.; Pandey, A.; Kumari, S.; Azam, Z.; Upmanyu, V.; Anwar, A.; Shukla, P.; Mishra, P.K.; et al. Lactobacillus rhamnosus attenuates bone loss and maintains bone health by skewing Treg-Th17 cell balance in Ovx mice. Sci. Rep. 2021, 11, 1807. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-Mediated Regulation of Osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Pietrosimone, K.M.; Jin, M.; Poston, B.; Liu, P. Collagen-Induced Arthritis: A model for Murine Autoimmune Arthritis. Bio. Protoc. 2015, 5, e1626. [Google Scholar] [CrossRef]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Cho, M.-L.; Min, S.-Y.; Kim, H.-Y. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun. Rev. 2007, 7, 65–70. [Google Scholar] [CrossRef]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: A review. APLAR J. Rheumatol. 2007, 10, 270–274. [Google Scholar] [CrossRef]
- Gaffen, S.L. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 365–370. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| GAPDH | ACCCAGAAGACTGTGGATGG | CACATTGGGGGTAGGAACAC |
| RANK | TGCAGCTCAACAAGGATACG | GAGCTGCAGACCACATCTGA |
| NF-κB | TCCTGGCCTCTAGCCTTGTA | GCCAAGGAAGAAAAGTGCTG |
| c-fos | CCAGTCAAGAGCATCAGCAA | AAGTAGTGCAGCCCGGAGTA |
| NFATc1 | GGTGCTGTCTGGCCATAACT | GCGGAAAGGTGGTATCTCAA |
| MMP9 | GAAGGCAAACCCTGTGTGTT | AGAGTACTGCTTGCCCAGGA |
| Atp6v0d2 | GACCCTGTGGCACTTTTTGT | GCTTGCATTTGGGGAATCTA |
| Calcr | CGGACTTTGACACAGCAGAA | GTCACCCTCTGGCAGCTAAG |
| Ctsk | CAGCTTCCCCAAGATGTGAT | AGCACCAACGAGAGGAGAAA |
| OPG | CTGCCTGGGAAGAAGATCAG | TTGTGAAGCTGTGCAGGAAC |
| RANKL | AGCCGAGACTACGGCAAGTA | GCGCTCGAAAGTACAGGAAC |
| IL-17 | TGAGTCCAGGGAGAGCTTCA | TTCATTGCGGTGGAGAGTCC |
| Group | Treatment | CIA Induction |
|---|---|---|
| Normal control | PBS | − |
| Model | PBS | + |
| LLMJ2 | Low-dose live P. freudenreichii MJ2 (1 × 107 CFU/mL) | + |
| HLMJ2 | High-dose live P. freudenreichii MJ2 (1 × 108 CFU/mL) | + |
| LDMJ2 | Low-dose dead P. freudenreichii MJ2 (1 × 107 cells/mL) | + |
| HDMJ2 | High-dose dead P. freudenreichii MJ2 (1 × 108 cells/mL) | + |
| Score | Condition |
|---|---|
| 0 | No signs |
| 1 | Mild but definite redness and swelling of the ankle or wrist, or apparent redness and swelling limited to individual digits, regardless of the number of affected digits |
| 2 | Moderate redness and swelling of ankle or wrist |
| 3 | Severe redness and swelling of the entire paw, including digits |
| 4 | Maximally inflamed limb with involvement of multiple joints |
| Group | BS/TV (1/mm) | BMD (g/cm3) | BV/TV (%) | Tb.Th (mm) | Tb.N (1/mm) | Tb.sp (mm) |
|---|---|---|---|---|---|---|
| Control | 12.513 ± 0.343 ### | 0.293 ± 0.007 ### | 34.293 ± 0.823 ### | 0.111 ± 0.002 ### | 3.152 ± 0.077 ### | 0.184 ± 0.007 ### |
| Model | 4.992 ± 0.834 *** | 0.131 ± 0.007 *** | 8.092 ± 1.118 *** | 0.082 ± 0.002 *** | 0.976 ± 0.115 *** | 0.340 ± 0.022 *** |
| LLMJ2 | 7.356 ± 0.864 | 0.186 ± 0.016 # | 13.131 ± 1.185 | 0.105 ± 0.003 ## | 1.353 ± 0.162 | 0.262 ± 0.013 # |
| HLMJ2 | 6.369 ± 0.295 | 0.168 ± 0.009 | 13.235 ± 1.161 | 0.098 ± 0.007 # | 1.344 ± 0.050 | 0.275 ± 0.021 |
| LDMJ2 | 6.500 ± 0.336 | 0.197 ± 0.008 ## | 12.792 ± 1.166 | 0.110 ± 0.001 ### | 1.373 ± 0.110 | 0.312 ± 0.016 |
| HDMJ2 | 8.376 ± 0.956 # | 0.193 ± 0.015 ## | 16.418 ± 3.474 # | 0.113 ± 0.002 ### | 1.930 ± 0.308 ## | 0.244 ± 0.007 ## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeom, J.; Yim, D.J.; Ma, S.; Lim, Y.-H. Propionibacterium freudenreichii Inhibits RANKL-Induced Osteoclast Differentiation and Ameliorates Rheumatoid Arthritis in Collagen-Induced Arthritis Mice. Microorganisms 2022, 10, 48. https://doi.org/10.3390/microorganisms10010048
Yeom J, Yim DJ, Ma S, Lim Y-H. Propionibacterium freudenreichii Inhibits RANKL-Induced Osteoclast Differentiation and Ameliorates Rheumatoid Arthritis in Collagen-Induced Arthritis Mice. Microorganisms. 2022; 10(1):48. https://doi.org/10.3390/microorganisms10010048
Chicago/Turabian StyleYeom, Jiah, Dong Joon Yim, Seongho Ma, and Young-Hee Lim. 2022. "Propionibacterium freudenreichii Inhibits RANKL-Induced Osteoclast Differentiation and Ameliorates Rheumatoid Arthritis in Collagen-Induced Arthritis Mice" Microorganisms 10, no. 1: 48. https://doi.org/10.3390/microorganisms10010048
APA StyleYeom, J., Yim, D. J., Ma, S., & Lim, Y.-H. (2022). Propionibacterium freudenreichii Inhibits RANKL-Induced Osteoclast Differentiation and Ameliorates Rheumatoid Arthritis in Collagen-Induced Arthritis Mice. Microorganisms, 10(1), 48. https://doi.org/10.3390/microorganisms10010048







