Identification of the Bacterial Pathogens in Children with Otitis Media: A Study in the Northwestern Portuguese District of Braga
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Bacterial Culture
2.3. Specific Quantitative PCR of MEF Samples
3. Results
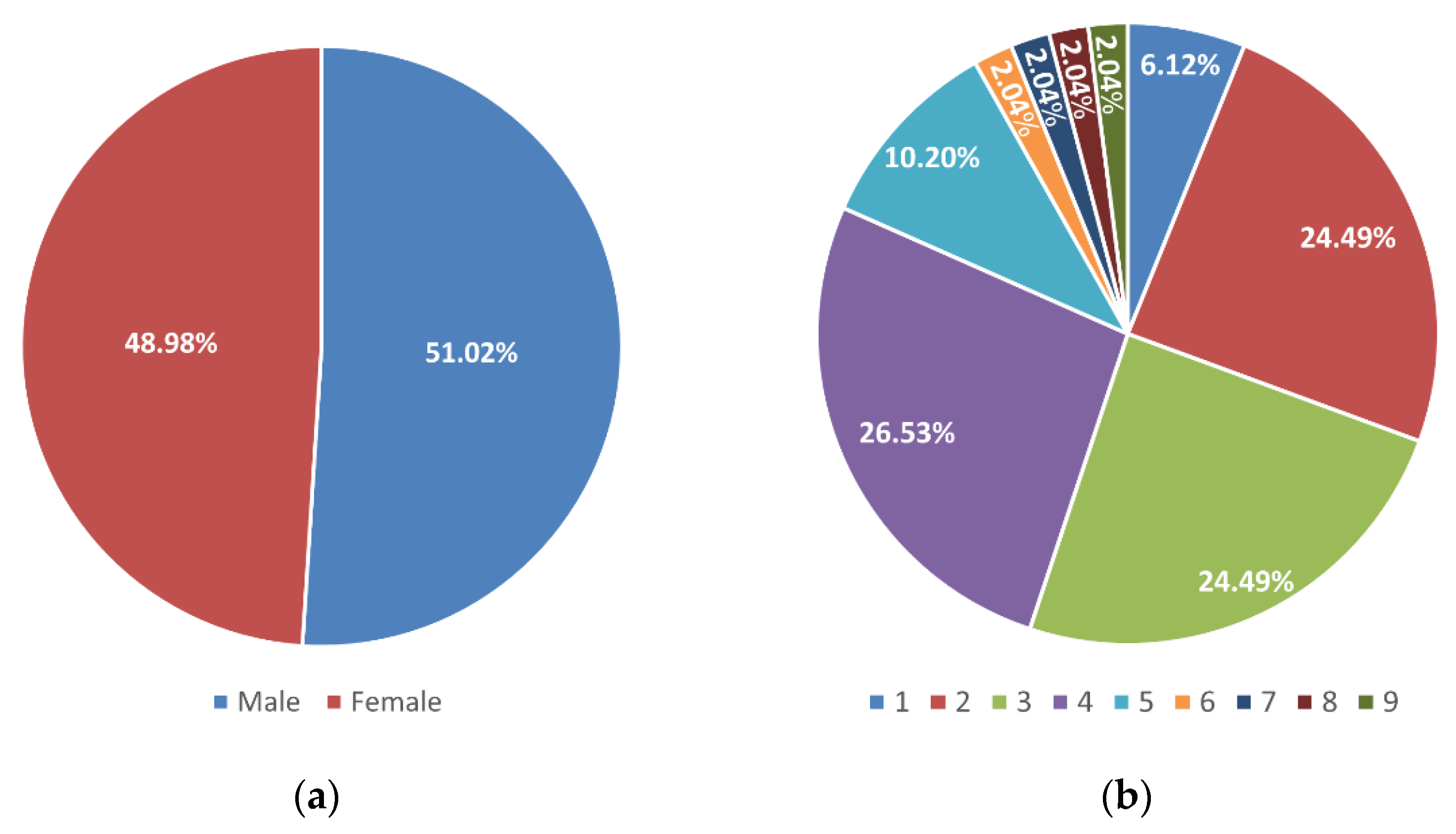
3.1. Study Population
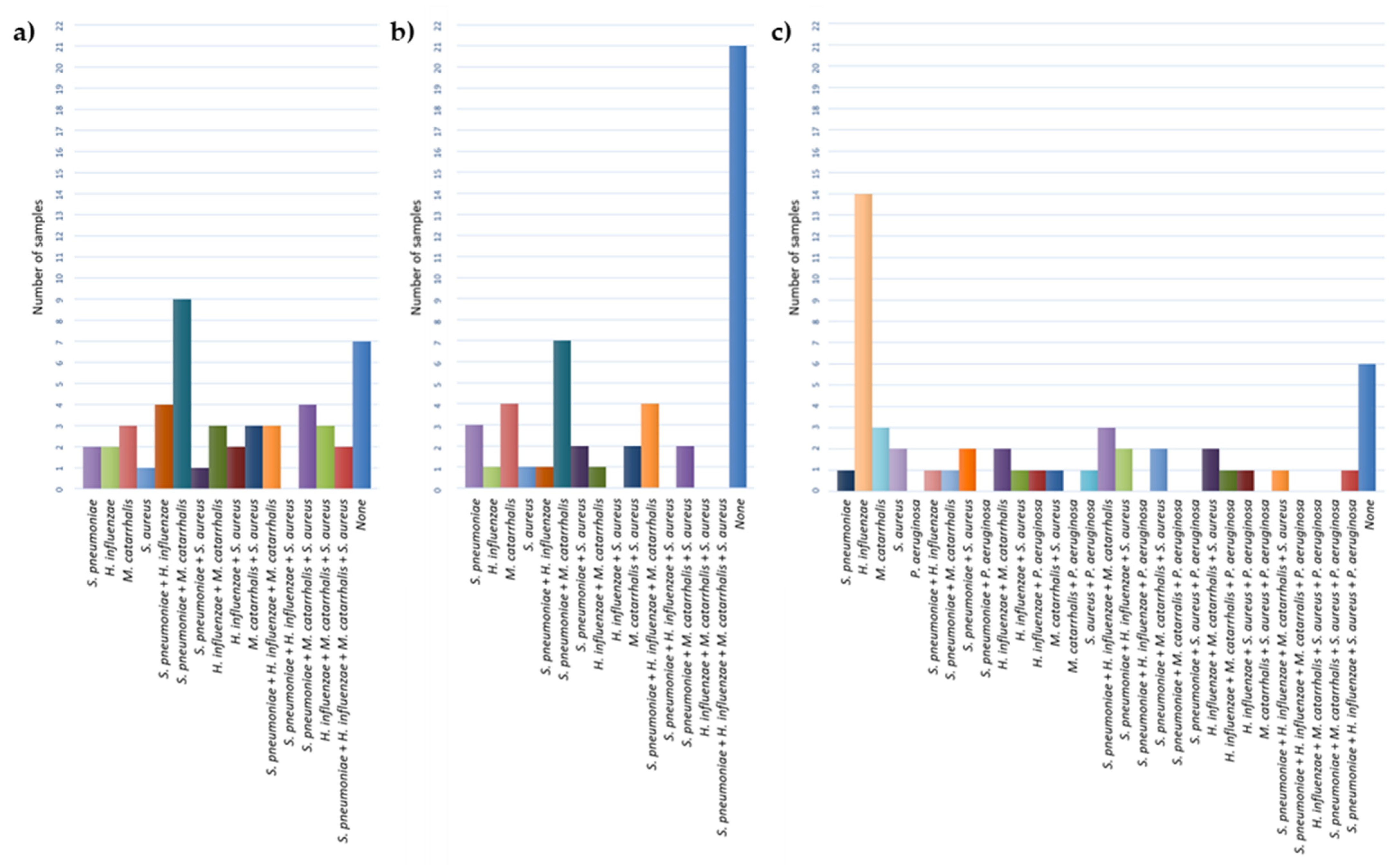
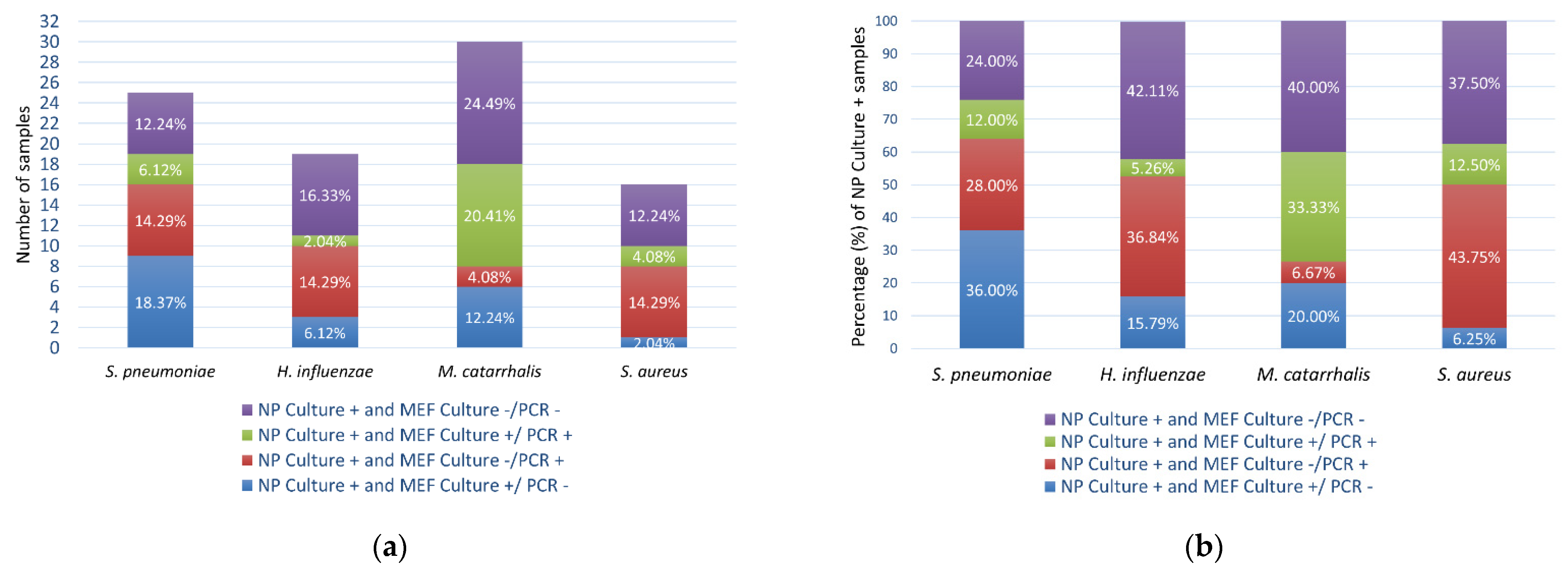
3.2. Identification of Bacterial Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, M.D.; Sillankorva, S. Otitis media pathogens–A life entrapped in biofilm communities. Crit. Rev. Microbiol. 2019, 45, 595–612. [Google Scholar] [CrossRef]
- Schilder, A.G.M.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis media. Nat. Rev. Dis. Prim. 2016, 2, 16063. [Google Scholar] [CrossRef] [PubMed]
- Speets, A.; Wolleswinkel, J.; Cardoso, C. Societal costs and burden of otitis media in Portugal. J. Multidiscip. Healthc. 2011, 4, 53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rovers, M.M. The burden of otitis media. Vaccine 2008, 26, G2–G4. [Google Scholar] [CrossRef]
- Stol, K.; Verhaegh, S.J.C.; Graamans, K.; Engel, J.A.M.; Sturm, P.D.J.; Melchers, W.J.G.; Meis, J.F.; Warris, A.; Hays, J.P.; Hermans, P.W.M. Microbial profiling does not differentiate between childhood recurrent acute otitis media and chronic otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Chonmaitree, T.; Jennings, K.; Golovko, G.; Khanipov, K.; Pimenova, M.; Patel, J.A.; McCormick, D.P.; Loeffelholz, M.J.; Fofanov, Y. Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media. PLoS ONE 2017, 12, e0180630. [Google Scholar] [CrossRef]
- Emaneini, M.; Gharibpour, F.; Khoramrooz, S.S.; Mirsalehian, A.; Jabalameli, F.; Darban-Sarokhalil, D.; Mirzaii, M.; Sharifi, A.; Taherikalani, M. Genetic similarity between adenoid tissue and middle ear fluid isolates of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis from Iranian children with otitis media with effusion. Int. J. Pediatric Otorhinolaryngol. 2013, 77, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.C.; Massa, H.M.; Thornton, R.B.; Cripps, A.W. Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PLoS ONE 2016, 11, e0150949. [Google Scholar] [CrossRef]
- Xu, Q.; Gill, S.; Xu, L.; Gonzalez, E.; Pichichero, M.E. Comparative Analysis of Microbiome in Nasopharynx and Middle Ear in Young Children With Acute Otitis Media. Front. Genet. 2019, 10, 1176. [Google Scholar] [CrossRef]
- Sillanpää, S.; Kramna, L.; Oikarinen, S.; Sipilä, M. Next-Generation Sequencing Combined with Specific PCR Assays To Determine the Bacterial 16S rRNA Gene Profiles of Middle Ear Fluid Collected from Children with Acute Otitis Media. mSphere 2017, 2, e00006-17. [Google Scholar] [CrossRef]
- Sillanpää, S.; Oikarinen, S.; Sipilä, M.; Kramna, L.; Rautiainen, M.; Huhtala, H.; Aittoniemi, J.; Laranne, J.; Hyöty, H.; Cinek, O. Moraxella catarrhalis Might Be More Common than Expected in Acute Otitis Media in Young Finnish Children. J. Clin. Microbiol. 2016, 54, 2373–2379. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Wabnitz, D.; Bardy, J.J.; Bassiouni, A.; Wormald, P.; Vreugde, S.; Psaltis, A.J. The Microbiome of Otitis Media With Effusion. Laryngoscope 2016, 126, 2844–2851. [Google Scholar] [CrossRef] [PubMed]
- Holder, R.C.; Kirse, D.J.; Evans, A.K.; Whigham, A.S.; Peters, T.R.; Poehling, K.A.; Swords, W.E.; Reid, S.D. Otopathogens detected in middle ear fluid obtained during tympanostomy tube insertion: Contrasting purulent and non-purulent effusions. PLoS ONE 2015, 10, e0128606. [Google Scholar] [CrossRef]
- Niedzielski, A.; Chmielik, L.P.; Stankiewicz, T. The Formation of Biofilm and Bacteriology in Otitis Media with Effusion in Children: A Prospective Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 3555. [Google Scholar] [CrossRef]
- Jervis-bardy, J.; Rogers, G.B.; Morris, P.S.; Smith-vaughan, H.C.; Nosworthy, E.; Leong, L.E.X.; Smith, R.J.; Weyrich, L.S.; De Haan, J.; Carney, A.S.; et al. The microbiome of otitis media with effusion in Indigenous Australian children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1548–1555. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; Kirkham, L.-A.S.; Corscadden, K.J.; Mowe, E.N.; Bowman, J.M.; Jacoby, P.; Francis, R.; Vijayasekaran, S.; Coates, H.L.; Riley, T.V.; et al. Predominance of nontypeable Haemophilus influenzae in children with otitis media following introduction of a 3 + 0 pneumococcal conjugate vaccine schedule. Vaccine 2011, 29, 5163–5170. [Google Scholar] [CrossRef]
- Hendolin, P.H.; Markkanen, A.; Ylikoski, J.; Wahlfors, J.J. Use of multiplex PCR for simultaneous detection of four bacterial species in middle ear effusions. J. Clin. Microbiol. 1997, 35, 2854–2858. [Google Scholar] [CrossRef]
- Hendolin, P.H.; Paulin, L.; Ylikoski, J.; Hendolin, P.H.; Paulin, L. Clinically Applicable Multiplex PCR for Four Middle Ear Pathogens. J. Clin. Microbiol. 2000, 38, 125–132. [Google Scholar] [CrossRef]
- Yatsyshina, S.; Mayanskiy, N.; Shipulina, O.; Kulichenko, T.; Alyabieva, N.; Katosova, L.; Lazareva, A.; Skachkova, T.; Elkina, M.; Matosova, S.; et al. Detection of respiratory pathogens in pediatric acute otitis media by PCR and comparison of fi ndings in the middle ear and nasopharynx. Diagn. Microbiol. Infect. Dis. 2016, 85, 125–130. [Google Scholar] [CrossRef]
- Rodrigues, F.; Morales-aza, B.; Turner, K.M.E.; Sikora, P.; Gould, K.; Hinds, J.; Finn, A. Multiple Streptococcus pneumoniae Serotypes in Aural Discharge Samples from Children with Acute Otitis Media with Spontaneous. J. Clin. Microbiol. 2013, 51, 3409–3411. [Google Scholar] [CrossRef]
- Dupont, D.; Mahjoub-Messai, F.; François, M.; Doit, C.; Mariani-Kurkdjian, P.; Bidet, P.; Bonacorsi, S.; Carol, A.; Bingen, E. Evolving microbiology of complicated acute otitis media before and after introduction of the pneumococcal conjugate vaccine in France. Diagn. Microbiol. Infect. Dis. 2010, 68, 89–92. [Google Scholar] [CrossRef]
- Stamboulidis, K.; Chatzaki, D.; Poulakou, G.; Ioannidou, S.; Lebessi, E.; Katsarolis, I.; Sypsa, V.; Tsakanikos, M.; Kafetzis, D.; Tsolia, M.N. The Impact of the Heptavalent Pneumococcal Conjugate Vaccine on the Epidemiology of Acute Otitis Media Complicated by Otorrhea. Pediatr. Infect. Dis. J. 2011, 30, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Grevers, G.; Wiedemann, S.; Bohn, J.-C.; Blasius, R.-W.; Harder, T.; Kroeniger, W.; Vetter, V.; Pirçon, J.-Y.; Marano, C. Identification and characterization of the bacterial etiology of clinically problematic acute otitis media after tympanocentesis or spontaneous otorrhea in German children. BMC Infect. Dis. 2012, 12, 312. [Google Scholar] [CrossRef]
- Pumarola, F.; Salamanca de la Cueva, I.; Sistiaga-Hernando, A.; García-Corbeira, P.; Moraga-Llop, F.A.; Cardelús, S.; McCoig, C.; Gómez Martínez, J.R.; Rosell Ferrer, R.; Iniesta Turpin, J.; et al. Etiología bacteriana de la otitis media aguda en España en la era de la vacuna neumocócica conjugada. An. Pediatr. 2016, 85, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Tin Tin Htar, M.; Christopoulou, D.; Schmitt, H.-J. Pneumococcal serotype evolution in Western Europe. BMC Infect. Dis. 2015, 15, 419. [Google Scholar] [CrossRef] [PubMed]
- Portuguese Directorate-General of Health. Programa Nacional de Vacinação. 2017. Available online: https://www.sns.gov.pt/wp-content/uploads/2016/06/programa_vacinacao_sns.pdf (accessed on 17 December 2021).
- European Centre for Disease Prevention and Control. Pneumococcal Disease: Recommended Vaccinations. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1 (accessed on 17 December 2021).
- Tendais-Almeida, M.; Ferreira-Magalhães, M.; Alves, I.; Tavares, M.; Azevedo, I. Vacinação Contra Infeções por Streptococcus pneumoniae em Crianças e Adolescentes de Alto Risco para Doença Invasiva Pneumocócica. Acta Med. Port. 2015, 28, 583. [Google Scholar] [CrossRef]
- Stewart, C. Share of Children Who Received the Pneumococcal Conjugate Vaccine* (PCV) in Selected European Countries in 2018. 2020. Available online: https://www.statista.com/statistics/1122694/pcv-immunization-in-europe/ (accessed on 17 December 2021).
- Pumarola, F.; Marès, J.; Losada, I.; Minguella, I.; Moraga, F.; Tarragó, D.; Aguilera, U.; Casanovas, J.M.; Gadea, G.; Trías, E.; et al. Microbiology of bacteria causing recurrent acute otitis media (AOM) and AOM treatment failure in young children in Spain: Shifting pathogens in the post-pneumococcal conjugate vaccination era. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Oliveira, R. Antibióticos em Portugal. 2016. Available online: https://www.infarmed.pt/documents/15786/2219894/Antibi%ff%ffticos%2bEspectro%2blargo%2be%2bestreito%2bem%2bambulat%ff%ffrio%2b%282014-2016%29/5817b82c-345c-4e7d-aea8-4b4072937a77 (accessed on 17 December 2021).
- Smith-Vaughan, H.C.; Binks, M.J.; Marsh, R.L.; Kaestli, M.; Ward, L.; Hare, K.M.; Pizzutto, S.J.; Thornton, R.B.; Morris, P.S.; Leach, A.J. Dominance of Haemophilus influenzae in ear discharge from Indigenous Australian children with acute otitis media with tympanic membrane perforation. BMC Ear Nose Throat Disord. 2013, 13, 12. [Google Scholar] [CrossRef]
- Ruohola, A.; Pettigrew, M.M.; Lindholm, L.; Jalava, J.; Räisänen, K.S.; Vainionpää, R.; Waris, M.; Tähtinen, P.A.; Laine, M.K.; Lahti, E.; et al. Bacterial and viral interactions within the nasopharynx contribute to the risk of acute otitis media. J. Infect. 2013, 66, 247–254. [Google Scholar] [CrossRef]
- Perez, A.C.; Pang, B.; King, L.B.; Tan, L.; Murrah, K.A.; Reimche, J.L.; Wren, J.T.; Richardson, S.H.; Ghandi, U.; Swords, W.E. Residence of Streptococcus pneumoniae and Moraxella catarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence In Vivo. Pathog. Dis. 2014, 70, 280–288. [Google Scholar] [CrossRef]
- Jörissen, J.; van den Broek, M.F.L.; De Boeck, I.; Van Beeck, W.; Wittouck, S.; Boudewyns, A.; Van de Heyning, P.; Topsakal, V.; Van Rompaey, V.; Wouters, I.; et al. Case-Control Microbiome Study of Chronic Otitis Media with Effusion in Children Points at Streptococcus salivarius as a Pathobiont-Inhibiting Species. mSystems 2021, 6, e00056-21. [Google Scholar] [CrossRef]
- Nogues, J.C.; Pérez-Losada, M.; Preciado, D. Review of otitis media microbiome studies: What do they tell us? Laryngoscope Investig. Otolaryngol. 2020, 5, 936–940. [Google Scholar] [CrossRef]
- Shishegar, M.; Faramarzi, A.; Kazemi, T.; Bayat, A.; Motamedifar, M. Polymerase chain reaction, bacteriologic detection and antibiogram of bacteria isolated from otitis media with effusion in children, Shiraz, Iran. Iran. J. Med. Sci. 2011, 36, 273–280. [Google Scholar] [PubMed]
- Khoramrooz, S.S.; Mirsalehian, A.; Emaneini, M.; Jabalameli, F.; Aligholi, M.; Saedi, B.; Bazargani, A.; Taherikalani, M.; Borghaei, P.; Razmpa, E. Frequency of Alloicoccus otitidis, Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae in children with otitis media with effusion (OME) in Iranian patients. Auris Nasus Larynx 2012, 39, 369–373. [Google Scholar] [CrossRef]
- Mills, N.; Best, E.J.; Murdoch, D.; Souter, M.; Neeff, M.; Anderson, T.; Salkeld, L.; Ahmad, Z.; Mahadevan, M.; Barber, C.; et al. What is behind the ear drum? The microbiology of otitis media and the nasopharyngeal flora in children in the era of pneumococcal vaccination. J. Paediatr. Child Health 2015, 51, 300–306. [Google Scholar] [CrossRef]
- Ueyama, T.; Kurono, Y.; Shirabe, K.; Takeshita, M.; Mogi, G. High incidence of Haemophilus influenzae in nasopharyngeal secretions and middle ear effusions as detected by PCR. J. Clin. Microbiol. 1995, 33, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Post, J.C.; Aul, J.J.; White, G.J.; Wadowsky, R.M.; Zavoral, T.; Tabari, R.; Kerber, B.; Doyle, W.J.; Ehrlich, G.D. PCR-based detection of bacterial DNA after antimicrobial treatment is indicative of persistent, viable bacteria in the chinchilla model of otitis media. Am. J. Otolaryngol. 1996, 17, 106–111. [Google Scholar] [CrossRef]
- Post, J.C. Direct Evidence of Bacterial Biofilms in Otitis Media. Laryngoscope 2001, 111, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202. [Google Scholar] [CrossRef]
- Dagan, R.; Pelton, S.; Bakaletz, L.; Cohen, R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect. Dis. 2016, 16, 480–492. [Google Scholar] [CrossRef]
- Chan, C.L.; Wabnitz, D.; Bassiouni, A.; Wormald, P.J.; Vreugde, S.; Psaltis, A.J. Identification of the bacterial reservoirs for the middle ear using phylogenic analysis. JAMA Otolaryngol.-Head Neck Surg. 2017, 143, 155–161. [Google Scholar] [CrossRef]
- Johnston, J.; Hoggard, M.; Biswas, K.; Astudillo-García, C.; Radcliff, F.J.; Mahadevan, M.; Douglas, R.G. Pathogen reservoir hypothesis investigated by analyses of the adenotonsillar and middle ear microbiota. Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Harimaya, A.; Takada, R.; Hendolin, P.H.; Fujii, N.; Ylikoski, J.; Himi, T. High Incidence of Alloiococcus otitidis in Children with Otitis Media, Despite Treatment with Antibiotics. J. Clin. Microbiol. 2006, 44, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Korona-Glowniak, I.; Wisniewska, A.; Juda, M.; Kielbik, K.; Niedzielska, G.; Malm, A. Bacterial aetiology of chronic otitis media with effusion in children-risk factors. J. Otolaryngol.-Head Neck Surg. 2020, 49, 24. [Google Scholar] [CrossRef] [PubMed]

| Species | Strain | Origin or Source | Growth Conditions | Culture Media |
|---|---|---|---|---|
| S. pneumoniae | R6st | Félix d’Hérelle Reference Center for Bacterial Viruses | 37 °C, 5% CO2 | Todd Hewitt Broth + 2% (w/v) yeast extract |
| H. influenzae | C894248 | Sputum, Hospital de Braga | 37 °C, 5% CO2 | Brain Heart Infusion Broth + 10 µg/mL NAD + 10 µg/mL Hemin |
| M. catarrhalis | U225012 | Ocular, Hospital de Braga | 37 °C, 5% CO2 | Brain Heart Infusion Broth |
| S. aureus | ATCC 6358 | Human lesion, American Type Culture Collection | 37 °C | Tryptic Soy Broth |
| P. aeruginosa | PAO1 (DSM 22644) | Infected wound, DSMZ—German Collection of Microorganisms and Cell Cultures GmbH | 37 °C | Tryptic Soy Broth |
| Target | Primer Name | Primer Sequence (5′ → 3′) | Product Size |
|---|---|---|---|
| S. pneumoniae, oralis, mitis and infantis 16S rRNA gene | Spomi_FW | AAGGTGCACTTGCATCACTACC | 484 |
| Common_RV | CTACGCATTTCACCGCTACAC | ||
| H. influenzae 16S rRNA gene | Hi_FW | CGTATTATCGGAAGATGAAAGTGC | 525 |
| Common_RV | CTACGCATTTCACCGCTACAC | ||
| M. catarrhalis 16S rRNA gene | Mc_FW | CCCATAAGCCCTGACGTTAC | 237 |
| Common_RV | CTACGCATTTCACCGCTACAC | ||
| S. pneumoniae lytA gene | Sp_FW | ACGCAATCTAGCAGATGAAGCA | 76 |
| Sp_RV | TCGTGCGTTTTAATTCCAGCT | ||
| S. aureus nucA gene | Sa_FW | CATCCTAAAAAAGGTGTAGAGA | 85 |
| Sa_RV | TTCAATTTTMTTTGCATTTTCTACCA | ||
| P. aeruginosa oprL gene | Pa_FW | GGGTTCATTAGGAGTTACATGA | 544 |
| Pa_RV | GGGCATAACGACTTCTTACTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.D.; Lima, A.; Marçal, N.; Dias, L.; Gama, M.; Sillankorva, S. Identification of the Bacterial Pathogens in Children with Otitis Media: A Study in the Northwestern Portuguese District of Braga. Microorganisms 2022, 10, 54. https://doi.org/10.3390/microorganisms10010054
Silva MD, Lima A, Marçal N, Dias L, Gama M, Sillankorva S. Identification of the Bacterial Pathogens in Children with Otitis Media: A Study in the Northwestern Portuguese District of Braga. Microorganisms. 2022; 10(1):54. https://doi.org/10.3390/microorganisms10010054
Chicago/Turabian StyleSilva, Maria Daniela, António Lima, Nuno Marçal, Luís Dias, Miguel Gama, and Sanna Sillankorva. 2022. "Identification of the Bacterial Pathogens in Children with Otitis Media: A Study in the Northwestern Portuguese District of Braga" Microorganisms 10, no. 1: 54. https://doi.org/10.3390/microorganisms10010054
APA StyleSilva, M. D., Lima, A., Marçal, N., Dias, L., Gama, M., & Sillankorva, S. (2022). Identification of the Bacterial Pathogens in Children with Otitis Media: A Study in the Northwestern Portuguese District of Braga. Microorganisms, 10(1), 54. https://doi.org/10.3390/microorganisms10010054









