New Biocalcifying Marine Bacterial Strains Isolated from Calcareous Deposits and Immediate Surroundings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
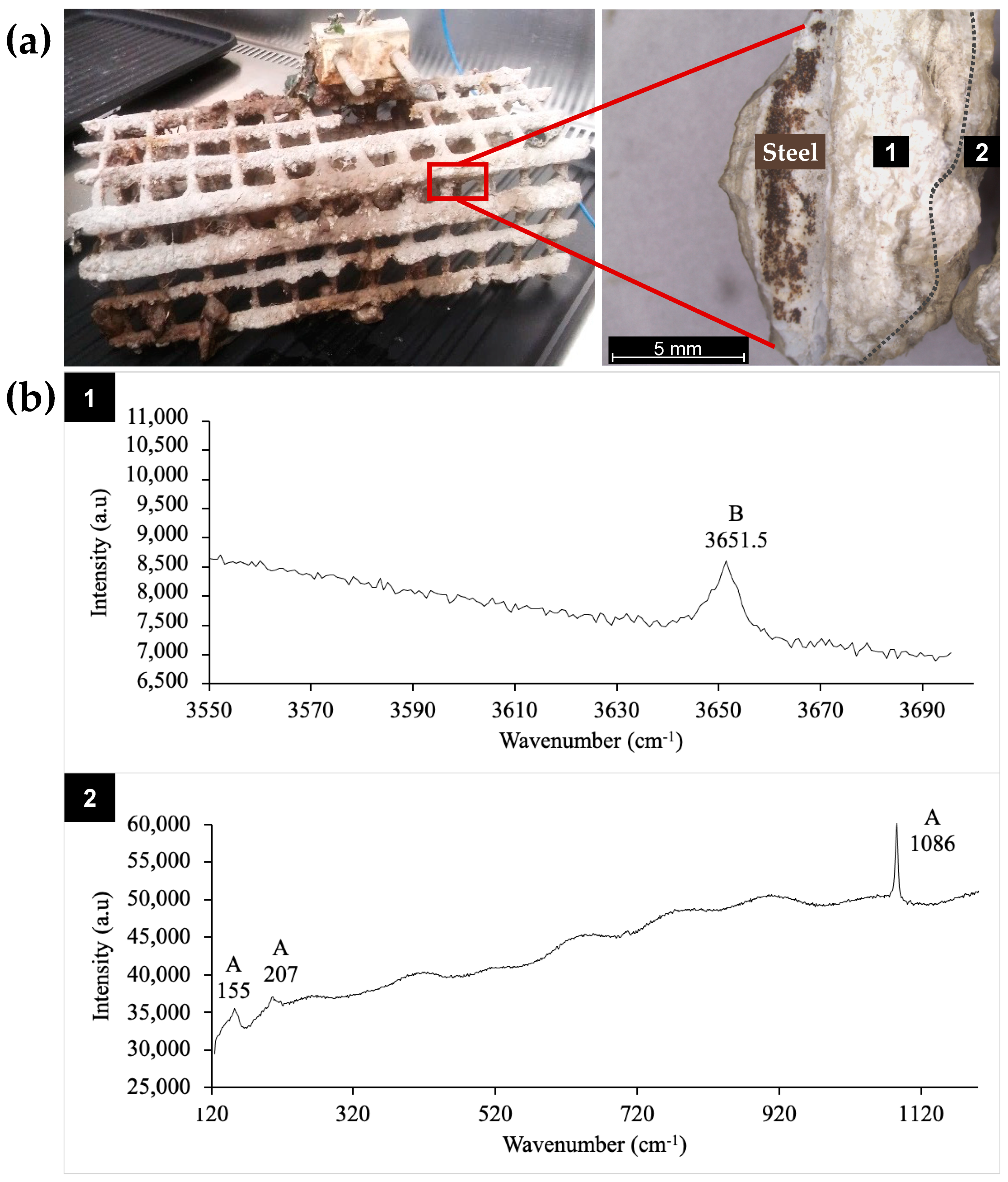
2.2. Sampling and Isolation of Biocalcifying Bacterial Strains
2.3. DNA Extraction, 16S rRNA Gene Amplification (PCR) and Sequencing, and Bacterial Strain Identification
2.4. Microbial Growth Kinetics and Calcium Carbonate Production
2.4.1. Culture Conditions
2.4.2. Collection of Calcium Carbonate Precipitates
2.4.3. Thermogravimetric Analysis
2.5. Morphological and Metabolic Characterisation of Bacteria
2.5.1. Morphological Characterisation
2.5.2. Urease Activity Assay
2.5.3. Carbonic Anhydrase Activity Assay
2.5.4. Inhibition of Carbonic Anhydrase Activity
2.6. Crystal Morphology and Identification
2.6.1. Binocular Magnifier
2.6.2. Fluorescence Microscopy
2.6.3. Raman Spectroscopy
2.7. Statistical Analyses
3. Results and Discussion
3.1. Biocalcifying Marine Bacteria
3.2. Phylogenetic Bacterial Identification
3.3. Metabolic Characterisation of the Biocalcifying Bacteria
3.4. Analysis of Biocalcification Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danjo, T.; Kawasaki, S. Formation Mechanisms of Beachrocks in Okinawa and Ishikawa, Japan, with a Focus on Cements. Mater. Trans. 2014, 55, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.P. Stromatolites. In Encyclopedia of Modern Coral Reefs: Structure, Form and Process; Hopley, D., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 1045–1051. ISBN 978-90-481-2639-2. [Google Scholar]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Zhao, H.; Tucker, M.E.; Zhao, Y.; Wu, G.; Zhou, J.; Yin, J.; Zhang, H.; Zhang, X.; et al. Mechanism of Biomineralization Induced by Bacillus subtilis J2 and Characteristics of the Biominerals. Minerals 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-Carbonates Precipitation and Limestone Genesis—The Microbiogeologist Point of View. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, A.D. Review on Microbial Induced Calcite Precipitation Mechanisms Leading to Bacterial Selection for Microbial Concrete. Constr. Build. Mater. 2019, 225, 67–75. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of Carbonate Precipitation in Modern Microbial Mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Lactose Mother Liquor as an Alternative Nutrient Source for Microbial Concrete Production by Sporosarcina pasteurii. J. Ind. Microbiol. Biotechnol. 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Original Research: Biocalcification by Sporosarcina pasteurii Using Corn Steep Liquor as the Nutrient Source. Ind. Biotechnol. 2010, 6, 170–174. [Google Scholar] [CrossRef]
- Wang, J.Y.; Soens, H.; Verstraete, W.; De Belie, N. Self-Healing Concrete by Use of Microencapsulated Bacterial Spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Characterization of Urease and Carbonic Anhydrase Producing Bacteria and Their Role in Calcite Precipitation. Curr. Microbiol. 2011, 62, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Urease-Aided Calcium Carbonate Mineralization for Engineering Applications: A Review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef]
- Bachmeier, K.L.; Williams, A.E.; Warmington, J.R.; Bang, S.S. Urease Activity in Microbiologically-Induced Calcite Precipitation. J. Biotechnol. 2002, 93, 171–181. [Google Scholar] [CrossRef]
- Konstantinou, C.; Wang, Y.; Biscontin, G.; Soga, K. The Role of Bacterial Urease Activity on the Uniformity of Carbonate Precipitation Profiles of Bio-Treated Coarse Sand Specimens. Sci. Rep. 2021, 11, 6161. [Google Scholar] [CrossRef]
- Henze, J.; Randall, D.G. Microbial Induced Calcium Carbonate Precipitation at Elevated pH Values (>11) Using Sporosarcina pasteurii. J. Environ. Chem. Eng. 2018, 6, 5008–5013. [Google Scholar] [CrossRef]
- Bhaduri, S.; Debnath, N.; Mitra, S.; Liu, Y.; Kumar, A. Microbiologically Induced Calcite Precipitation Mediated by Sporosarcina pasteurii. J. Vis. Exp. 2016, e53253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of Bacteria to Repair Cracks in Concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Dick, J.; De Windt, W.; De Graef, B.; Saveyn, H.; Van der Meeren, P.; De Belie, N.; Verstraete, W. Bio-Deposition of a Calcium Carbonate Layer on Degraded Limestone by Bacillus Species. Biodegradation 2006, 17, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Synergistic Role of Bacterial Urease and Carbonic Anhydrase in Carbonate Mineralization. Appl. Biochem. Biotechnol. 2014, 172, 2552–2561. [Google Scholar] [CrossRef]
- Park, I.; Hausinger, R. Requirement of Carbon Dioxide for in Vitro Assembly of the Urease Nickel Metallocenter. Science 1995, 267, 1156–1158. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. Prokaryotic Carbonic Anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef]
- Zheng, T.; Qian, C. Influencing Factors and Formation Mechanism of CaCO3 Precipitation Induced by Microbial Carbonic Anhydrase. Process Biochem. 2020, 91, 271–281. [Google Scholar] [CrossRef]
- Lee, S.-W.; Park, S.-B.; Jeong, S.-K.; Lim, K.-S.; Lee, S.-H.; Trachtenberg, M.C. On Carbon Dioxide Storage Based on Biomineralization Strategies. Micron 2010, 41, 273–282. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, H.; Tucker, M.E.; Zhou, J.; Jiang, M.; Wang, Y.; Zhao, Y.; Sun, B.; Han, Z.; Yan, H. Biomineralization of Monohydrocalcite Induced by the Halophile Halomonas smyrnensis WMS-3. Minerals 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Carré, C.; Zanibellato, A.; Achgare, N.; Mahieux, P.-Y.; Turcry, P.; Jeannin, M.; Sabot, R. Electrochemical Limestone Synthesis in Seawater Binds Metal Grids and Sediments for Coastal Protection. Environ. Chem. Lett. 2020, 18, 1685–1692. [Google Scholar] [CrossRef]
- Zanibellato, A. Synthèse Et Etudes Physico-Chimiques D’un Agglomérat Calcomagnésien Formé Sur Acier En Milieu Marin: Un Eco-Matériau Pour La Protection Du Littoral. Ph.D. Thesis, Université de La Rochelle, La Rochelle, France, 2016. [Google Scholar]
- Carré, C.; Zanibellato, A.; Jeannin, M.; Sabot, R.; Gunkel-Grillon, P.; Serres, A. Electrochemical Calcareous Deposition in Seawater. A Review. Environ. Chem. Lett. 2020, 18, 1193–1208. [Google Scholar] [CrossRef]
- Vincent, J.; Sabot, R.; Lanneluc, I.; Refait, P.; Turcry, P.; Mahieux, P.Y.; Jeannin, M.; Sablé, S. Biomineralization of calcium carbonate by marine bacterial strains isolated from calcareous deposits. Matériaux Tech. 2020, 108, 302. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Uad, I.; Gonzalez-Martinez, A.; Rivadeneyra, A.; Gonzalez-Lopez, J.; Rivadeneyra, M.A. Bioprecipitation of Calcium Carbonate Crystals by Bacteria Isolated from Saline Environments Grown in Culture Media Amended with Seawater and Real Brine. BioMed Res. Int. 2015, 2015, 816102. [Google Scholar] [CrossRef] [Green Version]
- Krause, S.; Liebetrau, V.; Löscher, C.R.; Böhm, F.; Gorb, S.; Eisenhauer, A.; Treude, T. Marine Ammonification and Carbonic Anhydrase Activity Induce Rapid Calcium Carbonate Precipitation. Geochim. Cosmochim. Acta 2018, 243, 116–132. [Google Scholar] [CrossRef]
- Lanneluc, I.; Langumier, M.; Sabot, R.; Jeannin, M.; Refait, P.; Sablé, S. On the Bacterial Communities Associated with the Corrosion Product Layer during the Early Stages of Marine Corrosion of Carbon Steel. Int. Biodeterior. Biodegrad. 2015, 99, 55–65. [Google Scholar] [CrossRef]
- Armstrong, J.M.; Myers, D.V.; Verpoorte, J.A.; Edsall, J.T. Purification and Properties of Human Erythrocyte Carbonic Anhydrases. J. Biol. Chem. 1966, 241, 5137–5149. [Google Scholar] [CrossRef]
- Pascale, F.; Tosoni, S.; Zicovich-Wilson, C.; Ugliengo, P.; Orlando, R.; Dovesi, R. Vibrational spectrum of brucite, Mg(OH)2: A periodic ab initio quantum mechanical calculation including OH anharmonicity. Chem. Phys. Lett. 2004, 396, 308–315. [Google Scholar] [CrossRef]
- Tomić, Z.; Makreski, P.; Gajić, B. Identification and Spectra-Structure Determination of Soil Minerals: Raman Study Supported by IR Spectroscopy and X-Ray Powder Diffraction. J. Raman Spectrosc. 2009, 41, 582–586. [Google Scholar] [CrossRef]
- Barchiche, C.; Deslouis, C.; Gil, O.; Refait, P.; Tribollet, B. Characterisation of Calcareous Deposits by Electrochemical Methods: Role of Sulphates, Calcium Concentration and Temperature. Electrochim. Acta 2004, 49, 2833–2839. [Google Scholar] [CrossRef]
- Lee, R.U.; Ambrose, J.R. Influence of Cathodic Protection Parameters on Calcareous Deposit Formation. Corrosion 1988, 44, 887–891. [Google Scholar] [CrossRef]
- Neville, A.; Morizot, A.P. Calcareous Scales Formed by Cathodic Protection—An Assessment of Characteristics and Kinetics. J. Cryst. Growth 2002, 243, 490–502. [Google Scholar] [CrossRef]
- Edyvean, R.G.J.; Maines, A.D.; Hutchinson, C.J.; Silk, N.J.; Evans, L.V. Interactions between Cathodic Protection and Bacterial Settlement on Steel in Seawater. Int. Biodeterior. Biodegrad. 1992, 29, 251–271. [Google Scholar] [CrossRef]
- Dexter, S.C.; Lin, S.-H. Effect of Marine Biofilms on Cathodic Protection. Int. Biodeterior. Biodegrad. 1992, 29, 231–249. [Google Scholar] [CrossRef]
- Faimali, M.; Chelossi, E.; Garaventa, F.; Corrà, C.; Greco, G.; Mollica, A. Evolution of Oxygen Reduction Current and Biofilm on Stainless Steels Cathodically Polarised in Natural Aerated Seawater. Electrochim. Acta 2008, 54, 148–153. [Google Scholar] [CrossRef]
- Permeh, S.; Lau, K.; Tansel, B.; Duncan, M. Surface Conditions for Microcosm Development and Proliferation of SRB on Steel with Cathodic Corrosion Protection. Constr. Build. Mater. 2020, 243, 118209. [Google Scholar] [CrossRef]
- Sarayu, K.; Iyer, N.R.; Murthy, A.R. Exploration on the Biotechnological Aspect of the Ureolytic Bacteria for the Production of the Cementitious Materials—A Review. Appl. Biochem. Biotechnol. 2014, 172, 2308–2323. [Google Scholar] [CrossRef]
- Ben Omar, N.; Arias, J.M.; González-Muñoz, M.T. Extracellular Bacterial Mineralization within the Context of Geomicrobiology. Microbiologia 1997, 13, 161–172. [Google Scholar] [PubMed]
- Buczynski, C.; Chafetz, H.S. Habit of Bacterially Induced Precipitates of Calcium Carbonate and the Influence of Medium Viscosity on Mineralogy. J. Sediment. Res. 1991, 61, 226–233. [Google Scholar] [CrossRef]
- Zhuang, D.; Yan, H.; Tucker, M.E.; Zhao, H.; Han, Z.; Zhao, Y.; Sun, B.; Li, D.; Pan, J.; Zhao, Y.; et al. Calcite Precipitation Induced by Bacillus Cereus MRR2 Cultured at Different Ca2+ Concentrations: Further Insights into Biotic and Abiotic Calcite. Chem. Geol. 2018, 500, 64–87. [Google Scholar] [CrossRef]
- Arias, D.; Cisternas, L.A.; Miranda, C.; Rivas, M. Bioprospecting of Ureolytic Bacteria From Laguna Salada for Biomineralization Applications. Front. Bioeng. Biotechnol. 2019, 6, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, S.; Beveridge, T.J. Mineral Formation by Bacteria in Natural Microbial Communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Wright, D.T.; Oren, A. Nonphotosynthetic Bacteria and the Formation of Carbonates and Evaporites Through Time. Geomicrobiol. J. 2005, 22, 27–53. [Google Scholar] [CrossRef]
- Zamarreño, D.V.; Inkpen, R.; May, E. Carbonate Crystals Precipitated by Freshwater Bacteria and Their Use as a Limestone Consolidant. Appl. Environ. Microbiol. 2009, 75, 5981–5990. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Cui, H.; Jiang, Z.; Liu, H.; He, H.; Fang, N. Biomineralization Processes of Calcite Induced by Bacteria Isolated from Marine Sediments. Braz. J. Microbiol. 2015, 46, 455–464. [Google Scholar] [CrossRef]
- Gauthier, G.; Gauthier, M.; Christen, R. Phylogenetic Analysis of the Genera Alteromonas, Shewanella, and Moritella Using Genes Coding for Small-Subunit RRNA Sequences and Division of the Genus Alteromonas into Two Genera, Alteromonas (Emended) and Pseudoalteromonas Gen. Nov., and Proposal of Twelve New Species Combinations. Int. J. Syst. Bacteriol. 1995, 45, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J. Bioactive Compound Synthetic Capacity and Ecological Significance of Marine Bacterial Genus Pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar] [CrossRef]
- Holmström, C.; Kjelleberg, S. Marine Pseudoalteromonas Species Are Associated with Higher Organisms and Produce Biologically Active Extracellular Agents. FEMS Microbiol. Ecol. 1999, 30, 285–293. [Google Scholar] [CrossRef]
- Torres-Crespo, N.; Martínez-Ruiz, F.; González-Muñoz, M.T.; Bedmar, E.J.; De Lange, G.J.; Jroundi, F. Role of Bacteria in Marine Barite Precipitation: A Case Study Using Mediterranean Seawater. Sci. Total Environ. 2015, 512–513, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Doghri, I.; Portier, E.; Desriac, F.; Zhao, J.M.; Bazire, A.; Dufour, A.; Rochette, V.; Sablé, S.; Lanneluc, I. Anti-Biofilm Activity of a Low Weight Proteinaceous Molecule from the Marine Bacterium Pseudoalteromonas Sp. IIIA004 against Marine Bacteria and Human Pathogen Biofilms. Microorganisms 2020, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, F.; Brunet, C.; van Zyl, L.J.; Trindade, M.; Golyshin, P.N.; Dell’Anno, A.; Ianora, A.; Sansone, C. Degradation of Hydrocarbons and Heavy Metal Reduction by Marine Bacteria in Highly Contaminated Sediments. Microorganisms 2020, 8, 1402. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Guo, Z.; Zeng, Z.; Guo, N.; Lei, Y.; Liu, T.; Sun, S.; Chang, X.; Yin, Y.; Wang, X. Marine Bacteria Provide Lasting Anticorrosion Activity for Steel via Biofilm-Induced Mineralization. ACS Appl. Mater. Interfaces 2018, 10, 40317–40327. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wang, Y.; Hui, X.; Zhao, Q.; Zeng, Z.; Pan, S.; Guo, Z.; Yin, Y.; Liu, T. Marine Bacteria Inhibit Corrosion of Steel via Synergistic Biomineralization. J. Mater. Sci. Technol. 2021, 66, 82–90. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Q.; Shao, Z. Genome-Based Analysis Reveals the Taxonomy and Diversity of the Family Idiomarinaceae. Front. Microbiol. 2018, 9, 2453. [Google Scholar] [CrossRef]
- González-Muñoz, M.T.; De Linares, C.; Martínez-Ruiz, F.; Morcillo, F.; Martín-Ramos, D.; Arias, J.M. Ca–Mg Kutnahorite and Struvite Production by Idiomarina Strains at Modern Seawater Salinities. Chemosphere 2008, 72, 465–472. [Google Scholar] [CrossRef]
- Wirth, J.S.; Whitman, W.B. Phylogenomic Analyses of a Clade within the Roseobacter Group Suggest Taxonomic Reassignments of Species of the Genera Aestuariivita, Citreicella, Loktanella, Nautella, Pelagibaca, Ruegeria, Thalassobius, Thiobacimonas and Tropicibacter, and the Proposal of Six Novel Genera. Int. J. Syst. Evol. Microbiol. 2018, 68, 2393–2411. [Google Scholar] [CrossRef]
- Brinkhoff, T.; Giebel, H.A.; Simon, M. Diversity, ecology, and genomics of the Roseobacter clade: A short overview. Arch. Microbiol. 2008, 189, 531–539. [Google Scholar] [CrossRef]
- Castanier, S.; Métayer-Levrel, G.L.; Perthuisot, J.-P. Bacterial Roles in the Precipitation of Carbonate Minerals. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 32–39. ISBN 978-3-662-04036-2. [Google Scholar]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wang, M.; Wang, H.; Tang, D.; Huang, J.; Sun, Y. Study on the Remediation of Cd Pollution by the Biomineralization of Urease-Producing Bacteria. Int. J. Environ. Res. Public Health 2019, 16, 268. [Google Scholar] [CrossRef] [Green Version]
- Priya, J.N.; Nan, M.K. Effect of Carbonic Anhydrase and Urease on Bacterial Calcium Carbonate Precipitation. Int. J. Pharma Bio Sci. 2017, 8, 609–614. [Google Scholar] [CrossRef]
- Effendi, S.S.W.; Ng, I.-S. The Prospective and Potential of Carbonic Anhydrase for Carbon Dioxide Sequestration: A Critical Review. Process Biochem. 2019, 87, 55–65. [Google Scholar] [CrossRef]
- Smith, K.S.; Jakubzick, C.; Whittam, T.S.; Ferry, J.G. Carbonic Anhydrase Is an Ancient Enzyme Widespread in Prokaryotes. Proc. Natl. Acad. Sci. USA 1999, 96, 15184–15189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, C.; Supuran, C.T. An Overview of the Alpha-, Beta- and Gamma-Carbonic Anhydrases from Bacteria: Can Bacterial Carbonic Anhydrases Shed New Light on Evolution of Bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Bond, G.M.; Stringer, J.; Brandvold, D.K.; Simsek, F.A.; Medina, M.-G.; Egeland, G. Development of Integrated System for Biomimetic CO2 Sequestration Using the Enzyme Carbonic Anhydrase. Energy Fuels 2001, 15, 309–316. [Google Scholar] [CrossRef]
- Jo, B.H.; Im, S.-K.; Cha, H.J. Halotolerant Carbonic Anhydrase with Unusual N-Terminal Extension from Marine Hydrogenovibrio marinus as Novel Biocatalyst for Carbon Sequestration under High-Salt Environments. J. CO2 Util. 2018, 26, 415–424. [Google Scholar] [CrossRef]
- Ghelani, A.D.; Bhagat, C.B.; Dudhagara, P.R.; Gondalia, S.V.; Patel, R.K. Biomimetic Sequestration of CO2 Using Carbonic Anhydrase from Calcite Encrust Forming Marine Actinomycetes. Sci. Int. 2015, 3, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Favre, N.; Christ, M.L.; Pierre, A.C. Biocatalytic Capture of CO2 with Carbonic Anhydrase and Its Transformation to Solid Carbonate. J. Mol. Catal. B Enzym. 2009, 60, 163–170. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon Sequestration via Carbonic Anhydrase Facilitated Magnesium Carbonate Precipitation. Int. J. Greenh. Gas Control 2013, 16, 145–155. [Google Scholar] [CrossRef]
- Sundaram, S.; Thakur, I.S. Induction of Calcite Precipitation through Heightened Production of Extracellular Carbonic Anhydrase by CO2 Sequestering Bacteria. Bioresour. Technol. 2018, 253, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Di Fonzo, P.; Carginale, V.; Donald, W.A.; Supuran, C.T.; Capasso, C. Comparison of the Sulfonamide Inhibition Profiles of the β- and γ-Carbonic Anhydrases from the Pathogenic Bacterium Burkholderia pseudomallei. Molecules 2017, 22, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, J.G. The γ Class of Carbonic Anhydrases. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 374–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, K.; Nishida, H.; Beppu, T. Dispensabilities of Carbonic Anhydrase in Proteobacteria. Int. J. Evol. Biol. 2012, 2012, 324549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, S.; Innocenti, A.; Casini, A.; Ferry, J.G.; Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Inhibition of the Prokariotic Beta and Gamma-Class Enzymes from Archaea with Sulfonamides. Bioorg. Med. Chem. Lett. 2004, 14, 6001–6006. [Google Scholar] [CrossRef]
- Bontognali, T.; Vasconcelos, C.; Warthmann, R.; Dupraz, C.; Bernasconi, S.; McKenzie, J. Microbes Produce Nanobacteria-like Structures, Avoiding Cell Entombment. Geology 2008, 36, 663–666. [Google Scholar] [CrossRef]
- Marvasi, M.; Casillas-Santiago, L.M.; Henríquez, T.; Casillas-Martinez, L. Involvement of EtfA Gene during CaCO3 Precipitation in Bacillus subtilis Biofilm. Geomicrobiol. J. 2017, 34, 722–728. [Google Scholar] [CrossRef]
- Perito, B.; Casillas-Martínez, L.; Marvasi, M. Factors Affecting Formation of Large Calcite Crystals (≥1 mm) in Bacillus subtilis 168 Biofilm. Geomicrobiol. J. 2018, 35, 385–391. [Google Scholar] [CrossRef]
- Hammes, F.; Boon, N.; de Villiers, J.; Verstraete, W.; Siciliano, S.D. Strain-Specific Ureolytic Microbial Calcium Carbonate Precipitation. Appl. Environ. Microbiol. 2003, 69, 4901–4909. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Ghosh, A. (Dey). Microbial Concrete as a Sustainable Option for Infrastructural Development in Emerging Economies. In Proceedings of the ASCE India Conference—Urbanization Challenges in Emerging Economies, New Delhi, India, 13 December 2018; pp. 413–423. [Google Scholar]
- De Muynck, W.; Leuridan, S.; Van Loo, D.; Verbeken, K.; Cnudde, V.; De Belie, N.; Verstraete, W. Influence of Pore Structure on the Effectiveness of a Biogenic Carbonate Surface Treatment for Limestone Conservation. Appl. Environ. Microbiol. 2011, 77, 6808–6820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhami, N.K.; Alsubhi, W.R.; Watkin, E.; Mukherjee, A. Bacterial Community Dynamics and Biocement Formation during Stimula-tion and Augmentation: Implications for Soil Consolidation. Front. Microbiol. 2017, 8, 1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omoregie, A.I.; Palombo, E.A.; Nissom, P.M. Bioprecipitation of calcium carbonate mediated by ureolysis: A review. Environ. Eng. Res. 2021, 26, 200379. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 2012, 42, 64–72. [Google Scholar] [CrossRef] [Green Version]






| Identification | Morphotype | Urease | Carbonic Anhydrase |
|---|---|---|---|
| CD1 Epibacterium mobile | Gram-negative bacilli | + | + |
| CD2 Bhargavaea ginsengi | Gram-positive bacilli | - | + |
| CD3 Pseudidiomarina maritima | Gram-negative bacilli | - | + |
| CD4 Epibacterium mobile | Gram-negative bacilli | - | + |
| CD5 Epibacterium mobile | Gram-negative bacilli | - | + |
| CD6 Virgibacillus halodenitrificans | Gram-positive bacilli | + | + |
| CD7 Bhargavaea beijingensis | Gram-positive bacilli | - | + |
| CD8 Planococcus maritimus | Gram-positive bacilli | - | + |
| CD9 Pseudoalteromonas sp. | Gram-negative bacilli | + | + |
| CD10 Pseudoalteromonas sp. | Gram-negative bacilli | + | + |
| MD1 Pseudoalteromonas sp. | Gram-negative bacilli | + | + |
| SW1 Pseudoalteromonas sp. | Gram-negative bacilli | - | + |
| SW2 Pseudoalteromonas sp. | Gram-negative bacilli | + | + |
| SW3 Pseudoalteromonas sp. | Gram-negative bacilli | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincent, J.; Colin, B.; Lanneluc, I.; Sabot, R.; Sopéna, V.; Turcry, P.; Mahieux, P.-Y.; Refait, P.; Jeannin, M.; Sablé, S. New Biocalcifying Marine Bacterial Strains Isolated from Calcareous Deposits and Immediate Surroundings. Microorganisms 2022, 10, 76. https://doi.org/10.3390/microorganisms10010076
Vincent J, Colin B, Lanneluc I, Sabot R, Sopéna V, Turcry P, Mahieux P-Y, Refait P, Jeannin M, Sablé S. New Biocalcifying Marine Bacterial Strains Isolated from Calcareous Deposits and Immediate Surroundings. Microorganisms. 2022; 10(1):76. https://doi.org/10.3390/microorganisms10010076
Chicago/Turabian StyleVincent, Julia, Béatrice Colin, Isabelle Lanneluc, René Sabot, Valérie Sopéna, Philippe Turcry, Pierre-Yves Mahieux, Philippe Refait, Marc Jeannin, and Sophie Sablé. 2022. "New Biocalcifying Marine Bacterial Strains Isolated from Calcareous Deposits and Immediate Surroundings" Microorganisms 10, no. 1: 76. https://doi.org/10.3390/microorganisms10010076
APA StyleVincent, J., Colin, B., Lanneluc, I., Sabot, R., Sopéna, V., Turcry, P., Mahieux, P.-Y., Refait, P., Jeannin, M., & Sablé, S. (2022). New Biocalcifying Marine Bacterial Strains Isolated from Calcareous Deposits and Immediate Surroundings. Microorganisms, 10(1), 76. https://doi.org/10.3390/microorganisms10010076






