Mineral Biofortification and Growth Stimulation of Lentil Plants Inoculated with Trichoderma Strains and Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fungal Strains and Metabolites
2.3. Experimental Design
2.3.1. EXPERIMENT #A: Application of Trichoderma Strains or BAMs in Greenhouse
2.3.2. EXPERIMENT #B: Application of Trichoderma Strains in Greenhouse
2.3.3. EXPERIMENT #C: Trichoderma Strains and/or BAMs Applied Singly or Combined in Greenhouse
2.3.4. EXPERIMENT #D: Plant Treatments in Field Conditions
2.4. Plant Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Trichoderma Strains or BAMs on Lentil Plants in Greenhouse (#Experiment A)
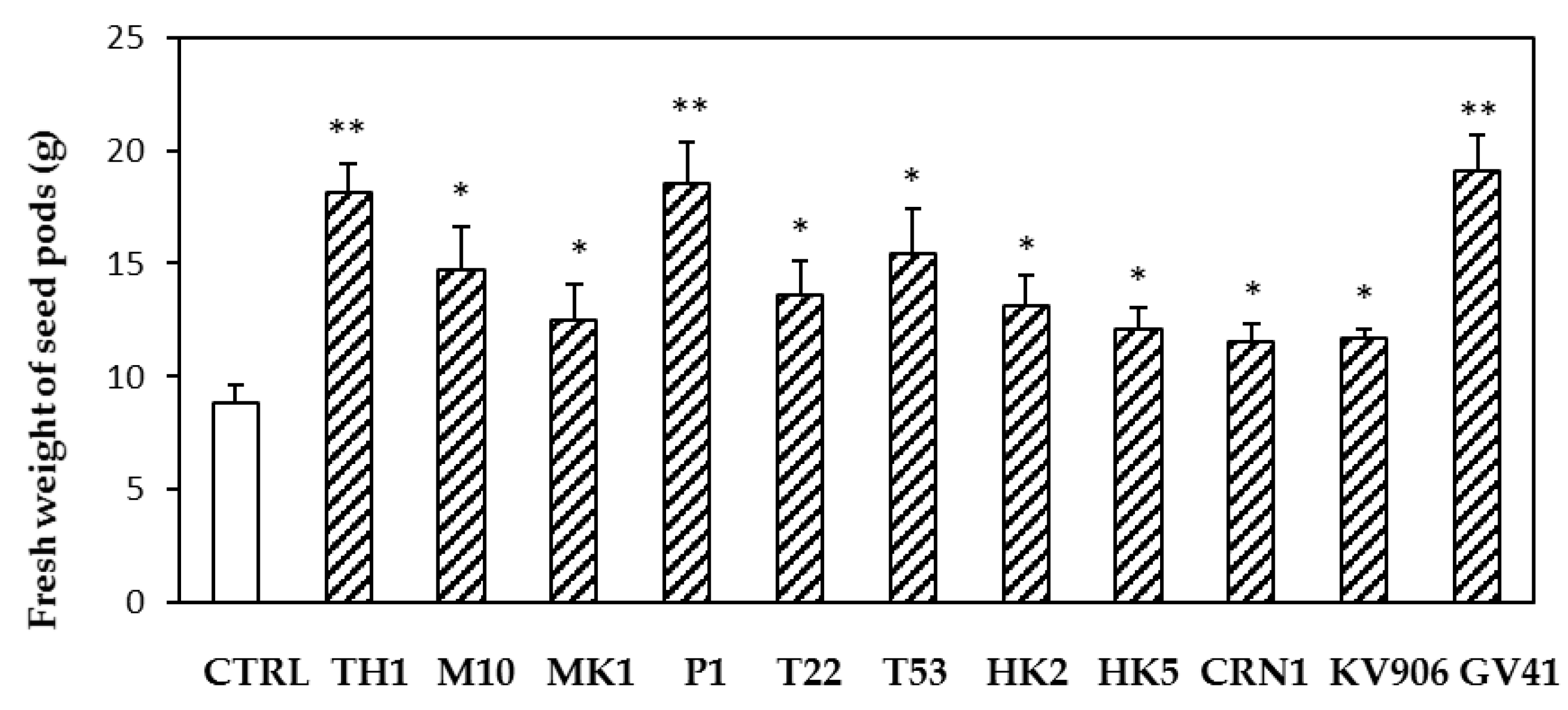
3.2. Effects of Trichoderma Strains on Lentil Plants in Greenhouse (#Experiment B)
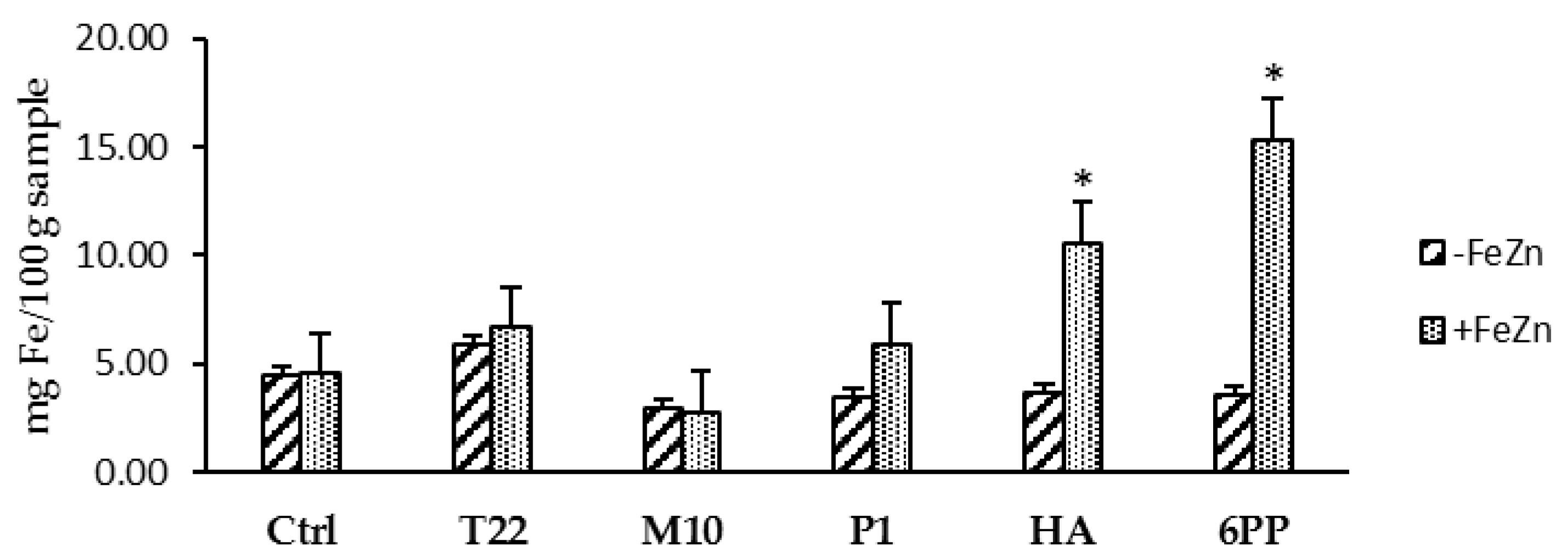
3.3. Effects of Trichoderma Strains or BAMs Applied Singly or Combined (#Experiment C)
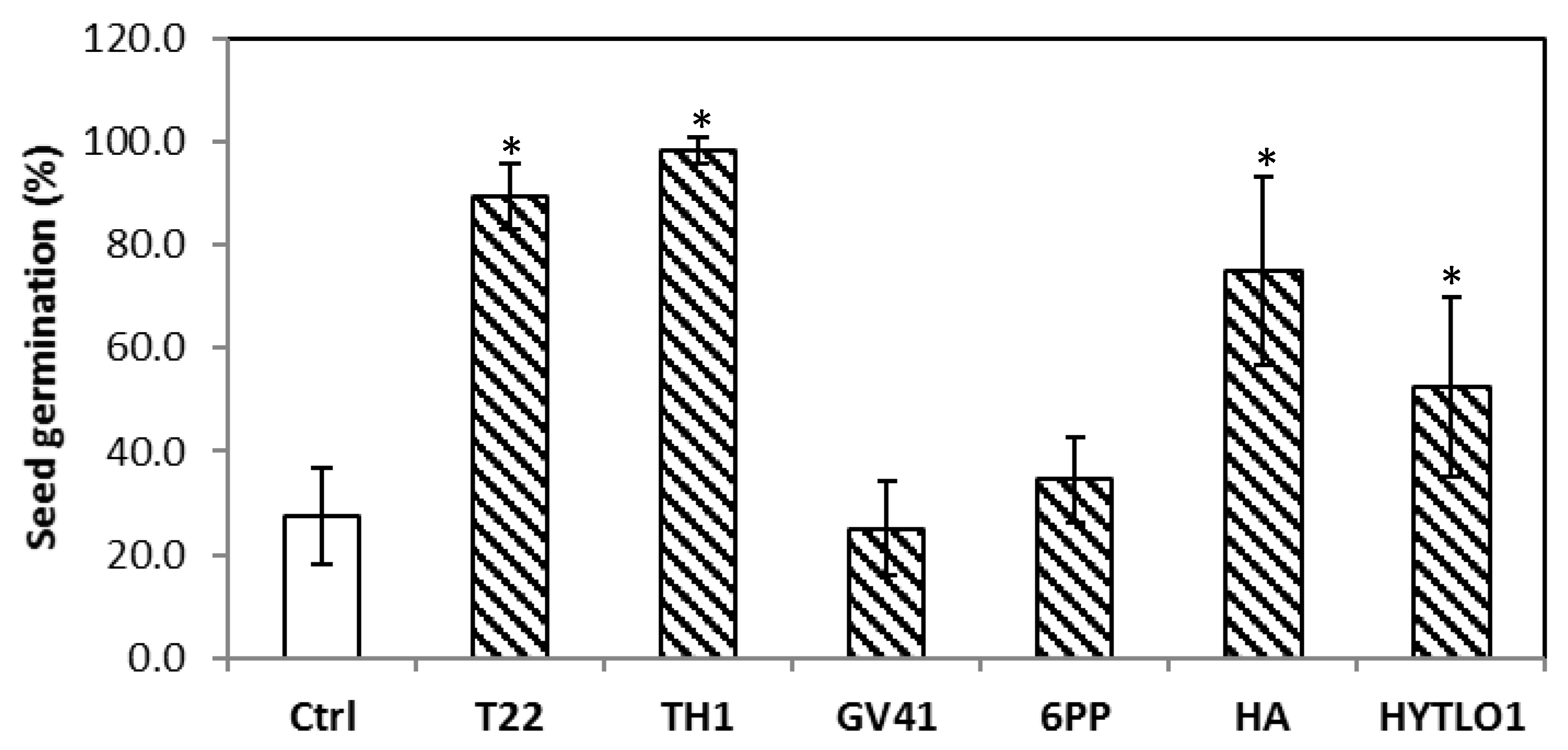
3.4. Effects of Trichoderma Strains and/or BAMs on Lentil Plants in Field Conditions (#Experiment D)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO) of the United Nations. The Future of Food and Agriculture. Trends and Challenges; FAO: Rome, Italy, 2017; p. 5. [Google Scholar]
- Strobbe, S.; Van Der Straeten, D. Toward Eradication of B-Vitamin Deficiencies: Considerations for Crop Biofortification. Front. Plant Sci. 2018, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Darnton-Hill, I.; Webb, P.; Harvey, P.W.; Hunt, J.M.; Dalmiya, N.; Chopra, M.; Ball, M.J.; Bloem, M.W.; de Benoist, B. Micronutrient deficiencies and gender: Social and economic costs. Am. J. Clin. Nutr. 2005, 81, 1198S–1205S. [Google Scholar] [CrossRef]
- Ruel-Bergeron, J.C.; Stevens, G.A.; Sugimoto, J.D.; Roos, F.F.; Ezzati, M.; Black, R.E.; Kraemer, K. Global update and trends of hidden hunger, 1995–2011: The hidden hunger index. PLoS ONE 2015, 10, e0143497. [Google Scholar] [CrossRef]
- Copenhagen Consensus. Solutions to Global Challenges. 2012. Available online: http://www.copenhagenconsensus.com/copenhagen-consensus-iii/outcome (accessed on 30 November 2021).
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Gangashetty, P.I.; Riyazaddin, M.; Sanogo, M.D.; Inousa, D.; Issoufou, K.A.; Asungre, P.A.; Sy, O.; Govindaraj, M.; Ignatius, A.I. Identification of High-Yielding Iron-Biofortified Open-Pollinated Varieties of Pearl Millet in West Africa. Front. Plant Sci. 2021, 12, 688937. [Google Scholar] [CrossRef]
- Reinbott, A.; Schelling, A.; Kuchenbecker, J.; Jeremias, T.; Russell, I.; Kevanna, O.; Krawinkel, M.B.; Jordan, I. Nutrition education linked to agricultural interventions improved child dietary diversity in rural Cambodia. Br. J. Nutr. 2016, 116, 1457–1468. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding Crops for Better Nutrition. Crop Sci. 2007, 47, S88–S105. [Google Scholar] [CrossRef]
- de Santiago, A.; Quintero, J.M.; Avilés, M.; Delgado, A. Effect of Trichoderma asperellum strain T34 on iron nutrition in white lupin. Soil Biol. Biochem. 2009, 41, 2453–2459. [Google Scholar] [CrossRef]
- de Santiago, A.; Quintero, J.M.; Avilés, M.; Delgado, A. Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant Soil 2011, 342, 97–104. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Abril-Urías, P.; Velasco, P. Endophytic fungi as direct plant growth promoters for sustainable agricultural production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Rigobelo, E.C.; Baron, N.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2021. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef]
- Gupta, R.; Bar, M. Plant immunity, priming, and systemic resistance as mechanisms for Trichoderma spp. biocontrol. In Trichoderma; Springer: Singapore, 2020; pp. 81–110. [Google Scholar]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 1004. [Google Scholar] [CrossRef]
- Velasco, P.; Rodríguez, V.M.; Soengas, P.; Poveda, J. Trichoderma hamatum Increases Productivity, Glucosinolate Content and Antioxidant Potential of Different Leafy Brassica Vegetables. Plants 2021, 10, 2449. [Google Scholar] [CrossRef]
- Marra, R.; Lombardi, N.; d’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma strains and metabolites enhances soybean productivity and nutrient content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Rehman, H.M.; Cooper, J.W.; Lam, H.-M.; Yang, S.H. Legume biofortification is an underexploited strategy for combatting hidden hunger. Plant Cell Environ. 2018, 42, 52–70. [Google Scholar] [CrossRef] [Green Version]
- Characteristics of Lentil (Lens culinaris L.) Variety CDC MAXIM CL. Available online: https://agriculture.basf.ca/west/products/seeds-and-systems/clearfield/clearfield-lentils/varieties.html (accessed on 15 October 2021).
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic Acid, an Antifungal and Plant Growth Promoting Metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Ruocco, M.; Lanzuise, S.; Lombardi, N.; Woo, S.L.; Vinale, F.; Marra, R.L.; Varlese, R.; Manganiello, G.; Pascale, A.; Scala, V.; et al. Multiple Roles and Effects of a Novel Trichoderma Hydrophobin. Mol. Plant Microbe Interact. 2015, 28, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Gericke, S.; Kurmies, B. Colorimetrische Bestimmung der Phosphorsäure mit Vanadat-Molybdat. Z. Anal. Chem. 1952, 137, 15–22. [Google Scholar] [CrossRef]
- FAOSTAT. 2021. Available online: https://www.fao.org/faostat/en/#data (accessed on 30 November 2021).
- US Department of Agriculture. National Nutrient Database for Standard Reference Release 28 (2016). “Full Report (All Nutrients): 16069, Lentils, Raw”. Retrieved 15 December 2015; US Department of Agriculture: Washington, DC, USA, 2016.
- Ye, Y.; Qu, J.; Pu, Y.; Rao, S.; Xu, F.; Wu, C. Selenium Biofortification of Crop Food by Beneficial Microorganisms. J. Fungi 2020, 6, 59. [Google Scholar] [CrossRef]
- Shahzad, R.; Jamil, S.; Ahmad, S.; Nisar, A.; Khan, S.; Amina, Z.; Kanwal, S.; Aslam, H.M.U.; Gill, R.A.; Zhou, W. Biofortification of Cereals and Pulses Using New Breeding Techniques: Current and Future Perspectives. Front. Nutr. 2021, 8, 721728. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, P.K.; Horwitz, B.A.; Zachow, C.; Berg, G.; Zeilinger, S. Trichoderma–Plant–Pathogen Interactions: Advances in Genetics of Biological Control. Indian J. Microbiol. 2012, 52, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Bazghaleh, N.; Prashar, P.; Woo, S.; Vandenberg, A. Effects of Lentil Genotype on the Colonization of Beneficial Trichoderma Species and Biocontrol of Aphanomyces Root Rot. Microorganisms 2020, 8, 1290. [Google Scholar] [CrossRef]
- Winkelmann, G. Ecology of siderophores with special reference to the fungi. Biometals 2007, 20, 379–392. [Google Scholar] [CrossRef]
- Lemanceau, P.; Bauer, P.; Kraemer, S.; Briat, J.F. Iron dynamics in the rizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 2009, 321, 513–535. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- Curie, C.; Briat, J.F. Iron transport and signaling in plants. Annu. Rev. Plant Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma applications on strawberry plants modulate the physiological processes positively affecting fruit production and quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; d’Errico, G.; et al. Effect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Profile of Strawberry Fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial Consortia: Promising Probiotics as Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Mishra, A.; Nautiyal, C.S. A novel Trichoderma fusant for enhancing nutritional value and defence activity in chickpea. Physiol. Mol. Biol. Plants 2018, 24, 411–422. [Google Scholar] [CrossRef]
- Poveda, J. Biological control of Fusarium oxysporum f. sp. ciceri and Ascochyta rabiei infecting protected geographical indication Fuentesaúco-Chickpea by Trichoderma species. Eur. J. Plant Pathol. 2021, 160, 825–840. [Google Scholar] [CrossRef]
- Silletti, S.; Di Stasio, E.; Van Oosten, M.J.; Ventorino, V.; Pepe, O.; Napolitano, M.; Marra, R.; Woo, S.L.; Cirillo, V.; Maggio, A. Biostimulant Activity of Azotobacter chroococcum and Trichoderma harzianum in Durum Wheat under Water and Nitrogen Deficiency. Agronomy 2021, 11, 380. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.M.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma Species: Versatile Plant Symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of phosphates and micronutrients by the Plant-Growth-Promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.K.; Singh, S.; Singh, H.B.; Sarma, B.K. Compatible rhizosphere-competent microbial consortium adds value to the nutritional quality in edible parts of chickpea. J. Agric. Food Chem. 2017, 65, 6122–6130. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J. Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plants. J. Basic Microbiol. 2014, 54, S115–S124. [Google Scholar] [CrossRef]
- Carvalho, S.M.P.; Vasconcelos, M.W. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013, 54, 961–971. [Google Scholar] [CrossRef]

| Variety | CDC MAXIM CL |
|---|---|
| Seed color | Red |
| Seed dimension | Small |
| Maximun yield (%) | 100 |
| Height (cm) | 34 |
| Flowering (days) | 51 |
| Seed weight (g/1000) | 40 |
| Aschochyta Resistance | Good |
| Anthracnose resistance (Race 1) | Good |
| No. | Trichoderma Species | Strain | Origin |
|---|---|---|---|
| 1 | harzianum | TH1 | Italy |
| 2 | harzianum | M10 | Australia |
| 3 | longibrachiatum | MK1 | Italy |
| 4 | atroviride | P1 | Norway |
| 5 | afroharzianum | T22 | USA |
| 6 | asperellum | T53 | Spain |
| 7 | harzianum | HK2 | USA |
| 8 | harzianum | HK5 | USA |
| 9 | asperellum | CRN1 | Costa Rica |
| 10 | asperellum | KV906 | Brazil |
| 11 | virens | GV41 | USA |
| Metabolite | Chemical Feature | Source | Properties | Reference |
|---|---|---|---|---|
| 6-pentyl-alpha-pyrone (6PP) | Pyrone | T. atroviride strain P1 | Antibiotic, volatile compound with a characteristic smell of coconut. | [25] |
| Harzianic acid (HA) | Tetramic acid | T. harzianum strain M10 | Antifungal activity, promotion of plant growth, ability to bind iron. | [26,27] |
| HYTLO1 | Hydrophobin | T. longibrachiatum strain MK1 | Involved in the aerial mycelium formation and stimulation of plant defenses and root growth, antifungal activity. | [28] |
| Mineral Content (mg/100 g Sample) | ||
|---|---|---|
| Strain | Fe | Zn |
| TH1 | 5.82 ± 0.65 bd | 7.345 ± 0.84 a |
| M10 | 4.02 ± 0.41 a | 7.245 ± 0.80 a |
| MK1 | 2.77 ± 0.28 c | 7.695 ± 0.69 a |
| P1 | 4.75 ± 0.39 ad | 6.845 ± 0.77 c |
| T22 | 6.47 ± 0.45 b | 9.395 ± 0.85 b |
| T53 | 3.99 ± 0.52 a | 7.295 ± 0.64 a |
| HK2 | 4.42 ± 0.68 a | 7.345 ± 0.25 a |
| HK5 | 4.01 ± 0.54 a | 7.245 ± 0.84 a |
| CRN1 | 3.15 ± 0.27 c | 7.745 ± 0.67 a |
| KV906 | 4.22 ± 0.51 a | 7.545 ± 0.64 a |
| GV41 | 6.88 ± 0.52 b | 7.695 ± 0.34 a |
| Control | 4.00 ± 0.63 a | 7.995 ± 0.40 a |
| Mineral Content (mg/100 g Sample) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Applied to Seed/Soil | IRON CONTENT | ZINC CONTENT | ||||||
| −FeZn | +FeZn | −FeZn | +FeZn | |||||
| CTRL | 5.60 | ±0.41 a | 12.06 | ±0.84 a | 6.00 | ±0.62 a | 4.28 | ±0.45 a |
| H2O/T22 | 13.59 | ±1.63 b | 4.57 | ±0.35 b | 7.95 | ±0.84 b | 3.81 | ±0.25 a |
| H2O/TH1 | 4.21 | ±0.58 c | 4.55 | ±0.65 b | 6.31 | ±0.36 a | 4.62 | ±0.41 a |
| H2O/GV41 | 13.88 | ±1.28 b | 10.77 | ±0.96 a | 4.85 | ±0.58 a | 4.46 | ±0.56 a |
| H2O/HA | 7.13 | ±0.63 d | 7.21 | ±0.78 c | 7.52 | ±0.89 ab | 4.28 | ±0.69 a |
| H2O/6PP | 4.38 | ±0.48 c | 24.99 | ±1.96 d | 6.40 | ±0.54 a | 4.47 | ±0.85 a |
| H2O/HYTLO1 | 3.36 | ±0.36 c | 47.89 | ±1.84 e | 5.82 | ±0.23 a | 3.79 | ±0.55 a |
| T22/H2O | 5.56 | ±0.69 ac | nd | 4.90 | ±0.85 a | nd | ||
| TH1/H2O | 7.73 | ±0.36 d | 19.53 | ±2.65 d | 5.53 | ±0.96 a | 5.29 | ±0.98 a |
| GV41/H2O | 5.73 | ±0.44 a | 2.81 | ±0.24 f | 6.08 | ±0.65 a | 4.04 | ±0.63 a |
| HA/H2O | 7.76 | ±0.18 d | 5.51 | ±0.75 c | 5.69 | ±0.41 a | 5.13 | ±0.35 a |
| 6PP/H2O | 9.13 | ±0.85 e | 8.57 | ±0.96 cg | 6.93 | ±0.21 a | 4.56 | ±0.14 a |
| HYTLO1/H2O | 7.02 | ±1.00 ad | 9.94 | ±1.45 ag | 6.61 | ±0.36 a | 5.01 | ±0.56 a |
| Mineral Content (mg/100 g Sample) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Applied to Seed/Soil | IRON CONTENT | ZINC CONTENT | ||||||
| −FeZn | −FeZn | −FeZn | +FeZn | |||||
| CTRL | 5.60 | ±0.41 a | 12.06 | ±2.56 a | 6.00 | ±0.62 a | 4.28 | ±0.44 a |
| HA/H2O | 7.76 | ±0.18 bc | 5.51 | ±0.75 b | 5.69 | ±0.41 ab | 5.13 | ±0.35 b |
| HA/T22 | 6.31 | ±0.56 a | 12.46 | ±1.69 a | 5.23 | ±0.63 ab | 4.00 | ±0.72 a |
| HA/TH1 | 6.26 | ±0.98 a | 13.21 | ±1.45 a | 5.28 | ±0.66 ab | 3.61 | ±0.35 a |
| HA/GV41 | 6.29 | ±0.68 a | 46.74 | ±6.51 c | 4.39 | ±0.58 b | 4.37 | ±0.68 a |
| 6PP/H2O | 9.13 | ±0.85 c | 8.57 | ±0.96 b | 6.93 | ±0.21 a | 4.56 | ±0.14 a |
| 6PP/T22 | 7.97 | ±0.77 bc | 13.29 | ±1.69 a | 5.36 | ±0.81 ab | 4.61 | ±0.35 a |
| 6PP/TH1 | 3.91 | ±0.45 d | 4.41 | ±0.87 b | 5.08 | ±0.80 ab | 4.49 | ±0.16 a |
| 6PP/GV41 | 7.77 | ±0.85 bc | 50.76 | ±5.12 c | 7.00 | ±0.96 a | 4.06 | ±0.41 a |
| HYTLO1/H2O | 7.02 | ±1.00 ab | 9.94 | ±1.45 ab | 6.61 | ±0.36 a | 4.56 | ±0.14 a |
| HYTLO1/T22 | 5.49 | ±0.66 a | 7.22 | ±0.98 b | 5.44 | ±0.63 ab | 3.94 | ±0.34 a |
| HYTLO1/TH1 | 9.75 | ±0.84 c | 8.04 | ±0.74 b | 5.61 | ±0.31 a | 4.79 | ±0.23 a |
| HYTLO1/GV41 | 3.95 | ±0.63 d | 7.61 | ±0.84 b | 4.16 | ±0.54 b | 5.10 | ±0.23 b |
| Treatment | Plant Dry Weight (g/Plant) | Seed Weight (g/Plant) | Mineral Content (ppm Solid) | |||
|---|---|---|---|---|---|---|
| Mean ± SE | % | Mean ± SE | % | Iron Mean ± SE (%) | Zinc Mean ± SE (%) | |
| CTRL | 16.9 ± 2.5 a | - | 5.6 ± 0.5 a | - | 83.1 ± 8.6 a | 66.33 ± 3.7 a |
| TH1 | 26.6 ± 2.7 b | +57 | 15.2 ± 1.0 b | +169 | 82.2 ± 6.6 a | 62.43 ± 1.8 a |
| M10 | 37.1 ± 0.4 c | +120 | 12.0 ± 0.4 c | +112 | 85.3 ± 10.8 a | 66.38 ± 5.5 a |
| P1 | nd | - | 4.8 ± 0.3 a | - | 73.6 ± 0.1 b | 59.94 ± 1.0 b |
| T22 | 7.3 ± 0.2 e | - | 5.8 ± 0.3 a | - | 70.4 ± 3.6 b | 56.40 ± 82.9 b |
| T53 | 13.3 ± 1.6 ad | - | 9.7 ± 0.5 d | +71 | 87.3 ± 1.4 a | 61.29 ± 2.7 a |
| HK5 | 16.5 ± 1.4 a | - | 8.7 ± 2.4 de | +53 | 83.5 ± 2.0 a | 68.82 ± 1.1 a |
| CRN1 | 17.5 ± 1.6 a | - | 5.3 ± 2.1 a | - | 82.1 ± 20.5 a | 66.44 ± 7.6 a |
| KV906 | 20.4 ± 2.1 a | - | 10.2 ± 0.7 cd | +81 | 98.4 ± 4.6 c (+11%) | 73.97 ± 6.7 c (+18%) |
| GV41 | 17.9 ± 4.5 a | - | 9.3 ± 1.9 cde | +65 | 76.1 ± 1.7 b | 62.28 ± 4.2 a |
| HA | 8.6 ± 0.6 e | - | 5.1 ± 0.7 a | - | 90.6 ± 0.1 a | 66.72 ± 4.2 a |
| 6PP | 12.9 ± 0.7 d | - | 7.6 ± 0.4 de | +36 | 73.6 ± 0.9 b | 62.12 ± 0.1 a |
| HYTLO1 | 21.5 ± 3.4 ab | - | 14.0 ± 2.9 b | +147 | 99.9 ± 2.0 c (+6%) | 70.55 ± 2.2 c (+20%) |
| T22 + HA | 16.3 ± 2.2 ad | - | 6.6 ± 1.3 ae | - | 88.6 ± 5.0 a | 67.45 ± 3.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marra, R.; Lombardi, N.; Piccolo, A.; Bazghaleh, N.; Prashar, P.; Vandenberg, A.; Woo, S. Mineral Biofortification and Growth Stimulation of Lentil Plants Inoculated with Trichoderma Strains and Metabolites. Microorganisms 2022, 10, 87. https://doi.org/10.3390/microorganisms10010087
Marra R, Lombardi N, Piccolo A, Bazghaleh N, Prashar P, Vandenberg A, Woo S. Mineral Biofortification and Growth Stimulation of Lentil Plants Inoculated with Trichoderma Strains and Metabolites. Microorganisms. 2022; 10(1):87. https://doi.org/10.3390/microorganisms10010087
Chicago/Turabian StyleMarra, Roberta, Nadia Lombardi, Alessandro Piccolo, Navid Bazghaleh, Pratibha Prashar, Albert Vandenberg, and Sheridan Woo. 2022. "Mineral Biofortification and Growth Stimulation of Lentil Plants Inoculated with Trichoderma Strains and Metabolites" Microorganisms 10, no. 1: 87. https://doi.org/10.3390/microorganisms10010087







