The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences
Abstract
1. Introduction
2. Analysis of Published Studies on the Impact of Introduced AMF Inoculants in the Field
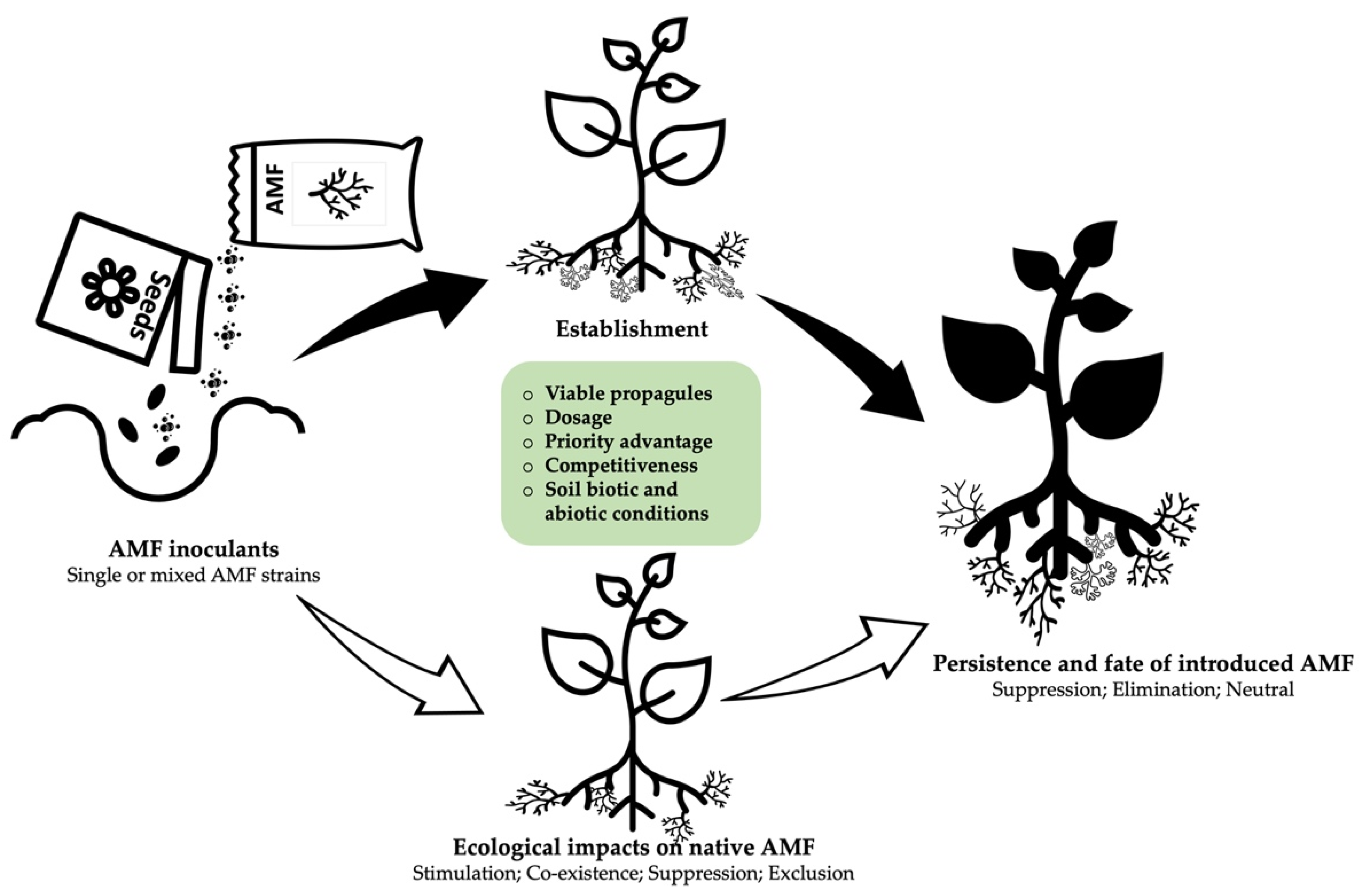
3. Establishment and Persistence of Introduced AMF in Field
3.1. Establishment
3.2. Persistence
4. What Determines the Establishment Success and Survival of an Inoculum?
4.1. Quality, Formulation, and Type of Inoculants
4.2. Priority advantage and Frequency of Application
4.3. Soil Abiotic Conditions
4.4. Soil Biotic Conditions
5. Effect of Inoculation on the Structure of Indigenous AMF Communities
5.1. The Impacts of Inoculation on Indigenous Communities Are Context-Specific
5.2. Inoculation mostly results into Shift in Structure, Rather Than in Composition
5.3. Niche Availability Influences Fate of Both Introduced and Indigenous AMF Communities
5.4. Alteration in the Functions of Indigenous AMF Communities Are Scarcely Reported
6. Monitoring Survival and Ecological Consequences of AMF Inoculants in the Field
7. Conclusions
8. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basiru, S.; Hijri, M. Does Commercial Inoculation Promote Arbuscular Mycorrhizal Fungi Invasion? Microorganisms 2022, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of Arbuscular Mycorrhizal Fungal Inoculant Benchmarks. Microorganisms 2021, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Read, D. Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Wang, X.-X.; Li, H.; Chu, Q.; Feng, G.; Kuyper, T.W.; Rengel, Z. Mycorrhizal impacts on root trait plasticity of six maize varieties along a phosphorus supply gradient. Plant Soil 2020, 448, 71–86. [Google Scholar] [CrossRef]
- Pepe, A.; Giovannetti, M.; Sbrana, C. Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan. Sci. Rep. 2018, 8, 10235. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Gui, H.; Gao, Y.; Wang, Z.; Shi, L.; Yan, K.; Xu, J. Arbuscular mycorrhizal fungi potentially regulate N2O emissions from agricultural soils via altered expression of denitrification genes. Sci. Total Env. 2021, 774, 145133. [Google Scholar] [CrossRef]
- Storer, K.; Coggan, A.; Ineson, P.; Hodge, A.J.N.P. Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol. 2018, 220, 1285–1295. [Google Scholar] [CrossRef]
- Teutscherova, N.; Vazquez, E.; Arango, J.; Arevalo, A.; Benito, M.; Pulleman, M. Native arbuscular mycorrhizal fungi increase the abundance of ammonia-oxidizing bacteria, but suppress nitrous oxide emissions shortly after urea application. Geoderma 2019, 338, 493–501. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, B. Arbuscular mycorrhizal fungi reduce soil nitrous oxide emission. Geoderma 2021, 402, 115179. [Google Scholar] [CrossRef]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Martelloni, L.; Sbrana, C.; Picciarelli, P.; Giovannetti, M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 2010, 335, 311–323. [Google Scholar] [CrossRef]
- El-Sawah, A.; El-Keblawy, A.; Ali, D.; Ibrahim, H.; El-Sheikh, M.; Sharma, A.; Alhaj Hamoud, Y.; Shaghaleh, H.; Brestic, M.; Skalicky, M.; et al. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture 2021, 11, 1033. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.; Krishnamoorthy, R.; Walitang, D.; Sundaram, S.; Joe, M.M.; Selvakumar, G.; Hu, S.; Oh, S.H.; Sa, T. Mycorrhizal Symbiotic Efficiency on C3 and C4 Plants under Salinity Stress—A Meta-Analysis. Front. Microbiol. 2016, 7, 1246. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Ceustermans, A.; Van Hemelrijck, W.; Van Campenhout, J.; Bylemans, D. Effect of Arbuscular Mycorrhizal Fungi on Pratylenchus penetrans Infestation in Apple Seedlings under Greenhouse Conditions. Pathogens 2018, 7, 76. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Gao, F.; Lv, C.; Li, L.; Han, T.; Chen, F. AMF Inoculation Can Enhance Yield of Transgenic Bt Maize and Its Control Efficiency Against Mythimna separata Especially Under Elevated CO2. Front. Plant Sci. 2021, 12, 655060. [Google Scholar] [CrossRef]
- Ceballos, I.; Ruiz, M.; Fernández, C.; Peña, R.; Rodríguez, A.; Sanders, I.R. The In Vitro Mass-Produced Model Mycorrhizal Fungus, Rhizophagus irregularis, Significantly Increases Yields of the Globally Important Food Security Crop Cassava. PLoS ONE 2013, 8, e70633. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.G.A.; Oehl, F. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.A.; Boller, T.; Wiemken, A. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 2004, 165, 273–283. [Google Scholar] [CrossRef]
- Lang, M.; Zhang, C.; Su, W.; Chen, X.; Zou, C.; Chen, X. Long-term P fertilization significantly altered the diversity, composition and mycorrhizal traits of arbuscular mycorrhizal fungal communities in a wheat-maize rotation. Appl. Soil Ecol. 2022, 170, 104261. [Google Scholar] [CrossRef]
- Van der Heyde, M.; Ohsowski, B.; Abbott, L.K.; Hart, M. Arbuscular mycorrhizal fungus responses to disturbance are context-dependent. Mycorrhiza 2017, 27, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Laczko, E.; Oberholzer, H.-R.; Jansa, J.; Egli, S. Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biol. Fertil. Soils 2017, 53, 777–797. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P.; Jones, J.; Bending, G.D. Evidence for functional redundancy in arbuscular mycorrhizal fungi and implications for agroecosystem management. Mycorrhiza 2016, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Duell, E.B.; Cobb, A.B.; Wilson, G.W.T. Effects of Commercial Arbuscular Mycorrhizal Inoculants on Plant Productivity and Intra-Radical Colonization in Native Grassland: Unintentional De-Coupling of a Symbiosis? Plants 2022, 11, 2276. [Google Scholar] [CrossRef]
- Faye, A.; Dalpé, Y.; Ndung’u-Magiroi, K.; Jefwa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of commercial arbuscular mycorrhizal inoculants. Can. J. Plant Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Faye, A.; Stewart, Z.P.; Ndung’u-Magiroi, K.; Diouf, M.; Ndoye, I.; Diop, T.; Dalpé, Y.; Prasad, P.V.V.; Lesueur, D. Testing of commercial inoculants to enhance P Uptake and grain yield of promiscuous soybean in Kenya. Sustainability 2020, 12, 3803. [Google Scholar] [CrossRef]
- Salomon, M.J.; Demarmels, R.; Watts-Williams, S.J.; McLaughlin, M.J.; Kafle, A.; Ketelsen, C.; Soupir, A.; Bücking, H.; Cavagnaro, T.R.; van der Heijden, M.G.A. Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl. Soil Ecol. 2022, 169, 104225. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.M.; Abbott, L.K. Exploring economic assessment of the arbuscular mycorrhizal symbiosis. Symbiosis 2020, 83, 143–152. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; van der Heijden, M.G.A.; et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.M.; Trexler, R.V.; Malik, R.J.; Hockett, K.L.; Bell, T.H. The Inherent Conflicts in Developing Soil Microbial Inoculants. Trends Biotechnol. 2019, 37, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.J.; Watts-Williams, S.J.; McLaughlin, M.J.; Bucking, H.; Singh, B.K.; Hutter, I.; Schneider, C.; Martin, F.M.; Vosatka, M.; Guo, L.; et al. Establishing a quality management framework for commercial inoculants containing arbuscular mycorrhizal fungi. iScience 2022, 25, 104636. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Martínez-Romero, E.; Banuelos, J.; Arteaga-Garibay, R.I. Tools and challenges to exploit microbial communities in agriculture. Curr. Res. Microb. Sci. 2021, 2, 100062. [Google Scholar] [CrossRef]
- Mummey, D.L.; Antunes, P.M.; Rillig, M.C. Arbuscular mycorrhizal fungi pre-inoculant identity determines community composition in roots. Soil Biol. Biochem. 2009, 41, 1173–1179. [Google Scholar] [CrossRef]
- Islam, M.N.; Germida, J.J.; Walley, F.L. Survival of a commercial AM fungal inoculant and its impact on indigenous AM fungal communities in field soils. Appl. Soil Ecol. 2021, 166, 103979. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K.; Field, K. Fungal inoculants in the field: Is the reward greater than the risk? Funct. Ecol. 2017, 32, 126–135. [Google Scholar] [CrossRef]
- Davidson, B.E.; Novak, S.J.; Serpe, M.D. Consequences of inoculation with native arbuscular mycorrhizal fungi for root colonization and survival of Artemisia tridentata ssp. wyomingensis seedlings after transplanting. Mycorrhiza 2016, 26, 595–608. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J.; Bardgett, R. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Pellegrino, E.; Nuti, M.; Ercoli, L. Multiple Arbuscular Mycorrhizal Fungal Consortia Enhance Yield and Fatty Acids of Medicago sativa: A Two-Year Field Study on Agronomic Traits and Tracing of Fungal Persistence. Front. Plant Sci. 2022, 13, 814401. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Li, Y.; Hamel, C.; Hanson, K.; Hart, M. Site specificity in establishment of a commercial arbuscular mycorrhizal fungal inoculant. Sci Total Env. 2019, 660, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Thioye, B.; Sanguin, H.; Kane, A.; Ndiaye, C.; Fall, D.; Sanogo, D.; Duponnois, R.; de Faria, S.M.; Sylla, S.N.; Bâ, A. Mycorrhizal inoculation increases fruit production without disturbance of native arbuscular mycorrhizal community in jujube tree orchards (Senegal). Symbiosis 2021, 83, 361–372. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S.; Avio, L.; Bonari, E.; Giovannetti, M. Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol. Biochem. 2011, 43, 367–376. [Google Scholar] [CrossRef]
- Alguacil Mdel, M.; Torrecillas, E.; Kohler, J.; Roldán, A. A molecular approach to ascertain the success of “in situ” AM fungi inoculation in the revegetation of a semiarid, degraded land. Sci Total Env. 2011, 409, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Tateishi, T.; Saito, M.; Marumoto, T. Application of a Molecular Method for the Identification of a Gigaspora margarita Isolate Released in a Field. Soil Sci. Plant Nutr. 2005, 51, 125–128. [Google Scholar] [CrossRef]
- Farmer, M.J.; Li, X.; Feng, G.; Zhao, B.; Chatagnier, O.; Gianinazzi, S.; Gianinazzi-Pearson, V.; van Tuinen, D. Molecular monitoring of field-inoculated AMF to evaluate persistence in sweet potato crops in China. Appl. Soil Ecol. 2007, 35, 599–609. [Google Scholar] [CrossRef]
- Sýkorová, Z.; Börstler, B.; Zvolenská, S.; Fehrer, J.; Gryndler, M.; Vosátka, M.; Redecker, D. Long-term tracing of Rhizophagus irregularis isolate BEG140 inoculated on Phalaris arundinacea in a coal mine spoil bank, using mitochondrial large subunit rDNA markers. Mycorrhiza 2012, 22, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Lumini, E.; Bianciotto, V. AMF components from a microbial inoculum fail to colonize roots and lack soil persistence in an arable maize field. Symbiosis 2017, 72, 73–80. [Google Scholar] [CrossRef]
- Victorino, I.M.M.; Voyron, S.; Caser, M.; Orgiazzi, A.; Demasi, S.; Berruti, A.; Scariot, V.; Bianciotto, V.; Lumini, E. Metabarcoding of Soil Fungal Communities Associated with Alpine Field-Grown Saffron (Crocus sativus L.) Inoculated with AM Fungi. J. Fungi 2021, 7, 45. [Google Scholar] [CrossRef]
- Akyol, T.Y.; Niwa, R.; Hirakawa, H.; Maruyama, H.; Sato, T.; Suzuki, T.; Fukunaga, A.; Sato, T.; Yoshida, S.; Tawaraya, K.; et al. Impact of Introduction of Arbuscular Mycorrhizal Fungi on the Root Microbial Community in Agricultural Fields. Microbes Env. 2019, 34, 23–32. [Google Scholar] [CrossRef]
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G.A. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2019, 273, 13–24. [Google Scholar] [CrossRef]
- Renaut, S.; Daoud, R.; Masse, J.; Vialle, A.; Hijri, M. Inoculation with Rhizophagus Irregularis Does Not Alter Arbuscular Mycorrhizal Fungal Community Structure within the Roots of Corn, Wheat, and Soybean Crops. Microorganisms 2020, 8, 83. [Google Scholar] [CrossRef]
- Hernádi, I.; Magurno, F.; Sasvári, Z.; Posta, K. Effects of mycorrhizal inoculants on cultivation of two spice pepper types and local arbuscular mycorrhizal fungal community. J. Landsc. Ecol. 2017, 10, 305–313. [Google Scholar]
- Hernádi, I.; Sasvári, Z.; Albrechtová, J.; Vosátka, M.; Posta, K. Arbuscular mycorrhizal inoculant increases yield of spice pepper and affects the indigenous fungal community in the field. HortScience 2012, 47, 603–606. [Google Scholar] [CrossRef]
- Li, Y.; Gan, Y.; Lupwayi, N.; Hamel, C. Influence of introduced arbuscular mycorrhizal fungi and phosphorus sources on plant traits, soil properties, and rhizosphere microbial communities in organic legume-flax rotation. Plant Soil 2019, 443, 87–106. [Google Scholar] [CrossRef]
- Li, Y.; Laterrière, M.; Lay, C.-Y.; Klabi, R.; Masse, J.; St-Arnaud, M.; Yergeau, É.; Lupwayi, N.Z.; Gan, Y.; Hamel, C. Effects of arbuscular mycorrhizal fungi inoculation and crop sequence on root-associated microbiome, crop productivity and nutrient uptake in wheat-based and flax-based cropping systems. Appl. Soil Ecol. 2021, 168, 104136. [Google Scholar] [CrossRef]
- Niwa, R.; Koyama, T.; Sato, T.; Adachi, K.; Tawaraya, K.; Sato, S.; Hirakawa, H.; Yoshida, S.; Ezawa, T. Dissection of niche competition between introduced and indigenous arbuscular mycorrhizal fungi with respect to soybean yield responses. Sci. Rep. 2018, 8, 7419. [Google Scholar] [CrossRef] [PubMed]
- Loján, P.; Senés-Guerrero, C.; Suárez, J.P.; Kromann, P.; Schüßler, A.; Declerck, S. Potato field-inoculation in Ecuador with Rhizophagus irregularis: No impact on growth performance and associated arbuscular mycorrhizal fungal communities. Symbiosis 2016, 73, 45–56. [Google Scholar] [CrossRef]
- Kinnunen, M.; Dechesne, A.; Proctor, C.; Hammes, F.; Johnson, D.; Quintela-Baluja, M.; Graham, D.; Daffonchio, D.; Fodelianakis, S.; Hahn, N.; et al. A conceptual framework for invasion in microbial communities. ISME J. 2016, 10, 2773–2775. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.N. Establishment, Persistence, and Spread of a Commercial Arbuscular Mycorrhizal Fungal Inoculant in Viticulture. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 2018. [Google Scholar]
- Thomsen, C.; Loverock, L.; Kokkoris, V.; Holland, T.; Bowen, P.A.; Hart, M. Commercial arbuscular mycorrhizal fungal inoculant failed to establish in a vineyard despite priority advantage. PeerJ 2021, 9, e11119. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.; Turrini, A.; Gamper, H.A.; Cafa, G.; Bonari, E.; Young, J.P.W.; Giovannetti, M. Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol. 2012, 194, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Buysens, C.; Alaux, P.L.; César, V.; Huret, S.; Declerck, S.; Cranenbrouck, S. Tracing native and inoculated Rhizophagus irregularis in three potato cultivars (Charlotte, Nicola and Bintje) grown under field conditions. Appl. Soil Ecol. 2017, 115, 1–9. [Google Scholar] [CrossRef]
- Thioye, B.; van Tuinen, D.; Kane, A.; de Faria, S.M.; Ndiaye, C.; Duponnois, R.; Sylla, S.N.; Ba, A.M. Tracing Rhizophagus irregularis isolate IR27 in Ziziphus mauritiana roots under field conditions. Mycorrhiza 2019, 29, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Desirò, A. Arbuscular Mycorrhizas: The Lives of Beneficial Fungi and Their Plant Hosts. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015; pp. 235–245. [Google Scholar] [CrossRef]
- Martignoni, M.M.; Garnier, J.; Hart, M.M.; Tyson, R.C. Investigating the impact of the mycorrhizal inoculum on the resident fungal community and on plant growth. Ecol. Model. 2020, 438, 109321. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Cheeke, T.E.; Zheng, C.; Koziol, L.; Gurholt, C.R.; Bever, J.D. Sensitivity to AMF species is greater in late-successional than early-successional native or nonnative grassland plants. Ecology 2019, 100, e02855. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef]
- Kohl, L.; Lukasiewicz, C.E.; van der Heijden, M.G. Establishment and effectiveness of inoculated arbuscular mycorrhizal fungi in agricultural soils. Plant Cell Env. 2016, 39, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Imperiali, N.; Chiriboga, X.; Schlaeppi, K.; Fesselet, M.; Villacrés, D.; Jaffuel, G.; Bender, S.F.; Dennert, F.; Blanco-Pérez, R.; van der Heijden, M.G.A.; et al. Combined field inoculations of Pseudomonas bacteria, arbuscular mycorrhizal fungi, and entomopathogenic nematodes and their effects on wheat performance. Front. Plant Sci. 2017, 8, 1809. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, M.-H.M.; Luis, S.-C.J.; Lino, S.-S.; Ricardo, S.-P.; Jabín, B.-B.J. Arbuscular Mycorrhizal Fungi: Inoculum Dose Affects Plant Development and Performance of Sugarcane (Saccharum spp.) Plantlets During Acclimatization Stage. J. Soil Sci. Plant Nutr. 2022, 1–10. [Google Scholar] [CrossRef]
- Werner, G.D.; Kiers, E.T. Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytol. 2015, 205, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.; Pogiatzis, A.; Bowen, P.; Kokkoris, V.; Richards, A.; Holland, T.; Hart, M. Performance and Establishment of a Commercial Mycorrhizal Inoculant in Viticulture. Agriculture 2020, 10, 539. [Google Scholar] [CrossRef]
- NicolÁS, E.; Maestre-Valero, J.F.; AlarcÓN, J.J.; Pedrero, F.; Vicente-SÁNchez, J.; BernabÉ, A.; GÓMez-Montiel, J.; HernÁNdez, J.A.; FernÁNdez, F. Effectiveness and persistence of arbuscular mycorrhizal fungi on the physiology, nutrient uptake and yield of Crimson seedless grapevine. J. Agric. Sci. 2015, 153, 1084–1096. [Google Scholar] [CrossRef]
- Janouskova, M.; Krak, K.; Vosatka, M.; Puschel, D.; Storchova, H. Inoculation effects on root-colonizing arbuscular mycorrhizal fungal communities spread beyond directly inoculated plants. PLoS ONE 2017, 12, e0181525. [Google Scholar] [CrossRef]
- Ketola, T.; Saarinen, K.; Lindstrom, L. Propagule pressure increase and phylogenetic diversity decrease community’s susceptibility to invasion. BMC Ecol. 2017, 17, 15. [Google Scholar] [CrossRef]
- Jansa, J.; Oberholzer, H.-R.; Egli, S. Environmental determinants of the arbuscular mycorrhizal fungal infectivity of Swiss agricultural soils. Eur. J. Soil Biol. 2009, 45, 400–408. [Google Scholar] [CrossRef]
- Ducousso-Detrez, A.; Fontaine, J.; Lounes-Hadj Sahraoui, A.; Hijri, M. Diversity of Phosphate Chemical Forms in Soils and Their Contributions on Soil Microbial Community Structure Changes. Microorganisms 2022, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Silva, U.C.; Medeiros, J.D.; Leite, L.R.; Morais, D.K.; Cuadros-Orellana, S.; Oliveira, C.A.; de Paula Lana, U.G.; Gomes, E.A.; Dos Santos, V.L. Long-Term Rock Phosphate Fertilization Impacts the Microbial Communities of Maize Rhizosphere. Front. Microbiol. 2017, 8, 1266. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Wang, D.; Guo, X.; Yang, T.; Xiang, X.; Walder, F.; Chu, H. Differential Responses of Arbuscular Mycorrhizal Fungal Communities to Long-Term Fertilization in the Wheat Rhizosphere and Root Endosphere. Appl. Env. Microbiol. 2021, 87, e0034921. [Google Scholar] [CrossRef] [PubMed]
- Monokrousos, N.; Papatheodorou, E.M.; Orfanoudakis, M.; Jones, D.G.; Scullion, J.; Stamou, G.P. The effects of plant type, AMF inoculation and water regime on rhizosphere microbial communities. Eur. J. Soil Sci. 2019, 71, 265–278. [Google Scholar] [CrossRef]
- Symanczik, S.; Courty, P.E.; Boller, T.; Wiemken, A.; Al-Yahya’ei, M.N. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species. Mycorrhiza 2015, 25, 639–647. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Wang, X.; Liu, F.; Li, X.; Zhu, X. Soil properties and geography shape arbuscular mycorrhizal fungal communities in black land of China. Appl. Soil Ecol. 2021, 167, 104109. [Google Scholar] [CrossRef]
- Ryan, M.H.; Kidd, D.R.; Sandral, G.A.; Yang, Z.; Lambers, H.; Culvenor, R.A.; Stefanski, A.; Nichols, P.G.H.; Haling, R.E.; Simpson, R.J. High variation in the percentage of root length colonised by arbuscular mycorrhizal fungi among 139 lines representing the species subterranean clover (Trifolium subterraneum). Appl. Soil Ecol. 2016, 98, 221–232. [Google Scholar] [CrossRef]
- Svenningsen, N.B.; Watts-Williams, S.J.; Joner, E.J.; Battini, F.; Efthymiou, A.; Cruz-Paredes, C.; Nybroe, O.; Jakobsen, I. Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J. 2018, 12, 1296–1307. [Google Scholar] [CrossRef]
- Rodriguez, A.; Sanders, I.R. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015, 9, 1053–1061. [Google Scholar] [CrossRef]
- Sato, T.; Cheng, W.; Tawaraya, K. Effects of indigenous and introduced arbuscular mycorrhizal fungi on the growth of Allium fistulosum under field conditions. J. Soil Sci. Plant Nutr. 2021, 21, 2781–2790. [Google Scholar] [CrossRef]
- Floc’h, J.B.; Hamel, C.; Laterriere, M.; Tidemann, B.; St-Arnaud, M.; Hijri, M. Inter-Kingdom Networks of Canola Microbiome Reveal Bradyrhizobium as Keystone Species and Underline the Importance of Bulk Soil in Microbial Studies to Enhance Canola Production. Microb Ecol 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Koch, A.; Dunfield, K.; Hart, M.; Downing, A.; Rillig, M.; Klironomos, J. Influence of commercial inoculation with Glomus intraradices on the structure and functioning of an AM fungal community from an agricultural site. Plant Soil 2009, 317, 257–266. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah Jerry, A.; Franken, O.; Verbruggen, E.; Fellbaum Carl, R.; Kowalchuk George, A.; Hart Miranda, M.; Bago, A.; et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.B.; Nolimal, S.; Sosa-Hernandez, M.A.; Egan, C.; Kastens, J. Trait-based aerial dispersal of arbuscular mycorrhizal fungi. New Phytol. 2020, 228, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Rottier, E.; Campos, C.; Victorino, G.; Costa, J.M.; Coito, J.L.; Pereira, H.S.; Viegas, W.; Lopes, C. The effects of field inoculation of arbuscular mycorrhizal fungi through rye donor plants on grapevine performance and soil properties. Agric. Ecosyst. Environ. 2021, 313, 107369. [Google Scholar] [CrossRef]
- Elliott, A.J.; Daniell, T.J.; Cameron, D.D.; Field, K.J. A commercial arbuscular mycorrhizal inoculum increases root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. Plants People Planet 2020, 3, 588–599. [Google Scholar] [CrossRef]
- Borstler, B.; Raab, P.A.; Thiery, O.; Morton, J.B.; Redecker, D. Genetic diversity of the arbuscular mycorrhizal fungus Glomus intraradices as determined by mitochondrial large subunit rRNA gene sequences is considerably higher than previously expected. New Phytol. 2008, 180, 452–465. [Google Scholar] [CrossRef]
- Formey, D.; Moles, M.; Haouy, A.; Savelli, B.; Bouchez, O.; Becard, G.; Roux, C. Comparative analysis of mitochondrial genomes of Rhizophagus irregularis—syn. Glomus irregulare—reveals a polymorphism induced by variability generating elements. New Phytol. 2012, 196, 1217–1227. [Google Scholar] [CrossRef]
- Badri, A.; Stefani, F.O.P.; Lachance, G.; Roy-Arcand, L.; Beaudet, D.; Vialle, A.; Hijri, M. Molecular diagnostic toolkit for Rhizophagus irregularis isolate DAOM-197198 using quantitative PCR assay targeting the mitochondrial genome. Mycorrhiza 2016, 26, 721–733. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef]
- Kohout, P.; Sudová, R.; Janoušková, M.; Čtvrtlíková, M.; Hejda, M.; Pánková, H.; Slavíková, R.; Štajerová, K.; Vosátka, M.; Sýkorová, Z. Comparison of commonly used primer sets for evaluating arbuscular mycorrhizal fungal communities: Is there a universal solution? Soil Biol. Biochem. 2014, 68, 482–493. [Google Scholar] [CrossRef]
- Kruger, M.; Stockinger, H.; Kruger, C.; Schussler, A. DNA-based species level detection of Glomeromycota: One PCR primer set for all arbuscular mycorrhizal fungi. New Phytol. 2009, 183, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Schlaeppi, K.; Bender, S.F.; Mascher, F.; Russo, G.; Patrignani, A.; Camenzind, T.; Hempel, S.; Rillig, M.C.; van der Heijden, M.G. High-resolution community profiling of arbuscular mycorrhizal fungi. New Phytol. 2016, 212, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Kolarikova, Z.; Slavikova, R.; Kruger, C.; Kruger, M.; Kohout, P. PacBio sequencing of Glomeromycota rDNA: A novel amplicon covering all widely used ribosomal barcoding regions and its applicability in taxonomy and ecology of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, D.; Nadimi, M.; Iffis, B.; Hijri, M. Rapid mitochondrial genome evolution through invasion of mobile elements in two closely related species of arbuscular mycorrhizal fungi. PLoS ONE 2013, 8, e60768. [Google Scholar] [CrossRef]
- De la Providencia, I.E.; Nadimi, M.; Beaudet, D.; Rodriguez Morales, G.; Hijri, M. Detection of a transient mitochondrial DNA heteroplasmy in the progeny of crossed genetically divergent isolates of arbuscular mycorrhizal fungi. New Phytol. 2013, 200, 211–221. [Google Scholar] [CrossRef]
- Croll, D.; Giovannetti, M.; Koch, A.M.; Sbrana, C.; Ehinger, M.; Lammers, P.J.; Sanders, I.R. Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2009, 181, 924–937. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, M.; Song, F.; Liu, S.; Li, X.; Zhu, X. Comparative Analysis of Arbuscular Mycorrhizal Fungal Communities between Farmland and Woodland in the Black Soil Region of Northeast China. Agriculture 2021, 11, 866. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Z.; Yang, M.; Lu, S.; Cao, L.; Wang, X. Molecular Diversity and Distribution of Arbuscular Mycorrhizal Fungi at Different Elevations in Mt. Taibai of Qinling Mountain. Front. Microbiol. 2021, 12, 609386. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W.; Song, F.; Li, X. Diversity and composition of arbuscular mycorrhizal fungal communities in the cropland black soils of China. Glob. Ecol. Conserv. 2020, 22, e00964. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W.; Sun, L.; Song, F.; Li, X. Anthropogenic land use changes diversity and structure of arbuscular mycorrhizal fungal communities at 100-m scale in northeast China. Arch. Agron. Soil Sci. 2020, 67, 778–792. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Rana, K.; Prasad Meena, R.; Chandra Joshi, D. A consortium of arbuscular mycorrizal fungi improves nutrient uptake, biochemical response, nodulation and growth of the pea (Pisum sativum L.) under salt stress. Rhizosphere 2020, 15, 100235. [Google Scholar] [CrossRef]
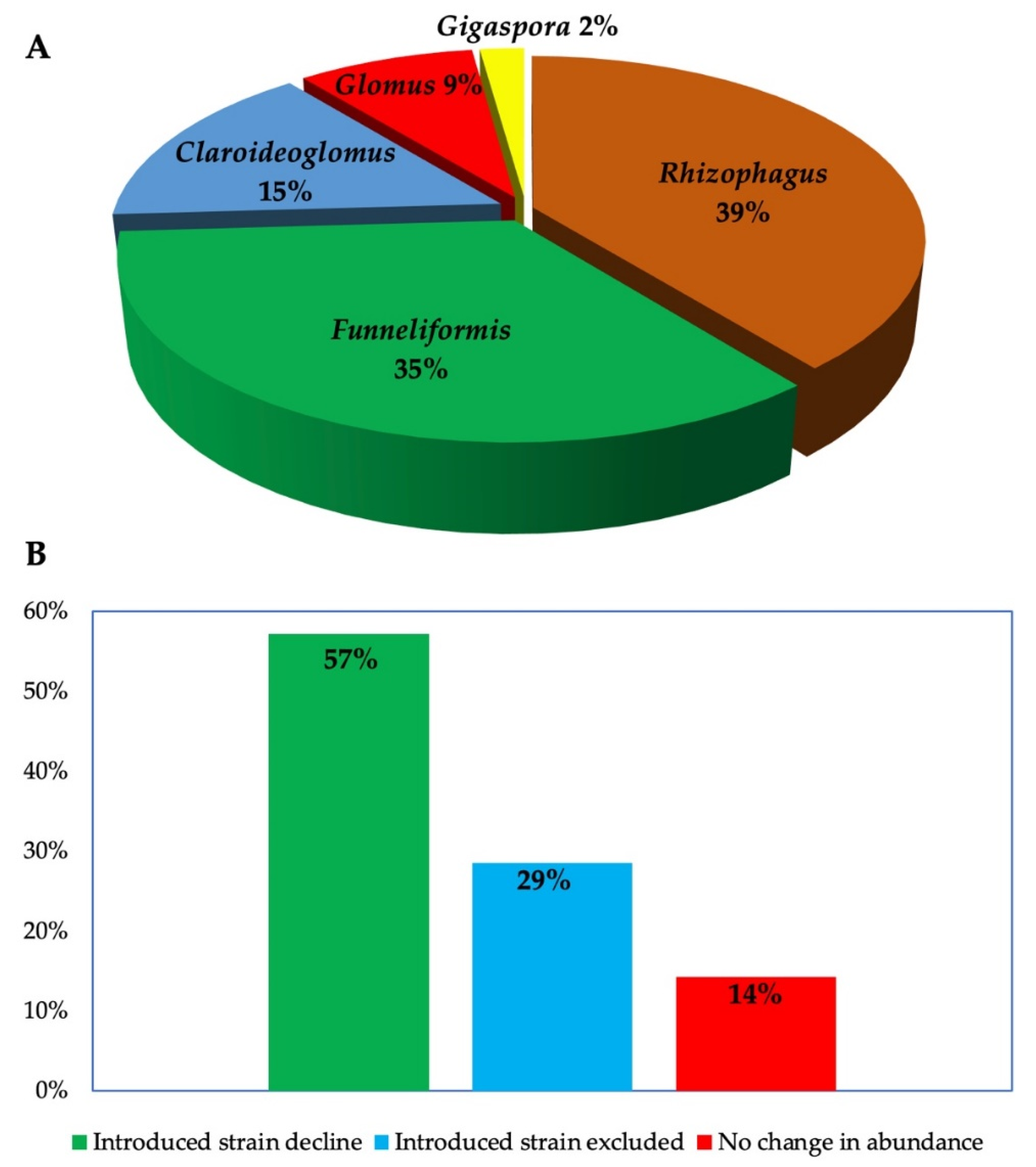

| AMF Name, Isolate (Brand, Manufacturer) | Location of Trial | Soil Type | Climate | Crop | Persistence Tracking Period | Change in Abundance of Introduced AMF over Time | References |
|---|---|---|---|---|---|---|---|
| Funneliformis mossaeae BEG12 and AZ225C, Rhizophagus irregularis BEG141 | Manciano (Grosseto) Italy | Haplic Calcisol or Inceptisol | Humid Mediterranean | Medicago sativa L. | 2 years | F. mosseae AZ225C was most persistent, followed by BEG12 and BEG141. Abundance of local F. mosseae cluster was reduced, while the abundance of native R. irregularis increased. | [45] |
| Rhizophagus Irregularis, DAOM 197198 (Myke Pro, Premier Tech) | Canada (Swift Current) | Brown Chernozem | Semi-arid | Pea-wheat | 2 years, 5 months | Inoculant decreased from 27% in first season to 15% in years two and three. | [41] |
| Canada (Outlook) | Dark Brown Chernozem | Semi-arid | 2 years, 5 months | Introduced AMF decreased from 17% in year one to 4% in years two and three. | |||
| Canada (Scott) | Chernozem | Sub-humid | 3 months | Relative abundance of introduced AMF was 33% but detection failed in subsequent years. | |||
| Canada (Melfort) | Black Chernozem | Sub-humid | 1 year, 3 months | AMF detected in year one with 10% abundance and 4% in year two but declined completely in year three. | |||
| Rhizophagus irregulare, DAOM 197198 (Myke Pro GR, Premier Tech) | Swift Current, Saskatchewan | _ | _ | Lentil Lens culinaris, Linum usitatissimum (Flax var. Bethune) rotation inoculation year 1 only | _ | Inoculation did not affect abundance of target AMF in roots. | [46] |
| Beaverlodge, Alberta | _ | _ | Pisum sativum, Linum usitatissimum (Flax var. Bethune) | _ | Inoculation did not affect abundance of target AMF in roots. | ||
| Melfort, Saskatchewan | _ | _ | _ | Abundance of introduced isolate was less in inoculated site than control | |||
| STR Saskatchewan | _ | _ | 2 years | Abundance of introduced isolate was higher in inoculated plots in year one, but did not differ in year two. | |||
| Rhizophagus Irregularis IR27 | Senegal | Tropical ferruginous | Semi-arid | Jujube (Ziziphus mauritiana lam., Tasset and Gola) | 1 years, 5 months | Abundance of R. irregularis was low (15% after 18 months). | [47] |
| Funneliformis Mosseae AZ225C and IMA1 | Pisa, Italy | Sandy loam | Mediterranea climate | M. sativa | 2 years | 2 years, relative proportion of F. mosseae decreased in favor of native species, abundance of isolate AZ225C dropped from 100% to 16.3%, while isolate IMA1 survived only three months. | [48] |
| Glomus sp., G. intraradices and a mixture of both | Vicente Banes, Molina de Segura, Southeastern Spain) | Typic Torriorthent (silty clay) | Semi-arid Mediteeranean climate | O. europaea | 1 year, 2 months | Abundance varied by AMF species: Glomus sp.: 20%, G. intraradices: 48.2%, and mixed (G. intraradices: 14%, Glomus sp.: 39.7%). | [49] |
| G. intraradices IMA6 and F. mosseae AZ 225C, and mixture of both | Italy | Mediterranean climate | Artichoke (Cynara cardunculus L. var. scolymus F.) | 3 months | Inoculation increased the abundance of Glomus OTUs in inoculated plants compared to control. | [12] | |
| Gigaspora margarita CK (cerakinkong, Central glass Co., Tokyo, Japan) | Mizunashi River, Mt Fugendake, Nagasaki Prefecture, Japan | Reforested soil | Eragrosis curvula (weeping love grass) and Miscanthus sinensis (Japanese Pampas grass) | 4 years | G. margariata isolate CK was detected in rhizosphere and root of E. curvula. | [50] | |
| Funneliformis (syn. Glomus) mosseae BEG12, 167 G. intraradices BEG 141 (IBG), G. eutenicatum BEG 168, (Endol, Biorize) | Daxing, Hebei Province, China | - | Sweet potato, (Ipomoea batatas L.) | 3 months | Contaminating AMF, G eutenicatum had longer persistence than G. mosseae, the establishment of which was not successful in year 2; similarly, low amounts of F. mosseae BEG 167 were detected compared to contaminating G. eutenicatum. | [51] | |
| R. irregularis GEG140, F. mosseae BEG95, C. claroideum BEG96, (Symbiom) | Coal mine spoil bank, Mekur, North Bohemia, Czech Republic | _ | _ | Phalaris arundinacea | 3 years | Introduced AMF persisted and co-existed with native strains. | [52] |
| AMF and Product Name | Molecular Method and Target Region | Crop | Location | Impacts on Native Community | References |
|---|---|---|---|---|---|
| Funneliformis mossaeae BEG12 and AZ225C, Rhizophagus irregularis BEG141 | SSU-ITS-LSU (8SrDNA) sequencing | Medicago sativa L. | Manciano (Grosseto) Italy | Local F. mosseae clusters was suppressed while native R. irregularis stimulated | [45] |
| R. irregularis DAOM197198, (Myke Pro) | SSU-ITS-LSU (18S rDNA) 454 Pyrosequencing | Pea–wheat rotation | Canada (Swift Current) | Indigenous Claroideglomus was suppressed in third season. | [41] |
| Canada (Outlook) | Abundance of Glomus and Funneliformis was decreased in year one, while Claroideglomus, Paraglomus, Archaeospora, and Diversispora were increased. Rhozophagus was excluded in third year. | ||||
| Canada (Scott) | Indigenous Claroideoglomus and Paraglomus were stimulated, while Funneliformis decreased | ||||
| Canada (Melfort) | Glomus, Funneliformis suppressed while Claoroideoglomus and Paraglomus stimulated. Rhizoglomus and Archaespora were excluded over three cropping seasons. | ||||
| R. irregularis IR27, lab-made inoculum propagated in greenhouse trap culture | LSU (18S rDNA) Illumina MiSeq | Jujube (Z. mauritiana | Senegal | Inoculation decreased Rhizophagus/Glomus ratio. | [47] |
| F. coronatum GO01, GU53, F. Caledonium GM24, R. intraradices GB6 and GG32, F. mosseae GP11 and GC11, and Septoglomus viscosum (GC41) | SSU (18S rDNA) 454 pyrosequencing | Fodder maize (Zea mays L. var. ‘Kalumet’ | Carmagnola, Italy | Inoculation induced an increase in alpha-diversity indices in roots by reducing species dominance. | [53] |
| R. intraradices, F. mosseae, and mixture of both (MycAgro Lab) | Illumina MiSeq (ITS2) | Saffron (Crocus sativus L.) | Saint Christophe, Italy (Morgex, Aosta Valley, Italy) | Inoculation did not impact field fungal communities; results differed by year of sampling and field. | [54] |
| Glomus sp. | Illumina MiSeq (18S rDNA) | Welsh onion cv. Motokura | Okasaki, Ayabe, Tsugaru, Japan | OTUs related to introduced AMF were decreased, while those belonging to distant taxa such as Gigaspora, and Acaulospora were consistently enriched. | [55] |
| R. irregularis GEG140, F. mosseae BEG95, C. claroideum BEG96 (Symbiom) | PCR-RFLP (25S rDNA) | Phalaris arundinacea | Coal mine spoil bank, Mekur, North Bohemia, Czech Republic | AMF persisted for 3 years and co-existed with native haplotypes of same species. | [52] |
| Rhizophagus irregularis SAF 22 Blazk, Wubet, Renker and Buscot | SSU-ITS-LSU (18S rDNA) SMRT and qPCR | Swiss corn | Eight farmers’ fields | Establishment of inoculated strains correlated negatively with root colonization. | [56] |
| R. irregularis DAOM 197198 (Myke Pro Liquid, Myke Pro Soybean Liquid, Myke Pro PS3, Premier Tech) | ITS-SSU (18S rDNA) Illumina MiSeq | Zea mays, cultivar Elite 49A12 Cruiser Max Quattro, | St-Elzear Quebec | Inoculation did not affect abundance or community diversity. | [57] |
| Glycine max cv. Pioneer, 90YO1, | Notre-Dame-du-Mont-Carmel, Quebec | ||||
| Wheat (T. aestivum), cv. Touran | Sainte-Helene-de-Kamoraska, Quebec | ||||
| Glomus spp. | SSU (18S rDNA) PCR-RFLP | Spice pepper (C. annuum L. var. longum), cv. Szegedi and cv. Kalocsai | Inoculation affected structure of resident AMF community, but there was no remarkable effect on AMF species composition. | [58] | |
| G. intraradices BEG140, G. mosseae BEG95, G. etunicatum BEG92, G. claroideum BEG96, G. microaggregatum BEG56, G. geoposporum BEG199 (Symbivit, Symbiom) | PCR-RFLP (18S rDNA) | Capsicum annuum L. var. longum and cv. Szegedi | Godollo, Hungary, Continental | Inoculation affected relative abundance of AMF ribotypes but did not influence composition. | [59] |
| R. irregularis DAOM 197198 (AGTIV) | Illumina MiSeq (ITS, mitochondrial rDNA) | Flax, lentil | Saskatchewan (Swift Current) | Single inoculation had no effect, but continuous AMF inoculation reduced Shannon diversity and Pielou’s evenness indices in flax rhizosphere in second rotation in Beaverlodge. | [60] |
| Alberta (Beaverlodge) | |||||
| R. irregularis GD50 | Illumina MiSeq (ITS) | Lentil–wheat, | Swift Current, Saskatchewan | No effect of inoculation in rotation phase 1, but AM altered fungal community structures of rhizosphere and root of flax grown in Swift Current in rotation phase 2. | [61] |
| Pea–flax | Beaverlodge | ||||
| R. irregularis R-10 | Illumina MiSeq (25S rDNA) | Glycine max (L.) Merrill. cv. Fukuyutaka | Kyushu, Okinawa Agricultural Research Center, Miyakonojo, Miyazaki Japan | Inoculation increased read abundance of inoculum R. irregularis (70%) compared to 30% in non-inoculated site, by competing for niche commonly distributed communities. | [62] |
| R. irregularis DAOM 19178 from four products: Myke Pro p-801, Myke Pro GR, Mycorise ASP, and Symplanta | 454 pyrosequencing (25S rDNA) | Solanum tuberosum c.v. INIAP-FIpapa | Zamora Huayco Research station, Loja | No effect on indigenous AMF community. Introduced AMFs were outcompeted by indigenous Acaulospora sp. | [63] |
| Santa Catalina Research Station, Ecuador |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiru, S.; Hijri, M. The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences. Microorganisms 2022, 10, 1897. https://doi.org/10.3390/microorganisms10101897
Basiru S, Hijri M. The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences. Microorganisms. 2022; 10(10):1897. https://doi.org/10.3390/microorganisms10101897
Chicago/Turabian StyleBasiru, Sulaimon, and Mohamed Hijri. 2022. "The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences" Microorganisms 10, no. 10: 1897. https://doi.org/10.3390/microorganisms10101897
APA StyleBasiru, S., & Hijri, M. (2022). The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences. Microorganisms, 10(10), 1897. https://doi.org/10.3390/microorganisms10101897







