Human Papillomavirus Infection during Pregnancy and Childhood: A Comprehensive Review
Abstract
:1. Introduction
2. HPV Transmission Routes
2.1. Vertical Transmission
2.1.1. Peri-Conceptual Transmission
2.1.2. Intrauterine Transmission
2.1.3. Perinatal Transmission
2.2. Non-Sexual Horizontal Transmission
2.3. Sexual Abuse
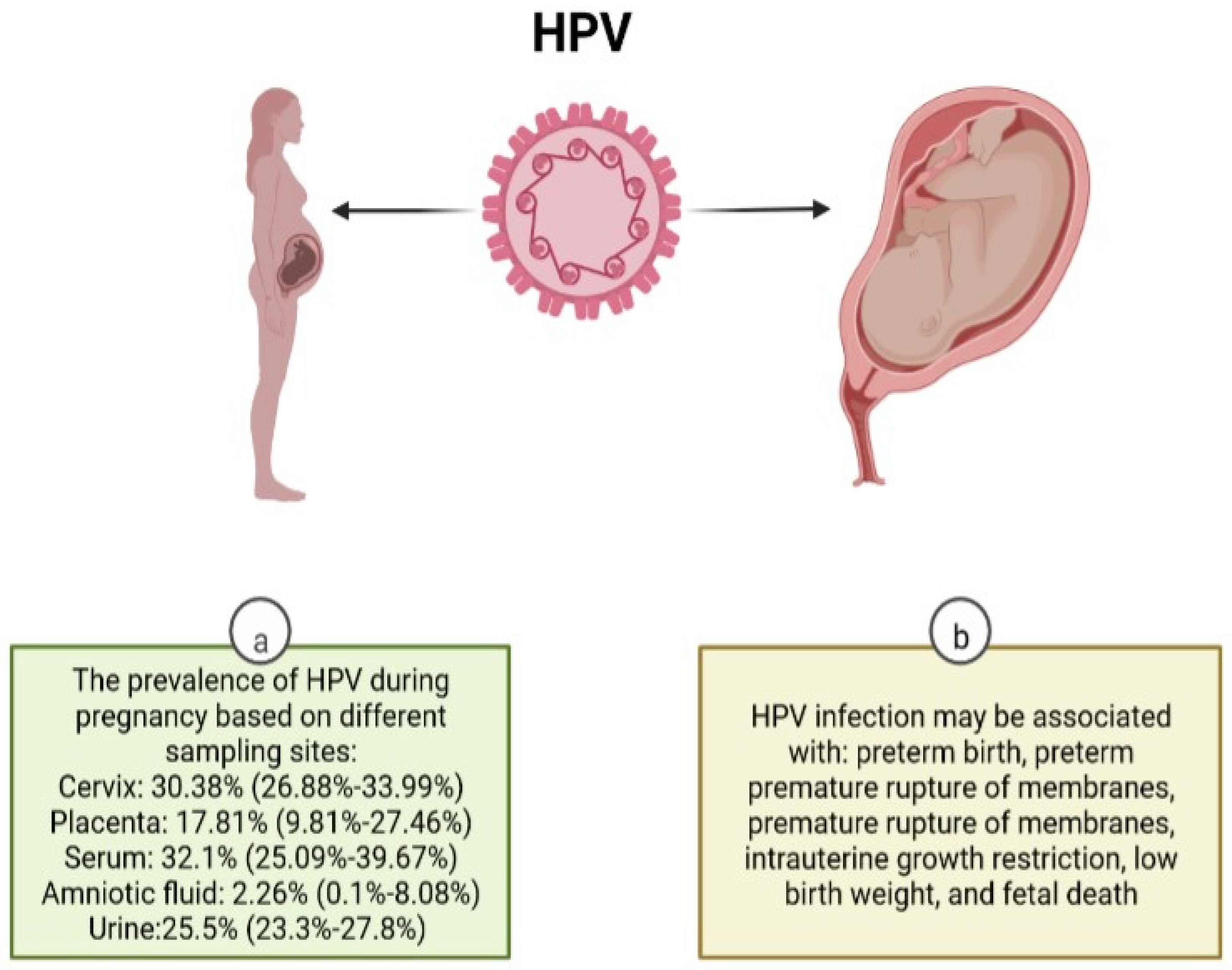
3. Pregnancy-Related Complications
4. HPV in Childhood
4.1. Skin Lesions
4.2. Mucosal Lesions
4.3. Juvenile Recurrent Respiratory Papillomatosis
4.4. Retinoblastoma
4.5. Conjunctival Papilloma
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Zandberg, D.P.; Bhargava, R.; Badin, S.; Cullen, K.J. The role of human papillomavirus in nongenital cancers. CA A Cancer J. Clin. 2013, 63, 57–81. [Google Scholar] [CrossRef]
- Steben, M.; Duarte-Franco, E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol. Oncol. 2007, 107, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.F.; Park, I.U. HPV and HPV-Associated Diseases. Infect. Dis. Clin. 2013, 27, 765–778. [Google Scholar] [CrossRef]
- Ardekani, A.; Sepidarkish, M.; Mollalo, A.; Afradiasbagharani, P.; Rouholamin, S.; Rezaeinejad, M.; Farid-Mojtahedi, M.; Mahjour, S.; Almukhtar, M.; Nourollahpour Shiadeh, M.; et al. Worldwide prevalence of human papillomavirus among pregnant women: A systematic review and meta-analysis. Rev. Med. Virol. 2022, e2374. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, J.; Zanré, N.; Mayrand, M.-H.; Trottier, H. Association between Maternal Human Papillomavirus Infection and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 221, 1925–1937. [Google Scholar] [CrossRef]
- Medeiros, L.R.; Ethur, A.B.; Hilgert, J.B.; Zanini, R.R.; Berwanger, O.; Bozzetti, M.C.; Mylius, L.C. Vertical transmission of the human papillomavirus: A systematic quantitative review. Cad. Saude Publica 2005, 21, 1006–1015. [Google Scholar] [CrossRef]
- Chatzistamatiou, K.; Sotiriadis, A.; Agorastos, T. Effect of mode of delivery on vertical human papillomavirus transmission—A meta-analysis. J. Obstet. Gynaecol. 2016, 36, 10–14. [Google Scholar] [CrossRef]
- Zouridis, A.; Kalampokas, T.; Panoulis, K.; Salakos, N.; Deligeoroglou, E. Intrauterine HPV transmission: A systematic review of the literature. Arch. Gynecol. Obstet. 2018, 298, 35–44. [Google Scholar] [CrossRef]
- LaCour, D.E.; Trimble, C. Human papillomavirus in infants: Transmission, prevalence, and persistence. J. Pediatr. Adolesc. Gynecol. 2012, 25, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Mammas, I.N.; Sourvinos, G.; Spandidos, D.A. Human papilloma virus (HPV) infection in children and adolescents. Eur. J. Pediatr. 2009, 168, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sabeena, S.; Bhat, P.; Kamath, V.; Arunkumar, G. Possible non-sexual modes of transmission of human papilloma virus. J. Obstet. Gynaecol. Res. 2017, 43, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Syrjanen, S.; Puranen, M. Human Papillomavirus Infections in Children: The Potential Role of Maternal Transmission. Crit. Rev. Oral Biol. Med. 2000, 11, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Park, J.S.; Norwitz, E.R.; Koo, J.N.; Oh, I.H.; Park, J.W.; Kim, S.M.; Kim, Y.H.; Park, C.-W.; Song, Y.S. Risk of Vertical Transmission of Human Papillomavirus throughout Pregnancy: A Prospective Study. PLoS ONE 2013, 8, e66368. [Google Scholar] [CrossRef] [PubMed]
- Zgura, A.F.; Bratila, E.; Vladareanu, S. Transplacental Transmission of Human Papillomavirus. Maedica 2015, 10, 159–162. [Google Scholar] [PubMed]
- Lyu, Z.; Feng, X.; Li, N.; Zhao, W.; Wei, L.; Chen, Y.; Yang, W.; Ma, H.; Yao, B.; Zhang, K.; et al. Human papillomavirus in semen and the risk for male infertility: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 714. [Google Scholar] [CrossRef]
- Foresta, C.; Garolla, A.; Zuccarello, D.; Pizzol, D.; Moretti, A.; Barzon, L.; Palù, G. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil. Steril. 2010, 93, 802–806. [Google Scholar] [CrossRef]
- Foresta, C.; Patassini, C.; Bertoldo, A.; Menegazzo, M.; Francavilla, F.; Barzon, L.; Ferlin, A. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS ONE 2011, 6, e15036. [Google Scholar] [CrossRef]
- Pereira, N.; Kucharczyk, K.M.; Estes, J.L.; Gerber, R.S.; Lekovich, J.P.; Elias, R.T.; Spandorfer, S.D. Human Papillomavirus Infection, Infertility, and Assisted Reproductive Outcomes. J. Pathog. 2015, 2015, 578423. [Google Scholar] [CrossRef]
- Rintala, M.A.M.; Pöllänen, P.P.; Nikkanen, V.P.; Grénman, S.E.; Syrjänen, S.M. Human Papillomavirus DNA Is Found in the Vas Deferens. J. Infect. Dis. 2002, 185, 1664–1667. [Google Scholar] [CrossRef] [Green Version]
- SyrjÄNen, S. Current concepts on human papillomavirus infections in children. APMIS 2010, 118, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; D’Adda, T.; Gnetti, L.; Froio, E.; Merisio, C.; Melpignano, M. Detection of human papillomavirus in organs of upper genital tract in women with cervical cancer. Int. J. Gynecol. Cancer 2006, 16, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.; Wong, L.; Xu, C.; Cheung, A.; Tsang, P.; Ngan, H. Detection of human papillomavirus DNA in malignant lesions from Chinese women with carcinomas of the upper genital tract. Gynecol. Oncol. 2002, 87, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cherif, S.; Amine, A.; Thies, S.; Taube, E.T.; Braicu, E.I.; Sehouli, J.; Kaufmann, A.M. Prevalence of human papillomavirus detection in ovarian cancer: A meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1791–1802. [Google Scholar] [CrossRef]
- Freitas, A.C.; Mariz, F.C.; Silva, M.A.R.; Jesus, A.L.S. Human Papillomavirus Vertical Transmission: Review of Current Data. Clin. Infect. Dis. 2013, 56, 1451–1456. [Google Scholar] [CrossRef]
- Rombaldi, R.L.; Serafini, E.P.; Mandelli, J.; Zimmermann, E.; Losquiavo, K.P. Transplacental transmission of Human Papillomavirus. Virol. J. 2008, 5, 106. [Google Scholar] [CrossRef]
- Trottier, H.; Mayrand, M.H.; Coutlée, F.; Monnier, P.; Laporte, L.; Niyibizi, J.; Carceller, A.M.; Fraser, W.D.; Brassard, P.; Lacroix, J.; et al. Human papillomavirus (HPV) perinatal transmission and risk of HPV persistence among children: Design, methods and preliminary results of the HERITAGE study. Papillomavirus Res. 2016, 2, 145–152. [Google Scholar] [CrossRef]
- Armbruster-Moraes, E.; Ioshimoto, L.M.; Leão, E.; Zugaib, M. Presence of human papillomavirus DNA in amniotic fluids of pregnant women with cervical lesions. Gynecol. Oncol. 1994, 54, 152–158. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Rao, H. Maternal-fetal transmission of human papillomavirus. Chin. Med. J. 1998, 111, 726–727. [Google Scholar]
- Weyn, C.; Thomas, D.; Jani, J.; Guizani, M.; Donner, C.; Van Rysselberge, M.; Hans, C.; Bossens, M.; Englert, Y.; Fontaine, V. Evidence of human papillomavirus in the placenta. J. Infect. Dis. 2011, 203, 341–343. [Google Scholar] [CrossRef]
- Sarkola, M.E.; Grénman, S.E.; Rintala, M.A.; Syrjänen, K.J.; Syrjänen, S.M. Human papillomavirus in the placenta and umbilical cord blood. Acta Obstet. Gynecol. Scand. 2008, 87, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Ermel, A. Human Papillomavirus Infections. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Cladel, N.M.; Jiang, P.; Li, J.J.; Peng, X.; Cooper, T.K.; Majerciak, V.; Balogh, K.K.; Meyer, T.J.; Brendle, S.A.; Budgeon, L.R.; et al. Papillomavirus can be transmitted through the blood and produce infections in blood recipients: Evidence from two animal models. Emerg. Microbes Infect. 2019, 8, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Pao, C.C.; Lin, S.S.; Lin, C.Y.; Maa, J.S.; Lai, C.H.; Hsieh, T.T. Identification of human papillomavirus DNA sequences in peripheral blood mononuclear cells. Am. J. Clin. Pathol. 1991, 95, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Koskimaa, H.M.; Waterboer, T.; Pawlita, M.; Grénman, S.; Syrjänen, K.; Syrjänen, S. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J. Pediatr. 2012, 160, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M.; Spong, C.Y. Neoplastic Disorders. In Williams Obstetrics, 25th ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Society, C.P. Skin care for your baby. Paediatr. Child. Health 2007, 12, 245–251. [Google Scholar] [CrossRef]
- Castellsagué, X.; Drudis, T.; Cañadas, M.P.; Goncé, A.; Ros, R.; Pérez, J.M.; Quintana, M.J.; Muñoz, J.; Albero, G.; de Sanjosé, S.; et al. Human Papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: A prospective study in Spain. BMC Infect. Dis. 2009, 9, 74. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Sánchez-Torices, M.S.; Corrales-Millan, R.; Hijona-Elosegui, J.J. Colonización orofaríngea perinatal por el virus del papiloma humano. Acta Otorrinolaringológica Española 2016, 67, 135–141. [Google Scholar] [CrossRef]
- Skoczyński, M.; Goździcka-Józefiak, A.; Kwaśniewska, A. The Prevalence of Human Papillomavirus between the Neonates and Their Mothers. Biomed. Res. Int. 2015, 2015, 126417. [Google Scholar] [CrossRef]
- Hahn, H.S.; Kee, M.K.; Kim, H.J.; Kim, M.Y.; Kang, Y.S.; Park, J.S.; Kim, T.J. Distribution of maternal and infant human papillomavirus: Risk factors associated with vertical transmission. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 202–206. [Google Scholar] [CrossRef]
- Hong, Y.; Li, S.Q.; Hu, Y.L.; Wang, Z.Q. Survey of human papillomavirus types and their vertical transmission in pregnant women. BMC Infect. Dis. 2013, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, S.W.; Lee, I.H.; Ryu, H.M.; Cho, A.R.; Kang, Y.S.; Hong, S.R.; Kim, S.S.; Seong, S.J.; Shin, S.M.; et al. Rate of vertical transmission of human papillomavirus from mothers to infants: Relationship between infection rate and mode of delivery. Virol. J. 2012, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.M.; Parker, M.A.; Rubenstein, L.M.; Haugen, T.H.; Hamsikova, E.; Turek, L.P. Evidence for vertical transmission of HPV from mothers to infants. Infect. Dis. Obstet. Gynecol. 2010, 2010, 326369. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, M.; Marianowski, L.; Wielgos, M.; Malejczyk, M.; Majewski, S. The occurrence of genital types of human papillomavirus in normal pregnancy and in pregnant women with pregestational insulin dependent diabetes mellitus. Neuro Endocrinol. Lett. 2005, 26, 766–770. [Google Scholar] [PubMed]
- Rintala, M.A.; Grénman, S.E.; Puranen, M.H.; Isolauri, E.; Ekblad, U.; Kero, P.O.; Syrjänen, S.M. Transmission of high-risk human papillomavirus (HPV) between parents and infant: A prospective study of HPV in families in Finland. J. Clin. Microbiol. 2005, 43, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Worda, C.; Huber, A.; Hudelist, G.; Schatten, C.; Leipold, H.; Czerwenka, K.; Eppel, W. Prevalence of cervical and intrauterine human papillomavirus infection in the third trimester in asymptomatic women. J. Soc. Gynecol. Investig. 2005, 12, 440–444. [Google Scholar] [CrossRef]
- Deng, D.; Wen, L.; Chen, W.; Ling, X. Asymptomatic genital infection of human papillomavirus in pregnant women and the vertical transmission route. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2005, 25, 343–345. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Sen, S.; Majumdar, L.; Chatterjee, R. Human papillomavirus infection among Indian mothers and their infants. Asian Pac. J. Cancer Prev. 2003, 4, 179–184. [Google Scholar]
- Peng, P.; Weng, X.; Gu, Z. Detection of the asymptomatic infection by human papillomavirus in pregnant women and neonates. Zhonghua Fu Chan Ke Za Zhi 2000, 35, 523–526. [Google Scholar]
- Tenti, P.; Zappatore, R.; Migliora, P.; Spinillo, A.; Belloni, C.; Carnevali, L. Perinatal transmission of human papillomavirus from gravidas with latent infections. Obstet. Gynecol. 1999, 93, 475–479. [Google Scholar] [CrossRef]
- Tseng, C.J.; Liang, C.C.; Soong, Y.K.; Pao, C.C. Perinatal transmission of human papillomavirus in infants: Relationship between infection rate and mode of delivery. Obstet. Gynecol. 1998, 91, 92–96. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.; Lu, S.; Ren, S. Clinical observation on vertical transmission of human papillomavirus. Chin. Med. Sci. J. Chung-Kuo I Hsueh K’o Hsueh Tsa Chih 1998, 13, 29–31. [Google Scholar] [PubMed]
- Watts, D.H.; Koutsky, L.A.; Holmes, K.K.; Goldman, D.; Kuypers, J.; Kiviat, N.B.; Galloway, D.A. Low risk of perinatal transmission of human papillomavirus: Results from a prospective cohort study. Am. J. Obstet. Gynecol. 1998, 178, 365–373. [Google Scholar] [CrossRef]
- Puranen, M.H.; Yliskoski, M.H.; Saarikoski, S.V.; Syrjänen, K.J.; Syrjänen, S.M. Exposure of an infant to cervical human papillomavirus infection of the mother is common. Am. J. Obstet. Gynecol. 1997, 176, 1039–1045. [Google Scholar] [CrossRef]
- Alberico, S.; Pinzano, R.; Comar, M.; Toffoletti, F.; Maso, G.; Ricci, G.; Guaschino, S. Maternal-fetal transmission of human papillomavirus. Minerva Ginecol. 1996, 48, 199–204. [Google Scholar] [PubMed]
- Cason, J.; Kaye, J.N.; Jewers, R.J.; Kambo, P.K.; Bible, J.M.; Kell, B.; Shergill, B.; Pakarian, F.; Raju, K.S.; Best, J.M. Perinatal infection and persistence of human papillomavirus types 16 and 18 in infants. J. Med. Virol. 1995, 47, 209–218. [Google Scholar] [CrossRef]
- Pakarian, F.; Kaye, J.; Cason, J.; Kell, B.; Jewers, R.; Derias, N.W.; Raju, K.S.; Best, J.M. Cancer associated human papillomaviruses: Perinatal transmission and persistence. Br. J. Obstet. Gynaecol. 1994, 101, 514–517. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef]
- Benjamin, L.T.; Levy, M.; Ofori, A. Condylomata Acuminata (Anogenital Warts) in Children; UpToDate: Waltham, MA, USA, 2014. [Google Scholar]
- Campbell, C.M.P.; Kreimer, A.R.; Lin, H.Y.; Fulp, W.; O’Keefe, M.T.; Ingles, D.J.; Abrahamsen, M.; Villa, L.L.; Lazcano-Ponce, E.; Giuliano, A.R. Long-term persistence of oral human papillomavirus type 16: The HPV Infection in Men (HIM) study. Cancer Prev. Res. 2015, 8, 190–196. [Google Scholar] [CrossRef]
- Rautava, J.; Willberg, J.; Louvanto, K.; Wideman, L.; Syrjänen, K.; Grénman, S.; Syrjänen, S. Prevalence, Genotype Distribution and Persistence of Human Papillomavirus in Oral Mucosa of Women: A Six-Year Follow-Up Study. PLoS ONE 2012, 7, e42171. [Google Scholar] [CrossRef]
- Cason, J.; Rice, P.; Best, J.M. Transmission of cervical cancer-associated human papilloma viruses from mother to child. Intervirology 1998, 41, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-W.; Wang, W.-J.; Li, C.-H.; Xu, L.; Liu, X.-Y.; Zheng, L.; Liu, D.-X. A Case of Condyloma Acuminatum on the Nipple Detected via Dermoscopy. Int. J. Dermatol. Venereol. 2020, 3, 125–126. [Google Scholar] [CrossRef]
- Saeki, Y.; Sato, S.; Okajima, K.; Ando, N.; Saeki, H.; Kawase, M.; Ito, K.; Nakagawa, H.; Ohtsuki, M. Condyloma acuminatum of the nipple and areola. Int. J. Dermatol. 2014, 53, e171–e172. [Google Scholar] [CrossRef] [PubMed]
- Sonnex, C.; Strauss, S.; Gray, J.J. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex. Transm. Infect. 1999, 75, 317–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryndock, E.J.; Meyers, C. A risk for non-sexual transmission of human papillomavirus? Expert Rev. Anti-Infect. Ther. 2014, 12, 1165–1170. [Google Scholar] [CrossRef]
- Gallay, C.; Miranda, E.; Schaefer, S.; Catarino, R.; Jacot-Guillarmod, M.; Menoud, P.A.; Guerry, F.; Achtari, C.; Sahli, R.; Vassilakos, P.; et al. Human papillomavirus (HPV) contamination of gynaecological equipment. Sex. Transm. Infect. 2016, 92, 19–23. [Google Scholar] [CrossRef]
- Strauss, S.; Sastry, P.; Sonnex, C.; Edwards, S.; Gray, J. Contamination of environmental surfaces by genital human papillomaviruses. Sex. Transm. Infect. 2002, 78, 135–138. [Google Scholar] [CrossRef]
- Johnson, L.W. Communal showers and the risk of plantar warts. J Fam Pract 1995, 40, 136–138. [Google Scholar]
- Houlihan, C.F.; Baisley, K.; Bravo, I.G.; Pavón, M.A.; Changalucha, J.; Kapiga, S.; De Sanjosé, S.; Ross, D.A.; Hayes, R.J.; Watson-Jones, D. Human papillomavirus DNA detected in fingertip, oral and bathroom samples from unvaccinated adolescent girls in Tanzania. Sex. Transm. Infect. 2019, 95, 374–379. [Google Scholar] [CrossRef]
- de Martino, M.; Haitel, A.; Wrba, F.; Schatzl, G.; Klatte, T.; Waldert, M. High-risk human papilloma virus infection of the foreskin in asymptomatic boys. Urology 2013, 81, 869–872. [Google Scholar] [CrossRef]
- Liel, C.; Ulrich, S.M.; Lorenz, S.; Eickhorst, A.; Fluke, J.; Walper, S. Risk factors for child abuse, neglect and exposure to intimate partner violence in early childhood: Findings in a representative cross-sectional sample in Germany. Child Abuse. Negl. 2020, 106, 104487. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T.M.; Kuiper, K.C.; van der Put, C.E.; Stams, G.J.M.; Assink, M. Risk factors for child neglect: A meta-analytic review. Child Abuse. Negl. 2018, 77, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.R.; Fajman, N.N.; Maloney, E.M.; Onyekwuluje, J.; Swan, D.C.; Howard, L.; Beck-Sague, C.M.; Sawyer, M.K.; Girardet, R.G.; Sautter, R.L.; et al. Anogenital Human Papillomavirus in Sexually Abused and Nonabused Children: A Multicenter Study. Pediatrics 2011, 128, e658–e665. [Google Scholar] [CrossRef] [PubMed]
- Hornor, G. Ano-genital warts in children: Sexual abuse or not? J. Pediatr. Health Care 2004, 18, 165–170. [Google Scholar] [CrossRef]
- Ingram, D.L.; Everett, V.D.; Lyna, P.R.; White, S.T.; Rockwell, L.A. Epidemiology of adult sexually transmitted disease agents in children being evaluated for sexual abuse. Pediatr. Infect. Dis. J. 1992, 11, 945–950. [Google Scholar] [CrossRef]
- Muram, D. Anal and perianal abnormalities in prepubertal victims of sexual abuse. Am. J. Obstet. Gynecol. 1989, 161, 278–281. [Google Scholar] [CrossRef]
- Awasthi, S.; Ornelas, J.; Armstrong, A.; Johnson, J.A.; Eisen, D.B. Anogenital warts and relationship to child sexual abuse: Systematic review and meta-analysis. Pediatric Dermatol. 2021, 38, 842–850. [Google Scholar] [CrossRef]
- Bussen, S.; Sütterlin, M.; Schmidt, U.; Bussen, D. Anogenital Warts in Childhood—Always a Marker for Sexual Abuse? Geburtshilfe Frauenheilkd 2012, 72, 43–48. [Google Scholar] [CrossRef]
- Obalek, S.; Jablonska, S.; Favre, M.; Walczak, L.; Orth, G. Condylomata acuminata in children: Frequent association with human papillomaviruses responsible for cutaneous warts. J. Am. Acad. Dermatol. 1990, 23, 205–213. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation during Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Akhavan, S.; Mousavi, A.; Modaresgilani, M.; Alibakhshi, A. Genital Warts. J. Obstet. Gynecol. Cancer Res. 2017, 2, e11440. [Google Scholar]
- Sugai, S.; Nishijima, K.; Enomoto, T. Management of Condyloma Acuminata in Pregnancy: A Review. Sex. Transm. Dis. 2021, 48, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Shehata, H.A.; Nelson-Piercy, C. Drugs to avoid. Best Pract. Res. Clin. Obstet. Gynaecol. 2001, 15, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). HPV Vaccination Recommendations. Available online: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html (accessed on 23 July 2022).
- Moreira, E.D., Jr.; Block, S.L.; Ferris, D.; Giuliano, A.R.; Iversen, O.E.; Joura, E.A.; Kosalaraksa, P.; Schilling, A.; Van Damme, P.; Bornstein, J.; et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics 2016, 138, e20154387. [Google Scholar] [CrossRef]
- Kharbanda, E.O.; Vazquez-Benitez, G.; DeSilva, M.B.; Naleway, A.L.; Klein, N.P.; Hechter, R.C.; Glanz, J.M.; Donahue, J.G.; Jackson, L.A.; Sheth, S.S.; et al. Association of Inadvertent 9-Valent Human Papillomavirus Vaccine in Pregnancy with Spontaneous Abortion and Adverse Birth Outcomes. JAMA Netw. Open 2021, 4, e214340. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, E.O.; Vazquez-Benitez, G.; Lipkind, H.S.; Sheth, S.S.; Zhu, J.; Naleway, A.L.; Klein, N.P.; Hechter, R.; Daley, M.F.; Donahue, J.G.; et al. Risk of Spontaneous Abortion after Inadvertent Human Papillomavirus Vaccination in Pregnancy. Obstet. Gynecol. 2018, 132, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lipkind, H.S.; Vazquez-Benitez, G.; Nordin, J.D.; Romitti, P.A.; Naleway, A.L.; Klein, N.P.; Hechter, R.C.; Jackson, M.L.; Hambidge, S.J.; Lee, G.M.; et al. Maternal and Infant Outcomes After Human Papillomavirus Vaccination in the Periconceptional Period or during Pregnancy. Obstet. Gynecol. 2017, 130, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.T.; Duun-Henriksen, A.K.; Dehlendorff, C.; Tatla, M.K.; Munk, C.; Kjaer, S.K. Adverse pregnancy outcomes and infant mortality after quadrivalent HPV vaccination during pregnancy. Vaccine 2019, 37, 265–271. [Google Scholar] [CrossRef]
- Sy, L.S.; Meyer, K.I.; Klein, N.P.; Chao, C.; Velicer, C.; Cheetham, T.C.; Ackerson, B.K.; Slezak, J.M.; Takhar, H.S.; Hansen, J.; et al. Postlicensure safety surveillance of congenital anomaly and miscarriage among pregnancies exposed to quadrivalent human papillomavirus vaccine. Hum. Vaccines Immunother. 2018, 14, 412–419. [Google Scholar] [CrossRef]
- Condrat, C.E.; Filip, L.; Gherghe, M.; Cretoiu, D.; Suciu, N. Maternal HPV Infection: Effects on Pregnancy Outcome. Viruses 2021, 13, 2455. [Google Scholar] [CrossRef]
- Popescu, S.D.; Boiangiu, A.G.; Sima, R.M.; Bilteanu, L.; Vladareanu, S.; Vladareanu, R. Maternal HPV Infection and the Estimated Risks for Adverse Pregnancy Outcomes-A Systematic Review. Diagnostics 2022, 12, 1471. [Google Scholar] [CrossRef]
- Nimrodi, M.; Kleitman, V.; Wainstock, T.; Gemer, O.; Meirovitz, M.; Maymon, E.; Benshalom-Tirosh, N.; Erez, O. The association between cervical inflammation and histologic evidence of HPV in PAP smears and adverse pregnancy outcome in low risk population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Capra, G.; Schillaci, R.; Bosco, L.; Roccheri, M.C.; Perino, A.; Ragusa, M.A. HPV infection in semen: Results from a new molecular approach. Epidemiol. Infect. 2019, 147, e177. [Google Scholar] [CrossRef] [PubMed]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.; Waterboer, T.; Rintala, M.; Pawlita, M.; Syrjänen, K.; Louvanto, K.; Grenman, S. Maternal HPV-antibodies and seroconversion to HPV in children during the first 3 years of life. Sci. Rep. 2022, 12, 2227. [Google Scholar] [CrossRef]
- Cason, J.; Mant, C.A. High-risk mucosal human papillomavirus infections during infancy & childhood. J. Clin. Virol. 2005, 32 (Suppl. S1), S52–S58. [Google Scholar] [CrossRef]
- Lee, B.; Lee, S.W.; Kim, D.I.; Kim, J.H. HPV prevalence in the foreskins of asymptomatic healthy infants and children: Systematic review and meta-analysis. Sci. Rep. 2017, 7, 7050. [Google Scholar] [CrossRef]
- Juckett, G.; Hartman-Adams, H. Human papillomavirus: Clinical manifestations and prevention. Am. Fam. Physician 2010, 82, 1209–1213. [Google Scholar]
- Leto, M.; Santos Júnior, G.F.; Porro, A.M.; Tomimori, J. Human papillomavirus infection: Etiopathogenesis, molecular biology and clinical manifestations. An. Bras. Dermatol. 2011, 86, 306–317. [Google Scholar] [CrossRef]
- Aguilera-Barrantes, I.; Magro, C.; Nuovo, G.J. Verruca Vulgaris of the Vulva in Children and Adults: A Nonvenereal Type of Vulvar Wart. Am. J. Surg. Pathol. 2007, 31, 529–535. [Google Scholar] [CrossRef]
- Van Haalen, F.M.; Bruggink, S.C.; Gussekloo, J.; Assendelft, W.J.J.; Eekhof, J.A.H. Warts in primary schoolchildren: Prevalence and relation with environmental factors. Br. J. Dermatol. 2009, 161, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, M.; Fernandes, I.; Rodrigues, A.G.; Lisboa, C. Anogenital warts in pediatric population. An. Bras. Dermatol. 2017, 92, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, Y.; Garland, S.M. Genital warts in children: What do they mean? Arch. Dis. Child. 2006, 91, 696–700. [Google Scholar] [CrossRef]
- Wiley, D.J.; Douglas, J.; Beutner, K.; Cox, T.; Fife, K.; Moscicki, A.-B.; Fukumoto, L. External Genital Warts: Diagnosis, Treatment, and Prevention. Clin. Infect. Dis. 2002, 35, S210–S224. [Google Scholar] [CrossRef] [PubMed]
- Zavras, N.; Christianakis, E.; Tsamoudaki, S.; Velaoras, K. Infantile perianal pyramidal protrusion: A report of 8 new cases and a review of the literature. Case Rep. Dermatol. 2012, 4, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Berbegal-DeGracia, L.; Betlloch-Mas, I.; DeLeon-Marrero, F.J.; Martinez-Miravete, M.T.; Miralles-Botella, J. Neonatal Molluscum contagiosum: Five new cases and a literature review. Australas. J. Dermatol. 2015, 56, e35–e38. [Google Scholar] [CrossRef]
- Pourang, A.; Fung, M.A.; Tartar, D.; Brassard, A. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021, 10, 18–21. [Google Scholar] [CrossRef]
- Allen, A.L.; Siegfried, E.C. The natural history of condyloma in children. J. Am. Acad. Dermatol. 1998, 39, 951–955. [Google Scholar] [CrossRef]
- Boull, C.; Groth, D. Update: Treatment of Cutaneous Viral Warts in Children. Pediatr. Dermatol. 2011, 28, 217–229. [Google Scholar] [CrossRef]
- Carroll, K.A.; Pierce, J.; Kovarik, C.L. Perianal Bowen disease in a child with human immunodeficiency virus. Pediatr. Dermatol. 2010, 27, 166–169. [Google Scholar] [CrossRef]
- Betz, S.J. HPV-Related Papillary Lesions of the Oral Mucosa: A Review. Head Neck Pathol. 2019, 13, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Martinelli-Kläy, C.P.; Lombardi, T. Clinical, histopathological and immunohistochemical study of oral squamous papillomas. Acta Odontol. Scand. 2015, 73, 508–515. [Google Scholar] [CrossRef]
- Ward, K.A.; Napier, S.S.; Winter, P.C.; Maw, R.D.; Dinsmore, W.W. Detection of human papilloma virus DNA sequences in oral squamous cell papillomas by the polymerase chain reaction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 63–66. [Google Scholar] [CrossRef]
- Bennett, L.K.; Hinshaw, M. Heck’s Disease: Diagnosis and Susceptibility. Pediatr. Dermatol. 2009, 26, 87–89. [Google Scholar] [CrossRef]
- Word Health Organization. Cervical Cancer. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 15 August 2022).
- Balarezo, F.S.; Joshi, V.V. Proliferative and neoplastic disorders in children with acquired immunodeficiency syndrome. Adv. Anat. Pathol. 2002, 9, 360–370. [Google Scholar] [CrossRef]
- Lee, N.V.; Kang, E.T.B.; Senger, C.; Poh, C.F. Oral cancer in a 5-year-old boy: A rare case report and review of literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, e10–e19. [Google Scholar] [CrossRef] [PubMed]
- Joos, B.; Joos, N.; Bumpous, J.; Burns, C.; French, C.A.; Farghaly, H. Laryngeal Squamous Cell Carcinoma in a 13 Year-Old Child Associated with Human Papillomaviruses 16 and 18: A Case Report and Review of the Literature. Head Neck Pathol. 2008, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, C.W.; Tabrizi, S.N.; Tiedemann, K.; Waters, K.D. Squamous cell carcinomas in children and young adults: A new wave of a very rare tumor? J. Pediatric Surg. 2007, 42, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Donne, A.J.; Hampson, L.; Homer, J.J.; Hampson, I.N. The role of HPV type in Recurrent Respiratory Papillomatosis. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 7–14. [Google Scholar] [CrossRef]
- Seedat, R.Y. Juvenile-Onset Recurrent Respiratory Papillomatosis Diagnosis and Management—A Developing Country Review. Pediatr. Health Med. Ther. 2020, 11, 39–46. [Google Scholar] [CrossRef]
- Scadding, G.K.; Bull, P.D.; Graham, J.M. (Eds.) Recurrent Respiratory Papillomatosis. In Pediatric ENT; Springer: Berlin/Heidelberg, Germany, 2007; pp. 255–265. [Google Scholar] [CrossRef]
- Quick, C.A.; Watts, S.L.; Krzyzek, R.A.; Faras, A.J. Relationship between condylomata and laryngeal papillomata. Clinical and molecular virological evidence. Ann. Otol Rhinol. Laryngol. 1980, 89, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.J.; Thorsen, P.; Lindeberg, H.; Grant, L.A.; Shah, K.V. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet. Gynecol. 2003, 101, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Amiling, R.; Meites, E.; Querec, T.D.; Stone, L.; Singh, V.; Unger, E.R.; Derkay, C.S.; Markowitz, L.E. Juvenile-Onset Recurrent Respiratory Papillomatosis in the United States, Epidemiology and HPV Types—2015–2020. J. Pediatric Infect. Dis. Soc. 2021, 10, 774–781. [Google Scholar] [CrossRef]
- Lépine, C.; Voron, T.; Berrebi, D.; Mandavit, M.; Nervo, M.; Outh-Gauer, S.; Péré, H.; Tournier, L.; Teissier, N.; Tartour, E.; et al. Juvenile-Onset Recurrent Respiratory Papillomatosis Aggressiveness: In Situ Study of the Level of Transcription of HPV E6 and E7. Cancers 2020, 12, 2836. [Google Scholar] [CrossRef]
- Bonagura, V.R.; Vambutas, A.; DeVoti, J.A.; Rosenthal, D.W.; Steinberg, B.M.; Abramson, A.L.; Shikowitz, M.J.; Gjertson, D.W.; Reed, E.F. HLA alleles, IFN-gamma responses to HPV-11 E6, and disease severity in patients with recurrent respiratory papillomatosis. Hum. Immunol. 2004, 65, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Bonagura, V.R.; Du, Z.; Ashouri, E.; Luo, L.; Hatam, L.J.; DeVoti, J.A.; Rosenthal, D.W.; Steinberg, B.M.; Abramson, A.L.; Gjertson, D.W.; et al. Activating killer cell immunoglobulin-like receptors 3DS1 and 2DS1 protect against developing the severe form of recurrent respiratory papillomatosis. Hum. Immunol. 2010, 71, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Gélinas, J.F.; Manoukian, J.; Côté, A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: A systematic review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 433–452. [Google Scholar] [CrossRef]
- Montaño-Velázquez, B.B.; Nolasco-Renero, J.; Parada-Bañuelos, J.E.; Garcia-Vázquez, F.; Flores-Medina, S.; García-Romero, C.S.; Jáuregui-Renaud, K. Quality of life of young patients with recurrent respiratory papillomatosis. J. Laryngol. Otol. 2017, 131, 425–428. [Google Scholar] [CrossRef]
- Ivancic, R.; Iqbal, H.; deSilva, B.; Pan, Q.; Matrka, L. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig. Otolaryngol. 2018, 3, 22–34. [Google Scholar] [CrossRef]
- Ouda, A.M.; Elsabagh, A.A.; Elmakaty, I.M.; Gupta, I.; Vranic, S.; Al-Thawadi, H.; Al Moustafa, A.E. HPV and Recurrent Respiratory Papillomatosis: A Brief Review. Life 2021, 11, 1279. [Google Scholar] [CrossRef]
- Rosen, C.A.; Bryson, P.C. Indole-3-carbinol for recurrent respiratory papillomatosis: Long-term results. J. Voice 2004, 18, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, T.; Philipsen, B.B.; Mehlum, C.S.; Dyrvig, A.K.; Wehberg, S.; Chirilǎ, M.; Godballe, C. Therapeutic Use of the Human Papillomavirus Vaccine on Recurrent Respiratory Papillomatosis: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2019, 219, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Best, S.R.; Mohr, M.; Zur, K.B. Systemic bevacizumab for recurrent respiratory papillomatosis: A national survey. Laryngoscope 2017, 127, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Evers, G.; Schliemann, C.; Beule, A.; Schmidt, L.-H.; Schulze, A.B.; Kessler, C.; Hoffmann, T.K.; Wiewrodt, R.; Groll, A.H.; Bleckmann, A.; et al. Long-Term Follow-Up on Systemic Bevacizumab Treatment in Recurrent Respiratory Papillomatosis. Laryngoscope 2021, 131, E1926–E1933. [Google Scholar] [CrossRef] [PubMed]
- Gerein, V.; Rastorguev, E.; Gerein, J.; Jecker, P.; Pfister, H. Use of interferon-alpha in recurrent respiratory papillomatosis: 20-year follow-up. Ann. Otol. Rhinol. Laryngol. 2005, 114, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Broekema, F.I.; Dikkers, F.G. Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur. Arch. Otorhinolaryngol. 2008, 265, 871–879. [Google Scholar] [CrossRef]
- Allen, C.T.; Lee, S.; Norberg, S.M.; Kovalovsky, D.; Ye, H.; Clavijo, P.E.; Hu-Lieskovan, S.; Schlegel, R.; Schlom, J.; Strauss, J.; et al. Safety and clinical activity of PD-L1 blockade in patients with aggressive recurrent respiratory papillomatosis. J. ImmunoTherapy Cancer 2019, 7, 119. [Google Scholar] [CrossRef]
- Limsukon, A.; Susanto, I.; Soo Hoo, G.W.; Dubinett, S.M.; Batra, R.K. Regression of recurrent respiratory papillomatosis with celecoxib and erlotinib combination therapy. Chest 2009, 136, 924–926. [Google Scholar] [CrossRef]
- Rojas-Lechuga, M.J.; Remacha, J.; González-Sánchez, N.; Grau, J.J.; Castillo, P.; Haag, O.; Vilaseca, I. Juvenile recurrent respiratory papillomatosis treated with combined erlotinib and celecoxib: Initial report. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110194. [Google Scholar] [CrossRef]
- Mitra, S.; Das, A.; Ghosh, D.; Sengupta, A. Postoperative Systemic Acyclovir in Juvenile-Onset Recurrent Respiratory Papillomatosis: The Outcome. Ear Nose Throat J. 2019, 98, 28–31. [Google Scholar] [CrossRef]
- Morrison, G.A.J.; Kotecha, B.; Evans, J.N.G. Ribavirin treatment for juvenile respiratory papillomatosis. J. Laryngol. Otol. 1993, 107, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Honavar, S.G. Retinoblastoma. Indian J. Pediatrics 2017, 84, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H.; Kimani, K.; Dimba, E.A.O.; Gronsdahl, P.; White, A.; Chan, H.S.L.; Gallie, B.L. Retinoblastoma. Lancet 2012, 379, 1436–1446. [Google Scholar] [CrossRef]
- Chauhan, S.; Sen, S.; Singh, N.; Sharma, A.; Chawla, B.; Kashyap, S. Human papillomavirus detection strategies in retinoblastoma. Pathol. Oncol. Res. 2020, 26, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Antoneli, C.B.; Ribeiro, K.B.; Sredni, S.T.; Arias, V.E.; Andreoli, M.A.; De Camargo, B.; Sobrinho, J.S.; Prado, J.C.; Soares, F.A.; Villa, L.L. Low prevalence of HPV in Brazilian children with retinoblastoma. J. Med. Virol. 2011, 83, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.-K.; Park, J.-S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2005, 37, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, S.; Tabibzadeh, A.; Yousefi, P.; Zandi, M.; Zakeri, A.; Akhavan Rezayat, S.; Ramezani, A.; Esghaei, M.; Farahani, A. HPV infections in retinoblastoma: A systematic review. J. Clin. Lab. Anal. 2021, 35, e23981. [Google Scholar] [CrossRef]
- Ryoo, N.-K.; Kim, J.-E.; Choung, H.-K.; Kim, N.; Lee, M.-J.; Khwarg, S.-I. Human papilloma virus in retinoblastoma tissues from Korean patients. Korean J. Ophthalmol. 2013, 27, 368–371. [Google Scholar] [CrossRef]
- Shetty, O.A.; Naresh, K.N.; Banavali, S.D.; Shet, T.; Joshi, R.; Qureshi, S.; Mulherkar, R.; Borges, A.; Desai, S.B. Evidence for the presence of high risk human papillomavirus in retinoblastoma tissue from nonfamilial retinoblastoma in developing countries. Pediatr. Blood Cancer 2012, 58, 185–190. [Google Scholar] [CrossRef]
- Bhuvaneswari, A.; Pallavi, V.; Jayshree, R.; Kumar, R.V. Maternal transmission of human papillomavirus in retinoblastoma: A possible route of transfer. Indian J. Med. Paediatr. Oncol. 2012, 33, 210–215. [Google Scholar] [CrossRef]
- Heck, J.E.; Lombardi, C.A.; Meyers, T.J.; Cockburn, M.; Wilhelm, M.; Ritz, B. Perinatal characteristics and retinoblastoma. Cancer Causes Control. 2012, 23, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, D.; Moein, M.; Esghaei, M.; Naseripour, M.; Monavari, S.H.; Bokharaei-Salim, F.; Sadeghipour, A. Molecular evidence of human papillomaviruses in the retinoblastoma tumor. Virusdisease 2019, 30, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chen, R.; Goshu, E.; Rushlow, D.; Chen, N.; Banister, C.; Creek, K.E.; Gallie, B.L. Human retinoblastoma is not caused by known pRb-inactivating human DNA tumor viruses. Int. J. Cancer 2007, 120, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.; Ramesh, C.; Appaji, L.; Kumari, B.A.; Shenoy, A.; Jayshree, R.; Kumar, R.V. Prevalence of high-risk human papillomavirus genotypes in retinoblastoma. Br. J. Ophthalmol. 2011, 95, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Theotoka, D.; Morkin, M.I.; Galor, A.; Karp, C.L. Update on diagnosis and management of conjunctival papilloma. Eye Vis. 2019, 6, 18. [Google Scholar] [CrossRef]
- Mammas, I.N.; Dalianis, T.; Doukas, S.G.; Zaravinos, A.; Achtsidis, V.; Thiagarajan, P.; Theodoridou, M.; Spandidos, D.A. Paediatric virology and human papillomaviruses: An update. Exp. Ther. Med. 2019, 17, 4337–4343. [Google Scholar] [CrossRef]
- Kaliki, S.; Arepalli, S.; Shields, C.L.; Klein, K.; Sun, H.; Hysenj, E.; Lally, S.E.; Shields, J.A. Conjunctival papilloma: Features and outcomes based on age at initial examination. JAMA Ophthalmol. 2013, 131, 585–593. [Google Scholar] [CrossRef]
- Li, Y.; Witte, D.; Myer, C.; Gluckman, J.; Pavelic, Z.; Pavelic, L.; Stambrook, P. Involvement of episomal hpv31 in a laryngeal carcinoma-persistent episomal maintenance of hpv DNA after passage through nude-mice. Int. J. Oncol. 1994, 4, 1377–1382. [Google Scholar] [CrossRef]
- Egbert, J.E.; Kersten, R.C. Female genital tract papillomavirus in conjunctival papillomas of infancy. Am. J. Ophthalmol. 1997, 123, 551–552. [Google Scholar] [CrossRef]

| Author, Year, Country | Features of the Participants of the Studies | Number of Pregnant Women Tested/Number of Positive Pregnant Women | Number of Newborns Tested/Number of Positive Newborns | Time and Sample for HPV Detection in Neonate | Type of HPV Screened (DNA PCR) | Positive Neonates/Positive Mother of Total Vaginal Delivery | Positive Neonates/Positive Mother of Total Cesarean Section |
|---|---|---|---|---|---|---|---|
| Trottier et al., 2016, Canada [27] | Pregnant women in the first trimester | 167/75 | 67/7 | At birth/3-month visits, conjunctival, oral, pharyngeal and genital areas | 6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89 | -/- of 136 | -/- of 31 |
| Sánchez-Torices et al., 2015, Spain [40] | HPV-positive pregnant women | 91/91 | 92/53 | Immediately after delivery, cord blood and oropharynx | 6, 11, 16, 18, 31, 33, 35, 38, 52 | 53/91 of 92 | None |
| SkoczyNski et al., 2015, Poland [41] | Healthy pregnant women prior to delivery with a singleton pregnancy | 152/29 | 152/16 | Immediately after delivery, oral area | 33 different HPV genotypes including 16 and 18 | NM | NM |
| Hahn et al., 2015, South Korea [42] | Pregnant women over 36 weeks of gestation | 469/72 | 469/15 | Immediately after delivery, oral area and secretions | 6, 11, 16, 18, 30, 31, 32, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 62, 66, 67, 68, 68a, 69, 70, 72, 81, 82, 84, 90, 91 | 14/51 of 300 | 1/21 of 169 |
| Lee et al., 2013, South Korea [14] | Healthy women with a singleton pregnancy | 153/37 | 153/8 | Immediately after delivery, cord blood and nasopharyngeal aspirate | 6, 11, 16, 18, 26, 30–35, 39, 40, 42–45, 51–56, 58, 59, 61, 62, 66–70, 72, 73, 81–84, 90, 91 | 3/- of 108 | 5/- of 45 |
| Hong et al., 2013, China [43] | HPV-positive pregnant women at delivery | 3139/422 | 233/35 | <24 h after birth, exfoliated oral and genital cells | 6, 11, 16, 18, 33, 43, 56, 58 | 19/136 of 136 | 16/97 of 97 |
| Park et al., 2012, South Korea [44] | Pregnant women over 36 weeks of gestation | 291/55 | 291/10 | Immediately after delivery, oral area | 24 different HPV genotype including 6, 11, 16, 18, 31, 33, 35, 39, 40, 44, 45, 51, 53, 56, 58, 66, 68, 70 | 10/- of 193 | None/- of 98 |
| Koskimaa et al., 2012, Finland [35] | Pregnant women in third trimester of pregnancy | 329/NM | 331/59 | At birth and till 2 months, oral area | 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, 82 | NM | NM |
| Smith et al., 2010, USA [45] | Healthy women with a singleton pregnancy | 333/99 | 333/5 | At a median of 42 h after birth, oral and genital areas, and cord blood | 6, 11, 16, 18, 31, 33, 39, 51, 53, 54, 56, 58, 59, 61, 69, 66, 70, 83, 84 | 3/86 of 295 | 2/13 of 38 |
| Castellsagué et al., 2009, Spain [38] | Pregnant women with potential risk of HPV exposure | 143/66 | 143/26 | From birth till 24 months of age, oral and genital areas | 6, 11, 16, 18, 31, 33, 39 | 22/- of 124 | 4/- of 19 |
| Rombaldi et al., 2008, Brazil [26] | HPV-positive pregnant women at delivery with prior history of HPV infection, abnormal smear, or genital warts | 49/49 | 49/11 | Immediately after delivery, oral, body, nasopharyngeal aspirates and cord blood | 6, 11, 16, 18, 31, 33, 42, 52, 58 | 5/24 of 24 | 6/25 of 25 |
| Gajewska et al., 2005, Poland [46] | Pregnant women with and without pregestational insulin-dependent diabetes mellitus | 45/12 | 45/9 | 48 h after birth, oral area, cord blood | 6, 11, 16 | NM | NM |
| Rintala et al., 2005, Finland [47] | Pregnant women in the third trimester | 76/57 | 77/56 | At birth till 2 years, oral and genital areas | 16, 18, 31, 33, 35, 39, 45, 51, 52, 54, 56, 58 | -/- of 63 | -/- of 13 |
| Worda et al., 2005, Austria [48] | Pregnant women underwent cesarean section between 37 and 40 weeks of pregnancy | 153/56 | NM | NM | 6, 11, 16, 18, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 56, 58, 59, 68 | None | -/56 of 153 |
| Deng et al., 2005, China [49] | Pregnant women without condylomata acuminata in the genital tract | 116/42 | 116/10 | 4 h after birth, cord blood, oropharyngeal secretions, amniotic fluid | 6, 11, 16, 18, 31, 33 | NM | NM |
| Bandyopadhyay et al., 2003, India [50] | Term pregnant women without a history of abnormal smears or genital warts | 135/38 | 135/14 | After birth, oral areas | 6, 11, 16, 18, 31, 33 | 3/11 of 59 | 11/27 of 76 |
| Peng et al., 2000, China [51] | Pregnant women in third trimester of pregnancy | 31/23 | 31/13 | Immediately after delivery, nasopharyngeal aspirates | 6, 11, 16, 18 | NM | NM |
| Tenti et al., 1999, Italy [52] | Pregnant women with negative Papanicolaou smear at first trimester | 711/37 | 711/11 | Immediately after delivery, nasopharyngeal aspirates | 6, 11, 16, 18, 33 | 11/29 of 557 | 0/8 of 154 |
| Wang et al., 1998, China [29] | Pregnant women on third trimester of pregnancy | 73/26 | 73/11 | Immediately after delivery, nasopharyngeal aspirates and amniotic fluid | 16, 18, 35 | 7/14 of 49 | 4/12 of 24 |
| Tseng et al., 1998, Taiwan [53] | Healthy women with a singleton pregnancy | 301/68 | 68/27 | At least 3 days after delivery, oral and genital area | 16, 18 | 18/35 of 160 | 9/33 of 141 |
| Xu et al., 1998, China [54] | Pregnant women on third trimester of pregnancy | 30/16 | 30/14 | 12–48 h after birth, pharyngeal secretions | 6, 11, 16, 18, 31, 33, 35, 38 | - of 17 | - of 13 |
| Watts et al., 1998, USA [55] | Pregnant women before 20 weeks of gestation | 151/112 | 151/8 | At birth and till 3 years, oral, genital and anal areas, nasopharyngeal aspirates | 6, 11, 16, 18, 31, 33, 35, 39, 45 | 6/- of - | 2/- of - |
| Puranen et al., 1997, Finland [56] | Pregnant women | 105/41 | 106/39 | Immediately after delivery, nasopharyngeal aspirates | 2, 6, 7, 11, 16, 18, 30, 31, 33, 53, 66 | 30/30 of 78 | 9/11 of 27 |
| Alberico et al., 1995, Italy [57] | Pregnant women in the first trimester | 170/53 | 170/37 | Immediately after delivery, oropharyngeal secretions | 6, 11, 16, 18, 31, 33, 52 | NM | NM |
| Cason et al., 1995, United Kingdom [58] | Pregnant women, some of them had history of abnormal smears and genital warts | 61/45 | 62/33 | After birth, oral and genital areas, nasopharyngeal aspirates | 16, 18 | NM | NM |
| Parkarin et al., 1994, United Kingdom [59] | Pregnant women, some with a history of abnormal smears or of previous genital warts | 31/20 | 32/12 | 24 h after birth, oral and genital areas | 16, 18, 31, 33 | NM | NM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardekani, A.; Taherifard, E.; Mollalo, A.; Hemadi, E.; Roshanshad, A.; Fereidooni, R.; Rouholamin, S.; Rezaeinejad, M.; Farid-Mojtahedi, M.; Razavi, M.; et al. Human Papillomavirus Infection during Pregnancy and Childhood: A Comprehensive Review. Microorganisms 2022, 10, 1932. https://doi.org/10.3390/microorganisms10101932
Ardekani A, Taherifard E, Mollalo A, Hemadi E, Roshanshad A, Fereidooni R, Rouholamin S, Rezaeinejad M, Farid-Mojtahedi M, Razavi M, et al. Human Papillomavirus Infection during Pregnancy and Childhood: A Comprehensive Review. Microorganisms. 2022; 10(10):1932. https://doi.org/10.3390/microorganisms10101932
Chicago/Turabian StyleArdekani, Ali, Erfan Taherifard, Abolfazl Mollalo, Emadeddin Hemadi, Amirhossein Roshanshad, Reza Fereidooni, Safoura Rouholamin, Mahroo Rezaeinejad, Maryam Farid-Mojtahedi, Maryam Razavi, and et al. 2022. "Human Papillomavirus Infection during Pregnancy and Childhood: A Comprehensive Review" Microorganisms 10, no. 10: 1932. https://doi.org/10.3390/microorganisms10101932
APA StyleArdekani, A., Taherifard, E., Mollalo, A., Hemadi, E., Roshanshad, A., Fereidooni, R., Rouholamin, S., Rezaeinejad, M., Farid-Mojtahedi, M., Razavi, M., & Rostami, A. (2022). Human Papillomavirus Infection during Pregnancy and Childhood: A Comprehensive Review. Microorganisms, 10(10), 1932. https://doi.org/10.3390/microorganisms10101932






