Biodiversity and Structure of Microbial Community in Glacial Melts and Soil in the High Arctic Ny-Ålesund, Svalbard
Abstract
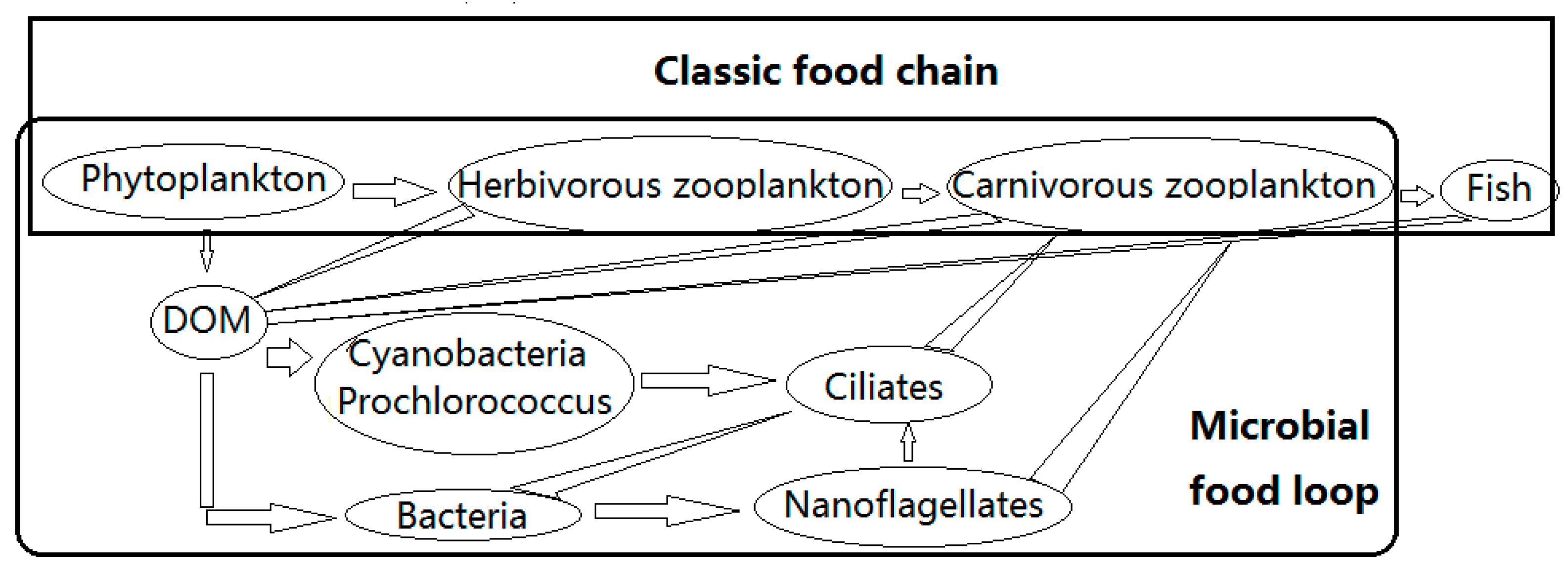
1. Introduction
2. Material and Methods
2.1. Sampling and Environmental Factor Analyses
2.2. Biodiversity and Taxonomy of Microbes (<20 μm) by Miseq Sequencing
2.3. Statistical Analysis between Microbial Community and Environmental Factors
3. Results and Discussion
3.1. Environmental Description of Sampling Sites
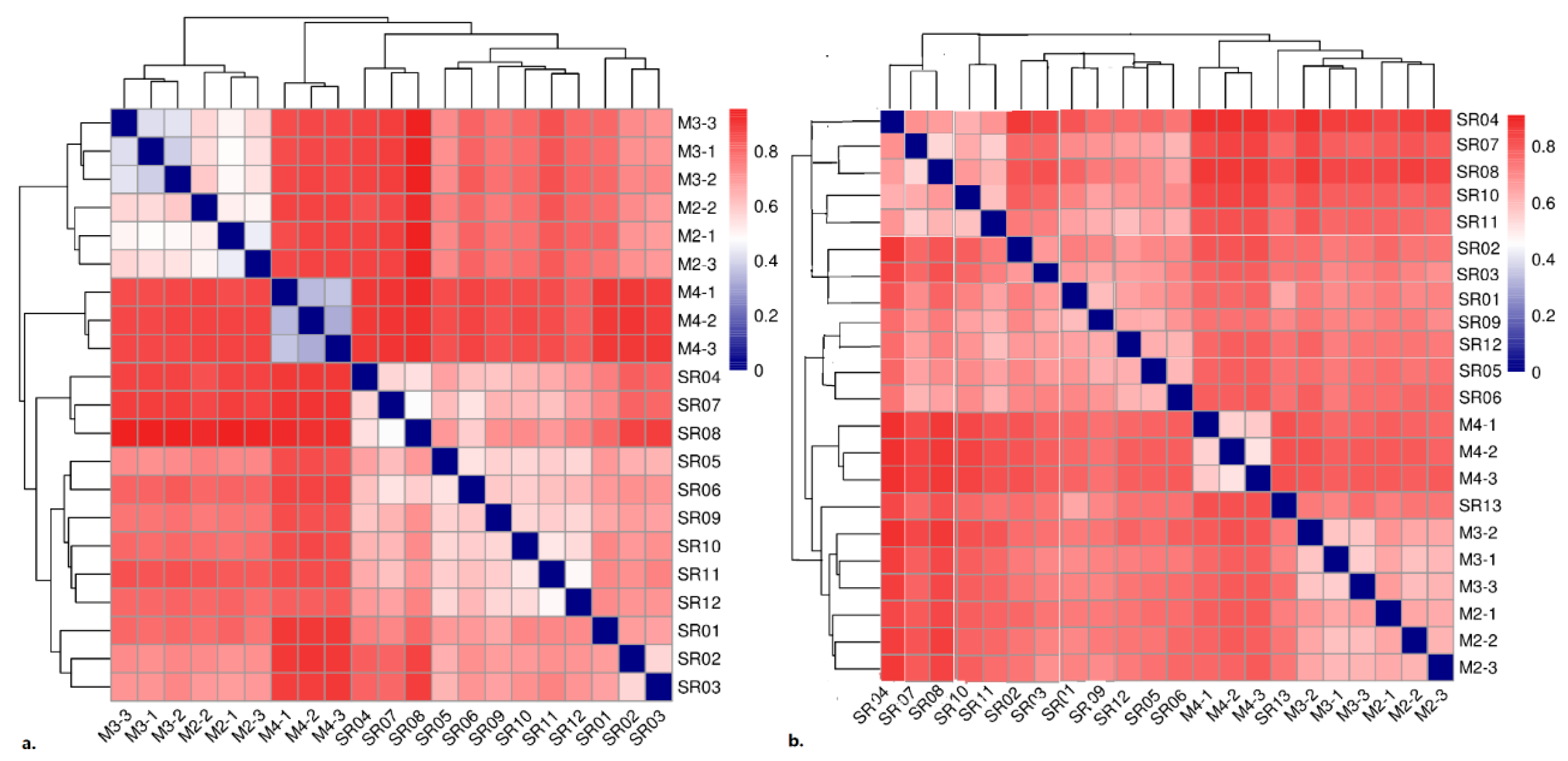
3.2. Microbial Community Diversity and Composition by High-Throughput Sequencing
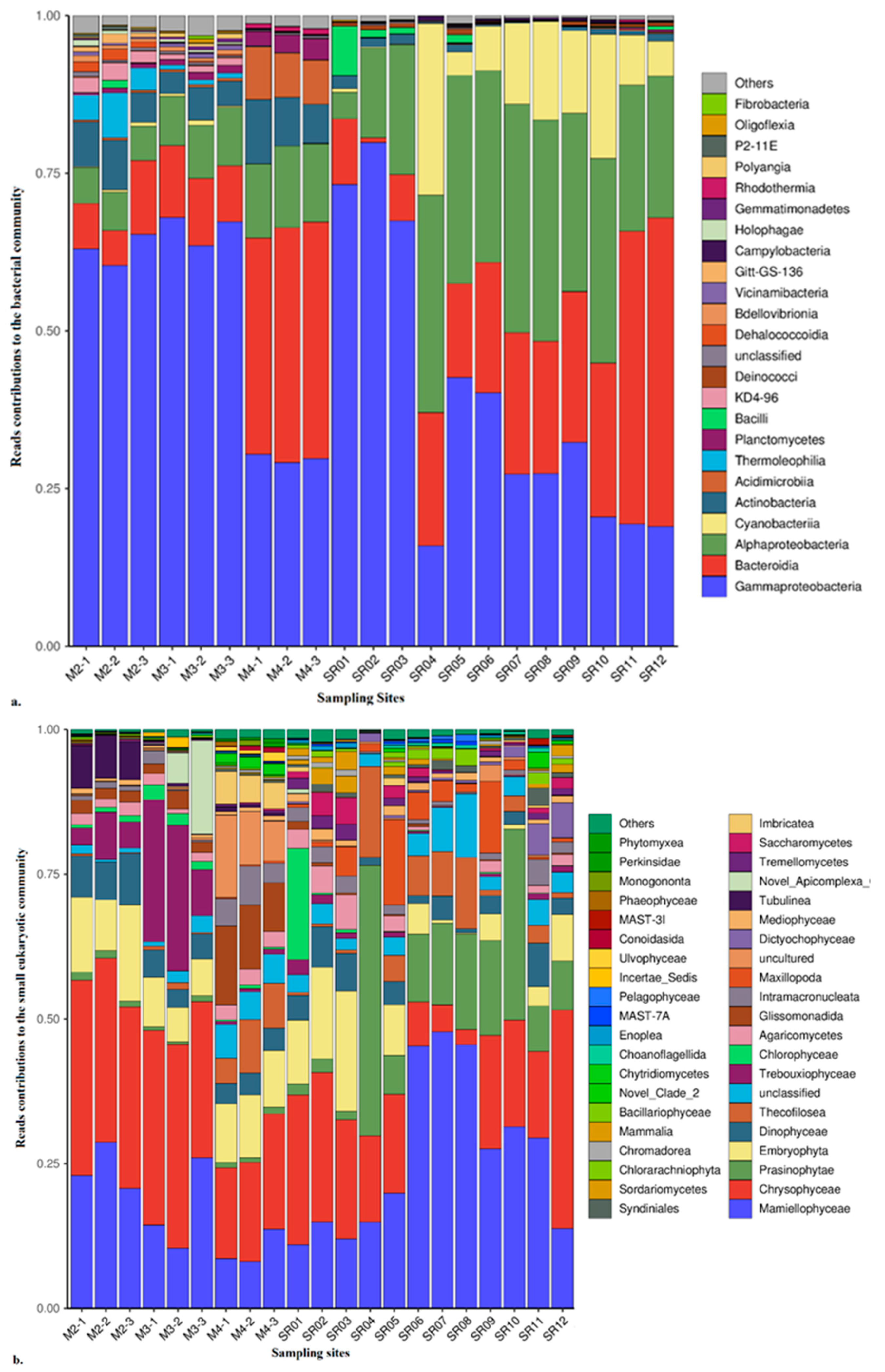
3.2.1. Community Diversity and Composition of Prokaryotes
3.2.2. Community Diversity and Composition of Eukaryotes
3.2.3. General Discussion for Prokaryotes and Eukaryotes in Ny-Ålesund, Svalbard
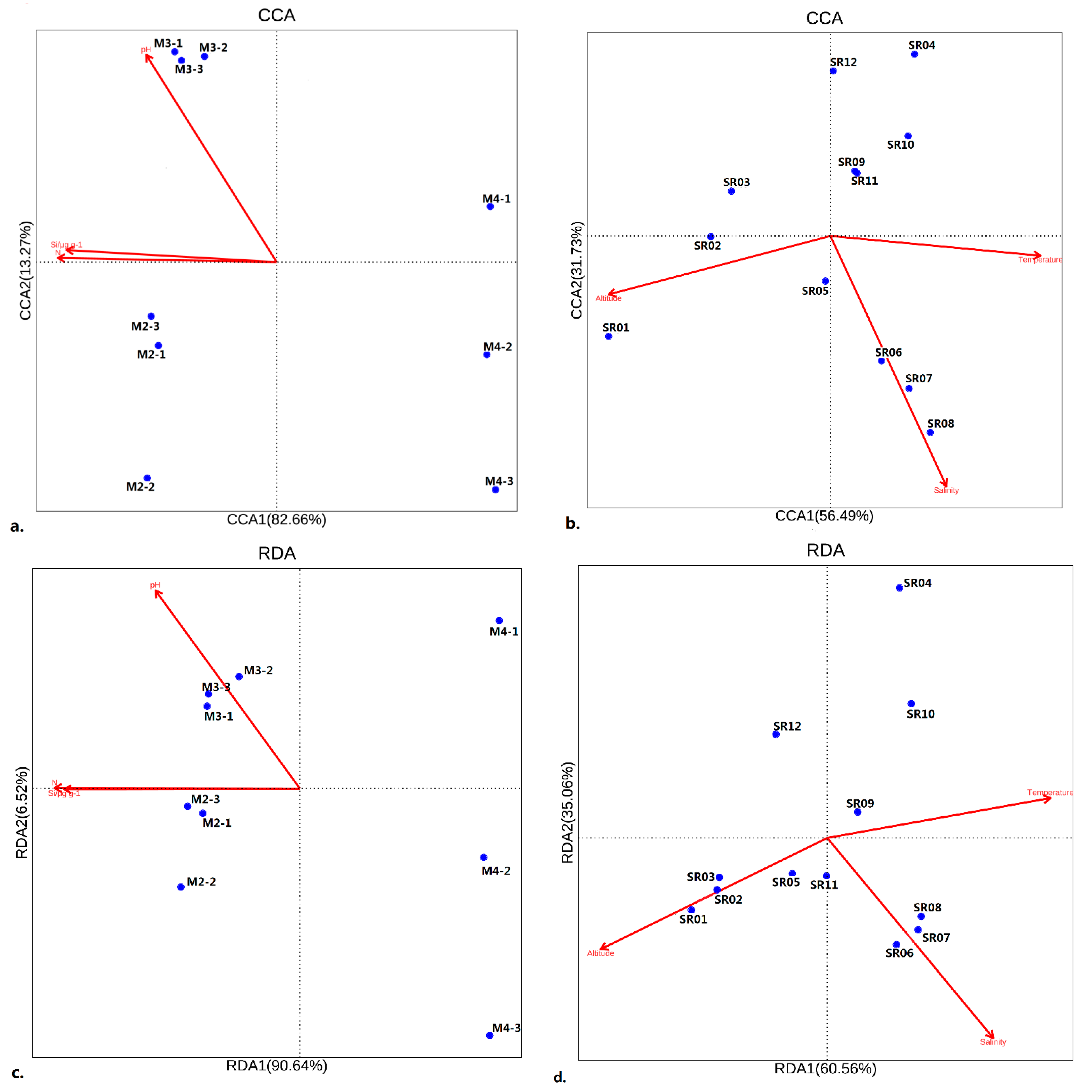
3.3. Environmental Correlations of Microbes in Ny-Ålesund
3.3.1. CCA for Prokaryotic Communities
3.3.2. RDA for Eukaryotic Communities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becking, L.G.M.B. Geobiologie of Inleiding Tot De Milieukunde; Van Stockum WP & Zoon: The Hague, The Netherlands, 1934. (In Dutch) [Google Scholar]
- Beijerinck, M.W. De Infusies En De Ontdekking Der Backteriën; Müller, reprinted in Verzamelde geschriften van M.W. Beijerinck vijfde deel; Jaarboek van de Koninklijke Akademie voor Wetenschappen: Amsterdam, The Netherlands, 1913; pp. 119–140. [Google Scholar]
- Zhang, F.; He, J.; Zhan, L.; Ye, W. Effects of Arctic Warming on Microbes and Methane in Different Land Types in Svalbard. Water 2021, 13, 3296. [Google Scholar] [CrossRef]
- Ye, W.; Wang, X.; Zhang, X.; Zhang, G. Methane production in oxic seawater of the western North Pacific and its marginal seas. Limnol. Oceanogr. 2020, 65, 2352–2365. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, S.; Gao, Y.; He, J. Distribution and environmental correlations of picoeukaryotes in an Arctic fjord (Kongsfjorden, Svalbard) during the summer. Polar Res. 2019, 3, 3390–3401. [Google Scholar] [CrossRef]
- Vary, P.; Johnson, M. Cell yields of bacteria grown on methane. Appl. Microbiol. 1967, 15, 1473–1478. [Google Scholar] [CrossRef]
- Altshuler, I.; Hamel, J.; Turney, S.; Magnuson, E.; Lévesque, R.; Greer, C.W.; Whyte, L.G. Species interactions and distinct microbial communities in high Arctic permafrost affected cryosols are associated with the CH4 and CO2 gas fluxes. Environ. Microbiol. 2019, 21, 3711–3727. [Google Scholar] [CrossRef]
- Stackhouse, B.T.; Vishnivetskaya, T.A.; Layton, A.; Chauhan, A.; Pfiffner, S.; Mykytczuk, N.C.; Sanders, R.; Whyte, L.G.; Hedin, L.; Saad, N.; et al. Effects of simulated spring thaw of permafrost from mineral cryosol on CO2 emissions and atmospheric CH4 uptake. J. Geophys. Res. Biogeosci. 2015, 120, 1764–1784. [Google Scholar] [CrossRef]
- Bourriquen, M.; Mercier, D.; Baltzer, A.; Fournier, J.; Costa, S.; Roussel, E. Paraglacial coasts responses to glacier retreat and associated shifts in river floodplains over decadal timescales (1966–2016), Kongsfjorden, Svalbard. Land Degrad. Dev. 2018, 29, 4173–4185. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, F.; He, J.; Ji, Z.; Zhou, Q. Water masses influence bacterioplankton community structure in summer Kongsfjorden. Extremophiles 2020, 24, 107–120. [Google Scholar] [CrossRef]
- Qiao, Z.Z.; Zeng, Y.X.; Dong, P.Y.; Zheng, T.L. Bacterial community composition and abundance of planktonic bacteria in Arctic Kongsforden in the summer of 2011. Chin. J. Polar Res. 2015, 27, 246. [Google Scholar]
- Zeng, Y.X.; Zhang, F.; He, J.F.; Lee, S.H.; Qiao, Z.Y.; Yu, Y.; Li, H.R. Bacterioplankton community structure in the Arctic waters as revealed by pyrosequencing of 16S rRNA genes. Antonie Van Leeuwenhoek 2013, 103, 1309–1319. [Google Scholar] [CrossRef]
- De Corte, D.; Sintes, E.; Yokokawa, T.; Herndl, G.J. Changes in viral and bacterial communities during the ice-melting season in the coastal Arctic (Kongsfjorden, Ny-Alesund). Environ. Microbiol. 2011, 13, 1827–1841. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.K.; Krishnan, K.P.; Kerkar, S.; Divya, D.T. Spatio-temporal monitoring and ecological signifcance of retrievable pelagic heterotrophic bacteria in Kongsforden, an Arctic Fjord. Indian J. Microbiol. 2016, 57, 116–120. [Google Scholar] [CrossRef][Green Version]
- Jain, A.; Krishnan, K.P. Diferences in free-living and particle associated bacterial communities and their spatial variation in Kongsforden, Arctic. J. Basic. Microb. 2017, 57, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, N.-F.; Zhang, Y.-Q.; Liu, H.-Y.; Yu, L.-Y. Diversity and Distribution of Aquatic Fungal Communities in the Ny-Ålesund Region, Svalbard (High Arctic). Microb. Ecol. 2016, 71, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tian, Y.; He, J. Occurrence of the Freshwater Chrysophyte Poterioochromonas malhamensis in a High Arctic Marine Ecosystem. Water 2021, 13, 2129. [Google Scholar] [CrossRef]
- Piwosz, K.; Spich, K.; Całkiewicz, J.; Weydmann, A.; Kubiszyn, A.M.; Wiktor, J.M. Distribution of small phytoflagellates along an Arctic fjord transect. Environ. Microbiol. 2015, 17, 2393–2406. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhang, W.; Xiao, T. Spatial and temporal distribution of tintinnid (Ciliophora: Tintinnida) communities in Kongsfjorden, Svalbard (Arctic), during summer. Polar Biol. 2014, 37, 291–296. [Google Scholar] [CrossRef]
- Hamilton, A.K.; Lovejoy, C.; Galand, P.E.; Ingram, R.G. Water masses and biogeography of picoeukaryote assemblages in a cold hydrographically complex system. Limnol. Oceanogr. 2008, 53, 922–935. [Google Scholar] [CrossRef]
- Keck, A.; Wiktor, J.; Hapter, R.; Nilsen, R. Phytoplankton assemblages related to physical gradients in an arctic, glacier-fed fjord in summer. ICES J. Mar. Sci. 1999, 56, 203–214. [Google Scholar] [CrossRef]
- Kilias, E.S.; Nöthig, E.-M.; Wolf, C.; Metfies, K. Picoeukaryote plankton composition off West Spitsbergen at the entrance to the Arctic Ocean. J. Eukaryot. Microbiol. 2014, 61, 569–579. [Google Scholar] [CrossRef] [PubMed]
- LaRose, C.; Berger, S.; Ferrari, C.; Navarro, E.; Dommergue, A.; Schneider, D.; Vogel, T. Microbial sequences retrieved from environmental samples from seasonal arctic snow and meltwater from Svalbard, Norway. Extremophiles 2010, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, H.; Gjessing, Y.; Hamran, S.-E.; Hagen, J.O.; Liestøl, O.; Pálsson, F.; Erlingsson, B. The thermal regime of sub-polar glaciers mapped by multi-frequency radio-echo sounding. J. Glaciol. 1996, 42, 23–32. [Google Scholar] [CrossRef]
- Wang, N.F.; Zhang, T.; Yang, X.; Wang, S.; Yu, Y.; Dong, L.L.; Guo, Y.D.; Ma, Y.X.; Zang, J.Y. Diversityand Composition of Bacterial Community in Soils and Lake Sediments from an Arctic Lake Area. Front. Microbiol. 2016, 7, 1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.F.; Zhang, T.; Zhang, F.; Wang, E.T.; He, J.F.; Ding, H.; Zhang, B.T.; Liu, J.; Bin Ran, X.; Zang, J.Y. Diversity and structure of soil bacterial communities in the Fildes Region (maritime Antarctica) as revealed by 454 pyrosequencing. Front. Microbiol. 2015, 6, 1188. [Google Scholar] [CrossRef] [PubMed]
- Kaštovská, K.; Elster, J.; Stibal, M.; Šantrůčková, H. Microbial Assemblages in Soil Microbial Succession After Glacial Retreat in Svalbard (High Arctic). Microb. Ecol. 2005, 50, 396. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Hansen, A.A.; Herbert, R.A.; Mikkelsen, K.; Jensen, L.L.; Kristoffersen, T.; Tiedje, J.M.; Lomstein, B.A.; Finster, K. Viability, diversity and composition of the bacterial community in a high Arctic permafrost soil from Spitsbergen, Northern Norway. Environ. Microbiol. 2007, 9, 2870–2884. [Google Scholar] [CrossRef] [PubMed]
- He, R.H.; Du, Z.J.; Yu, Y.; Li, H.R. Isolation of antibacterial and culturable bacteria from Arctic tundra soils. Acta Microbiol. Sinica 2019, 59, 1050–1062. [Google Scholar] [CrossRef]
- Vishnivetskaya, T.A.; Petrova, M.; Urbance, J.; Ponder, M.; Moyer, C.L.; Gilichinsky, D.A.; Tiedje, J.M. Bacterial community in ancient Siberian permafrost as characterized by culture and culture-independent methods. Astrobiology 2006, 6, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.W.; Pearce, D.A.; Convey, P.; Tan, G.A.; Wong, R.C.; Tan, I.K. High levels of spatial heterogeneity in the biodiversity of soil prokaryotes on Signy Island, Antarctica. Soil Biol. Biochem. 2010, 42, 601–610. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Liu, Y.; Xie, S.; Liu, Y. Bacterioplankton communities in a high-altitude freshwater wetland. Ann. Microbiol. 2014, 64, 1405–1411. [Google Scholar] [CrossRef]
- Rodriguez, H.M.; Robertson, A.D.; Gregoret, L.M. Native state EX2 and EX1 hydrogen exchange of Escherichia coli CspA, a small β-sheet protein. Biochemistry 2002, 41, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Piquet, A.M.-T.; van de Poll, W.H.; Visser, R.J.W.; Wiencke, C.; Bolhuis, H.; Buma, A.G.J. Springtime phytoplankton dynamics in Arctic Krossfjorden and Kongsfjorden (Spitsbergen) as a function of glacier proximity. Biogeosciences 2014, 11, 2263–2279. [Google Scholar] [CrossRef]
- Schnepf, E.; Kühn, S.F. Food uptake and fine structure of Cryothecomonas longipes sp. nov., a marine nanoflagellate incertae sedis feeding phagotrophically on large diatoms. Helgol. Mar. Res. 2000, 54, 18–32. [Google Scholar] [CrossRef]
- Zhang, F.; He, J.; Lin, L.; Jin, H. Dominance of picophytoplankton in the newly open surface water of the central Arctic Ocean. Polar. Biol. 2015, 38, 1081–1089. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, L.; Gao, Y.; Cao, S.; He, J. Ecophysiology of picophytoplankton in different water masses of the northern Bering Sea. Polar Biol. 2016, 39, 1381–1397. [Google Scholar] [CrossRef]

| Sampling Sites | Latitude and Longitude | Altitude/m | Temperature/°C | Salinity |
|---|---|---|---|---|
| SR01 (Glacial water) | 78°53′40.88″ N 12°02′38.67″ E | 78 | −1.0 | 0 |
| SR02 (Subglacial soil water) | 78°53′40.85″ N 12°02′33.15″ E | 58 | 0.0 | 0 |
| SR03 (Subglacial lake water) | 78°53′51.36″ N 12°02′50.25″ E | 21 | 5.0 | 0 |
| SR04 (Glacial runoff into estuary water) | 78°54′51.18″ N 12°02′29.93″ E | 0 | 6.0 | 0 |
| SR05 (Sea water in estuary #1) | 78°54′52.50″ N 12°02′29.70″ E | 0 | 6.0 | 17 |
| SR06 (Sea water in estuary #2) | 78°54′53.42″ N 12°02′29.34″ | 0 | 6.5 | 27 |
| SR07 (Sea water in estuary #3) | 78°54′55.07″ N 12°02′29.01″ E | 0 | 7.0 | 36 |
| SR08 (Sea water in estuary #4) | 78°54′56.43″ N 12°02′28.64″ E | 0 | 7.0 | 38 |
| SR09 (glacial melt in estuary #1) | 78°54′53.10″ N 12°02′35.70″ E | 0 | 4.5 | 0 |
| SR10 (glacial melt in estuary #2) | 78°54′53.42″ N 12°02′38.34″ E | 0 | 4.5 | 0 |
| SR11 (glacial melt in estuary #3) | 78°55′55.07″ N 12°02′37.51″ E | 0 | 5.0 | 0 |
| SR12 (glacial melt in estuary #4) | 78°54′58.47″ N 12°02′35.15″ E | 0 | 5.0 | 0 |
| Sample | Latitude and Longitude | pH ± S.D. | NH4+ ± S.D. (μg/g) | SiO42− ± S.D. (μg/g) | NO2− ± S.D. (μg/g) | NO3− ± S.D. (μg/g) | |
|---|---|---|---|---|---|---|---|
| M2 | M2-1 | 78°53′40.85″ N 12°02′33.15″ E | 8.55 ± 0.03 | 1.56 ± 0.06 | 10.36 ± 0.01 | 0.33 ± 0.02 | 0.30 ± 0.02 |
| M2-2 | 8.55 ± 0.03 | 1.96 ± 0.05 | 10.62 ± 0.01 | 0.32 ± 0.02 | 0.31 ± 0.02 | ||
| M2-3 | 8.55 ± 0.03 | 1.85 ± 0.04 | 10.56 ± 0.01 | 0.31 ± 0.02 | 0.30 ± 0.02 | ||
| M3 | M3-1 | 78°53′51.36″ N 12°02′50.25″ E | 8.69 ± 0.04 | 1.31 ± 0.06 | 10.67 ± 0.01 | 0.20 ± 0.03 | 0.23 ± 0.03 |
| M3-2 | 8.50 ± 0.02 | 1.37 ± 0.04 | 7.63 ± 0.03 | 0.18 ± 0.03 | 0.21 ± 0.03 | ||
| M3-3 | 8.70 ± 0.02 | 2.28 ± 0.02 | 10.19 ± 0.01 | 0.17 ± 0.02 | 0.25 ± 0.03 | ||
| M4 | M4-1 | 78°54′51.18″ N 12°02′29.93″ E | 8.58 ± 0.01 | 1.74 ± 0.05 | 6.56 ± 0.03 | 0.16 ± 0.02 | 0.64 ± 0.01 |
| M4-2 | 8.56 ± 0.04 | 1.26 ± 0.06 | 9.66 ± 0.02 | 0.13 ± 0.03 | 0.73 ± 0.01 | ||
| M4-3 | 8.35 ± 0.05 | 1.58 ± 0.04 | 6.67 ± 0.03 | 0.18 ± 0.02 | 0.79 ± 0.01 | ||
| Coverage (%) | Reads | OTUs | ACE | Shannon | |
|---|---|---|---|---|---|
| M2-1 | 99.16 | 44,063 | 508 | 660.47 | 3.97 |
| M2-2 | 99.21 | 46,998 | 428 | 589.03 | 3.70 |
| M2-3 | 99.03 | 40,992 | 482 | 686.26 | 3.67 |
| M3-1 | 98.95 | 49,590 | 603 | 794.23 | 4.01 |
| M3-2 | 98.91 | 48,208 | 627 | 821.67 | 4.26 |
| M3-3 | 98.91 | 49,815 | 588 | 796.23 | 4.04 |
| M4-1 | 98.98 | 55,531 | 560 | 771.87 | 4.44 |
| M4-2 | 99.01 | 53,353 | 609 | 783.71 | 4.68 |
| M4-3 | 98.91 | 78,053 | 649 | 842.38 | 4.71 |
| SR01 | 99.33 | 25,870 | 313 | 470.04 | 3.04 |
| SR02 | 99.03 | 73,053 | 402 | 643.31 | 3.12 |
| SR03 | 99.25 | 71,130 | 347 | 515.29 | 3.09 |
| SR04 | 99.33 | 23,095 | 274 | 500.28 | 3.29 |
| SR05 | 98.60 | 77,477 | 649 | 977.53 | 4.33 |
| SR06 | 99.13 | 27,951 | 424 | 610.93 | 3.89 |
| SR07 | 99.29 | 27,049 | 329 | 526.63 | 3.50 |
| SR08 | 99.56 | 23,616 | 238 | 339.76 | 3.36 |
| SR09 | 98.98 | 25,251 | 465 | 711.80 | 3.82 |
| SR10 | 98.79 | 29,946 | 499 | 832.23 | 3.83 |
| SR11 | 98.93 | 29,691 | 489 | 718.61 | 3.59 |
| SR12 | 98.80 | 71,431 | 554 | 834.28 | 3.71 |
| F-test | 0.06 | 0.013 | 0.0082 | 0.024 | 0.007 |
| Coverage (%) | Reads | OTUs | Chao1 | Shannon | |
|---|---|---|---|---|---|
| M2-1 | 99.64 | 56,520 | 330 | 378.88 | 2.74 |
| M2-2 | 99.47 | 63,412 | 295 | 400.32 | 2.64 |
| M2-3 | 99.54 | 78,284 | 342 | 410.36 | 2.87 |
| M3-1 | 99.44 | 98,071 | 357 | 449.97 | 2.75 |
| M3-2 | 99.39 | 114,701 | 328 | 449.39 | 2.93 |
| M3-3 | 99.47 | 59,915 | 326 | 417.67 | 2.61 |
| M4-1 | 99.38 | 48,574 | 611 | 689.91 | 4. 10 |
| M4-2 | 99.42 | 51,680 | 641 | 703.65 | 4.23 |
| M4-3 | 99.30 | 71,068 | 695 | 774.08 | 4.18 |
| SR01 | 99.28 | 38,693 | 644 | 730.26 | 3.71 |
| SR02 | 99.74 | 39,070 | 419 | 453.03 | 3.64 |
| SR03 | 99.54 | 37,452 | 428 | 499.94 | 3.52 |
| SR04 | 99.56 | 38,194 | 223 | 314.74 | 2.43 |
| SR05 | 99.45 | 36,135 | 501 | 571.00 | 3.83 |
| SR06 | 99.27 | 35,166 | 442 | 604.56 | 3.36 |
| SR07 | 99.21 | 35,580 | 444 | 605.79 | 3.22 |
| SR08 | 99.31 | 35,130 | 342 | 502.67 | 3.09 |
| SR09 | 99.07 | 37,275 | 515 | 677.11 | 3.14 |
| SR10 | 99.45 | 36,202 | 317 | 426.31 | 2.79 |
| SR11 | 99.44 | 36,203 | 501 | 608.00 | 4.06 |
| SR12 | 99.39 | 37,932 | 487 | 580.94 | 3.56 |
| F-test | 0.10 | 0.025 | 0.0074 | 0.034 | 0.0065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Lv, F.; Chen, M. Biodiversity and Structure of Microbial Community in Glacial Melts and Soil in the High Arctic Ny-Ålesund, Svalbard. Microorganisms 2022, 10, 1941. https://doi.org/10.3390/microorganisms10101941
Zhang F, Lv F, Chen M. Biodiversity and Structure of Microbial Community in Glacial Melts and Soil in the High Arctic Ny-Ålesund, Svalbard. Microorganisms. 2022; 10(10):1941. https://doi.org/10.3390/microorganisms10101941
Chicago/Turabian StyleZhang, Fang, Fenglin Lv, and Mianrun Chen. 2022. "Biodiversity and Structure of Microbial Community in Glacial Melts and Soil in the High Arctic Ny-Ålesund, Svalbard" Microorganisms 10, no. 10: 1941. https://doi.org/10.3390/microorganisms10101941
APA StyleZhang, F., Lv, F., & Chen, M. (2022). Biodiversity and Structure of Microbial Community in Glacial Melts and Soil in the High Arctic Ny-Ålesund, Svalbard. Microorganisms, 10(10), 1941. https://doi.org/10.3390/microorganisms10101941







