Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention
Abstract
:1. Introduction
2. Etiology
3. Virulence Factors
3.1. Clostridium perfringens Alpha (CPA) Toxin
3.2. Clostridium perfringens Beta (CPB) Toxin
3.3. C. perfringens Enterotoxin (CPE)
3.4. Necrotic Enteritis Like Toxin (NetB)
3.5. TpeL
4. Culture and Detection
5. Predisposing Factors
5.1. Coccidiosis
5.2. Dietary Factors
5.3. Feed Mycotoxins
5.4. Immunosuppression
5.5. Stocking Density
5.6. Temperature
6. Pathogenesis
7. Immune Response to Necrotic Enteritis in Broilers
7.1. Gut-Associated Lymphoid Tissue and Immune Response in Chicken
7.2. Innate Immunity
7.3. Adaptive Immunity
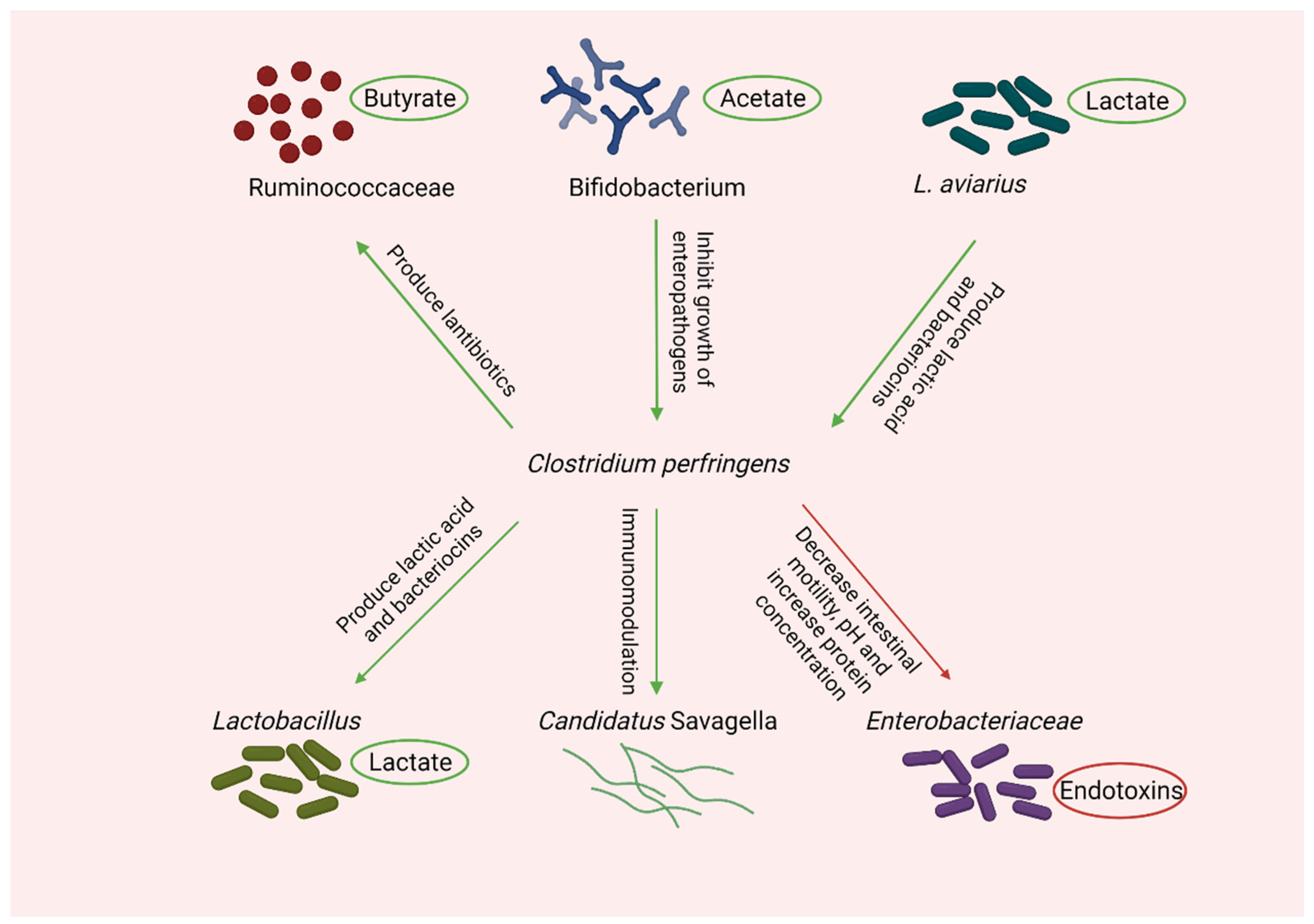
8. Microbial Shift during Necrotic Enteritis
9. Zoonosis
10. Antibiotics Resistance in C. perfringens
11. Control of Necrotic Enteritis in the Post-Antibiotic Era
11.1. Probiotics
11.2. Prebiotics
11.3. Phytobiotics
11.4. Organic Acids
11.5. Immunoglobulins
11.6. Bacteriophages
11.7. Vaccination
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ficken, M.D. Necrotic enteritis. In Diseases of Poultry; Iowa State University Press: Uppsala, IA, USA, 1991; pp. 264–267. [Google Scholar]
- Parish, W.E. Necrotic enteritis in the fowl (Gall Us Gall Us D Omes Ticus): I. Histopathology of the disease and isolation of a strain of clostridium welchii. J. Comp. Pathol. Ther. 1961, 71, 377-IN33. [Google Scholar] [CrossRef]
- Hughes, L.; Hermans, P.; Morgan, K. Risk factors for the use of prescription antibiotics on UK broiler farms. J. Antimicrob. Chemother. 2008, 61, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lillehoj, H.S.; Gadde, U.D.; Ritter, D.; Oh, S. Characterization of Clostridium perfringens strains isolated from healthy and necrotic enteritis-afflicted broiler chickens. Avian Dis. 2017, 61, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hermans, P.G.; Morgan, K.L. Prevalence and associated risk factors of necrotic enteritis on broiler farms in the United Kingdom; a cross-sectional survey. Avian Pathol. 2007, 36, 43–51. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Impacts of antimicrobial growth promoter termination in Denmark. In Proceedings of the WHO International Review Panel’s Evaluation of the Termination of the Use of Antimicrobial Growth Promoters in Denmark, Foulum, Denmark, 6–9 November 2002; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Gaucher, M.L.; Quessy, S.; Letellier, A.; Arsenault, J.; Boulianne, M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poult. Sci. 2015, 94, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ohtani, K.; Hirakawa, H.; Ohshima, K.; Yamashita, A.; Shiba, T.; Ogasawara, N.; Hattori, M.; Kuhara, S.; Hayashi, H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 2002, 99, 996–1001. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 5: Characteristics of Microbial Pathogens; Springer Science & Business Media: Cham, Switzerland, 1996; Volume 5. [Google Scholar]
- Bacon, R.T.; Sofos, J.N. Food Safety Handbook; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Wrigley, D.M. Clostridium perfringens. In Diseases Caused by Bacteria; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Smith, L. The genus Clostridium—Medical. In The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community; Springer: Cham, Switzerland, 1992. [Google Scholar]
- Ohtani, K.; Shimizu, T. Regulation of toxin gene expression in Clostridium perfringens. Res. Microbiol. 2015, 166, 280–289. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.E.; Stevens, D.L. Phospholipase C and perfringolysin O from Clostridium perfringens upregulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured human umbilical vein endothelial cells. Infect. Immun. 1996, 64, 358–362. [Google Scholar] [PubMed]
- Nagahama, M.; Ochi, S.; Oda, M.; Miyamoto, K.; Takehara, M.; Kobayashi, K. Recent insights into Clostridium perfringens beta-toxin. Toxins 2015, 7, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Allaart, J.G.; van Asten, A.J.; Vernooij, J.C.; Gröne, A. Beta2 toxin is not involved in in vitro cell cytotoxicity caused by human and porcine cpb2-harbouring Clostridium perfringens. Vet. Microbiol. 2014, 171, 132–138. [Google Scholar] [CrossRef]
- Farzan, A.; Kircanski, J.; DeLay, J.; Soltes, G.; Songer, J.G.; Friendship, R.; Prescott, J.F. An investigation into the association between cpb2-encoding Clostridium perfringens type A and diarrhea in neonatal piglets. Can. J. Vet. Res. 2013, 77, 45–53. [Google Scholar]
- Seike, S.; Takehara, M.; Kobayashi, K.; Nagahama, M. Clostridium perfringens Delta-Toxin Damages the Mouse Small Intestine. Toxins 2019, 11, 232. [Google Scholar] [CrossRef]
- Freedman, J.C.; Shrestha, A.; McClane, B.A. Clostridium perfringens enterotoxin: Action, genetics, and translational applications. Toxins 2016, 8, 73. [Google Scholar] [CrossRef]
- Clark, A.; Savva, C.G.; Naylor, C.E.; Popoff, M.R.; Moss, D.S.; Basak, A.K.; Titball, R.W.; Bokori-Brown, M. The pore structure of Clostridium perfringensepsilon toxin. Nat. Commun. 2019, 10, 2641. [Google Scholar]
- Lee, K.; Lillehoj, H.S. Role of Clostridium perfringens necrotic enteritis B-like toxin in disease pathogenesis. Vaccines 2021, 10, 61. [Google Scholar] [CrossRef]
- Sindern, N.; Suchodolski, J.S.; Leutenegger, C.M.; Mehdizadeh Gohari, I.; Prescott, J.F.; Proksch, A.; Mueller, R.S.; Busch, K.; Unterer, S. Prevalence of Clostridium perfringens netE and netF toxin genes in the feces of dogs with acute hemorrhagic diarrhea syndrome. J. Vet. Intern. Med. 2019, 33, 100–105. [Google Scholar] [CrossRef]
- Stevens, D.L.; Tweten, R.K.; Awad, M.M.; Rood, J.I.; Bryant, A.E. Clostridial gas gangrene: Evidence that α and θ toxins differentially modulate the immune response and induce acute tissue necrosis. J. Infect. Dis. 1997, 176, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Stiles, B.G.; Wigelsworth, D.J.; Popoff, M.R.; Barth, H. Clostridial binary toxins: Iota and C2 family portraits. Front. Cell. Infect. Microbiol. 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coursodon, C.F.; Glock, R.D.; Moore, K.L.; Cooper, K.K.; Songer, J.G. TpeL-producing strains of Clostridium perfringens type A are highly virulent for broiler chicks. Anaerobe 2012, 18, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Yonogi, S.; Matsuda, S.; Kawai, T.; Yoda, T.; Harada, T.; Kumeda, Y.; Gotoh, K.; Hiyoshi, H.; Nakamura, S.; Kodama, T. BEC, a novel enterotoxin of Clostridium perfringens found in human clinical isolates from acute gastroenteritis outbreaks. Infect. Immun. 2014, 82, 2390–2399. [Google Scholar] [CrossRef]
- Chiarezza, M.; Lyras, D.; Pidot, S.J.; Flores-Díaz, M.; Awad, M.M.; Kennedy, C.L.; Cordner, L.M.; Phumoonna, T.; Poon, R.; Hughes, M.L. The NanI and NanJ sialidases of Clostridium perfringens are not essential for virulence. Infect. Immun. 2009, 77, 4421–4428. [Google Scholar] [CrossRef]
- Oda, M.; Terao, Y.; Sakurai, J.; Nagahama, M. Membrane-binding mechanism of Clostridium perfringens alpha-toxin. Toxins 2015, 7, 5268–5275. [Google Scholar] [CrossRef]
- Oda, M.; Kabura, M.; Takagishi, T.; Suzue, A.; Tominaga, K.; Urano, S.; Nagahama, M.; Kobayashi, K.; Furukawa, K.; Furukawa, K. Clostridium perfringens alpha-toxin recognizes the GM1a-TrkA complex. J. Biol. Chem. 2012, 287, 33070–33079. [Google Scholar] [CrossRef]
- Monturiol-Gross, L.; Flores-Díaz, M.; Campos-Rodríguez, D.; Mora, R.; Rodríguez-Vega, M.; Marks, D.L.; Alape-Girón, A. Internalization of C lostridium perfringens α-toxin leads to ERK activation and is involved on its cytotoxic effect. Cell. Microbiol. 2014, 16, 535–547. [Google Scholar] [CrossRef]
- Goossens, E.; Valgaeren, B.R.; Pardon, B.; Haesebrouck, F.; Ducatelle, R.; Deprez, P.R.; Van Immerseel, F. Rethinking the role of alpha toxin in Clostridium perfringens-associated enteric diseases: A review on bovine necro-haemorrhagic enteritis. Vet. Res. 2017, 48, 1–17. [Google Scholar] [CrossRef]
- Monti, M.; Iommelli, F.; De Rosa, V.; Carriero, M.V.; Miceli, R.; Camerlingo, R.; Di Minno, G.; Del Vecchio, S. Integrin-dependent cell adhesion to neutrophil extracellular traps through engagement of fibronectin in neutrophil-like cells. PLoS ONE 2017, 12, e0171362. [Google Scholar]
- Keyburn, A.L.; Sheedy, S.A.; Ford, M.E.; Williamson, M.M.; Awad, M.M.; Rood, J.I.; Moore, R.J. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 2006, 74, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.E.; Brown, J.E.; Oyston, P.C.; Sakurai, J.; Titball, R.W. Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus. Infect. Immun. 1993, 61, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Kokai-Kun, J.F.; Songer, J.G.; Czeczulin, J.R.; Chen, F.; McClane, B.A. Comparison of Western immunoblots and gene detection assays for identification of potentially enterotoxigenic isolates of Clostridium perfringens. J. Clin. Microbiol. 1994, 32, 2533–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. Enterotoxic clostridia: Clostridium perfringens enteric diseases. Microbiol. Spectr. 2018, 6, 6–7. [Google Scholar] [CrossRef]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe 2016, 41, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Bannam, T.L.; Moore, R.J.; Rood, J.I. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins 2010, 2, 1913–1927. [Google Scholar] [CrossRef]
- Martin, T.G.; Smyth, J.A. Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States. Vet. Microbiol. 2009, 136, 202–205. [Google Scholar] [CrossRef]
- Amimoto, K.; Noro, T.; Oishi, E.; Shimizu, M. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 2007, 153, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Guttenberg, G.; Hornei, S.; Jank, T.; Schwan, C.; Lü, W.; Einsle, O.; Papatheodorou, P.; Aktories, K. Molecular characteristics of Clostridium perfringens TpeL toxin and consequences of mono-O-GlcNAcylation of Ras in living cells. J. Biol. Chem. 2012, 287, 24929–24940. [Google Scholar] [CrossRef] [PubMed]
- Schorch, B.; Song, S.; Van Diemen, F.R.; Bock, H.H.; May, P.; Herz, J.; Brummelkamp, T.R.; Papatheodorou, P.; Aktories, K. LRP1 is a receptor for Clostridium perfringens TpeL toxin indicating a two-receptor model of clostridial glycosylating toxins. Proc. Natl. Acad. Sci. USA 2014, 111, 6431–6436. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.K.; Bueschel, D.M.; Songer, J.G. Presence of Clostridium perfringens in retail chicken livers. Anaerobe 2013, 21, 67–68. [Google Scholar]
- Wilkie, D.C.; Van Kessel, A.G.; White, L.J.; Laarveld, B.; Drew, M.D. Dietary amino acids affect intestinal Clostridium perfringens populations in broiler chickens. Can. J. Anim. Sci. 2005, 85, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Rodgers, N.; Choct, M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010, 54, 1058–1065. [Google Scholar] [CrossRef]
- Samples, C.M. Isolation, identification and characterization of clostridium perfringens from cooked meat-poultry samples and in silico biomodeling of its delta enterotoxin. Delta 2010, 4, 028. [Google Scholar]
- Fader, R.C.; Mahon, C.R.; Lehman, D.C.; Manuselis, G. Textbook of Diagnostic Microbiology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Wise, M.G.; Siragusa, G.R. Quantitative detection of Clostridium perfringens in the broiler fowl gastrointestinal tract by real-time PCR. Appl. Environ. Microbiol. 2005, 71, 3911–3916. [Google Scholar] [CrossRef]
- Wu, S.; Rodgers, N.; Choct, M. Real-time PCR assay for Clostridium perfringens in broiler chickens in a challenge model of necrotic enteritis. Appl. Environ. Microbiol. 2011, 77, 1135–1139. [Google Scholar] [CrossRef]
- Moore, R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef]
- Rodgers, N.J.; Swick, R.A.; Geier, M.S.; Moore, R.J.; Choct, M.; Wu, S. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015, 59, 38–45. [Google Scholar] [CrossRef]
- Stanley, D.; Wu, S.; Rodgers, N.; Swick, R.A.; Moore, R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS ONE 2014, 9, e104739. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef]
- Wu, S.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef]
- Alnassan, A.A.; Kotsch, M.; Shehata, A.A.; Krüger, M.; Daugschies, A.; Bangoura, B. Necrotic enteritis in chickens: Development of a straightforward disease model system. Vet. Rec. 2014, 174, 555. [Google Scholar] [CrossRef]
- Williams, R.B.; Marshall, R.N.; La Ragione, R.M.; Catchpole, J. A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens. Parasitol. Res. 2003, 90, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Riddell, C.; Kong, X. The influence of diet on necrotic enteritis in broiler chickens. Avian Dis. 1992, 499–503. [Google Scholar] [CrossRef]
- Palliyeguru, M.; Rose, S.P.; Mackenzie, A.M. Effect of dietary protein concentrates on the incidence of subclinical necrotic enteritis and growth performance of broiler chickens. Poult. Sci. 2010, 89, 34–43. [Google Scholar] [CrossRef]
- Barnes, D.M.; Kirby, Y.K.; Oliver, K.G. Effects of biogenic amines on growth and the incidence of proventricular lesions in broiler chickens. Poult. Sci. 2001, 80, 906–911. [Google Scholar] [CrossRef]
- Chakrabarty, A.K.; Boro, B.R. Prevalence of food-poisoning (enterotoxigenic) Clostridium perfringens type A in blood and fish meal. Zentralblatt fur Bakteriologie, Mikrobiologie und Hygiene. Hygiene 1981, 172, 427–433. [Google Scholar]
- Antonissen, G.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F.; Timbermont, L.; Verlinden, M.; Janssens, G.P.J.; Eeckhaut, V.; Eeckhout, M. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS ONE 2014, 9, e108775. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Böhm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2006, 90, 32–37. [Google Scholar] [CrossRef]
- Antonissen, G.; Croubels, S.; Pasmans, F.; Ducatelle, R.; Eeckhaut, V.; Devreese, M.; Verlinden, M.; Haesebrouck, F.; Eeckhout, M.; De Saeger, S. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet. Res. 2015, 46, 98. [Google Scholar] [CrossRef] [PubMed]
- Hoerr, F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010, 54, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Lovland, A.; Kaldhusdal, M.; Redhead, K.; Skjerve, E.; Lillehaug, A. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. 2004, 33, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shivaramaiah, S.; Wolfenden, R.E.; Barta, J.R.; Morgan, M.J.; Wolfenden, A.D.; Hargis, B.M.; Téllez, G. The role of an early Salmonella Typhimurium infection as a predisposing factor for necrotic enteritis in a laboratory challenge model. Avian Dis. 2011, 55, 319–323. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, J.L.; Byrd, J.A.; Anderson, R.C.; Moore, R.W.; Edrington, T.S.; Genovese, K.J.; Poole, T.L.; Kubena, L.F.; Nisbet, D.J. Evaluation of immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poult. Sci. 2004, 83, 1948–1952. [Google Scholar] [CrossRef]
- Directive, C. 43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Off. J. Eur. Union L 2007, 182, 19–28. [Google Scholar]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. High stocking density as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2015, 44, 59–66. [Google Scholar] [CrossRef]
- Shane, S.M. Hot climates and ventilation. In Managing Poultry in Hot Climates; Zootecnica International: Scandicci, Italy, 1988; pp. 37–40. [Google Scholar]
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.J.; Patterson, J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2018, 47, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Hangalapura, B.N. Cold Stress and Immunity: Do Chickens Adapt to Cold by Trading-off Immunity for Thermoregulation? Wageningen University and Research: Wageningen, The Netherlands, 2006. [Google Scholar]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. The effect of cold stress on the pathogenesis of necrotic enteritis in broiler chicks. Avian Pathol. 2015, 44, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Regnier, J.A.; Kelley, K.W. Heat-and cold-stress suppresses in vivo and in vitro cellular immune responses of chickens. Am. J. Vet. Res. 1981, 42, 294–299. [Google Scholar]
- Van der Poll, T.; Opal, S.M. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 2008, 8, 32–43. [Google Scholar] [CrossRef]
- Prescott, J.F.; Parreira, V.R.; Mehdizadeh Gohari, I.; Lepp, D.; Gong, J. The pathogenesis of necrotic enteritis in chickens: What we know and what we need to know: A review. Avian Pathol. 2016, 45, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; De Smet, L.; Van Nieuwerburgh, F.; Parreira, V.R.; Van Driessche, G.; Haesebrouck, F.; Ducatelle, R.; Prescott, J.; Deforce, D.; Devreese, B. Perfrin, a novel bacteriocin associated with netB positive Clostridium perfringens strains from broilers with necrotic enteritis. Vet. Res. 2014, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Tsuchida, A.; Hirata, A.; Kobayashi, N.; Goto, K.; Osumi, K.; Hirose, Y.; Nakayama, J.; Yamanoi, T.; Ashida, H. Glycoside hydrolase family 89 α-N-acetylglucosaminidase from Clostridium perfringens specifically acts on GlcNAcα1, 4Galβ1R at the non-reducing terminus of O-glycans in gastric mucin. J. Biol. Chem. 2011, 286, 6479–6489. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, R.F.; Paspaliari, D.K.; Larsen, T.; Storgaard, B.G.; Larsen, M.H.; Ingmer, H.; Palcic, M.M.; Leisner, J.J. Bacterial chitinases and chitin-binding proteins as virulence factors. Microbiology 2013, 159, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Ficko-Blean, E.; Stuart, C.P.; Suits, M.D.; Cid, M.; Tessier, M.; Woods, R.J.; Boraston, A.B. Carbohydrate recognition by an architecturally complex α-N-acetylglucosaminidase from Clostridium perfringens. PLoS ONE 2012, 7, e33524. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Lepp, D.; Mehdizadeh Gohari, I.; Wu, T.; Zhou, H.; Yin, X.; Yu, H.; Prescott, J.F.; Nie, S.; Xie, M. The Agr-like quorum sensing system is required for pathogenesis of necrotic enteritis caused by Clostridium perfringens in poultry. Infect. Immun. 2017, 85, 975. [Google Scholar] [CrossRef] [PubMed]
- Olkowski, A.A.; Wojnarowicz, C.; Chirino-Trejo, M.; Drew, M.D. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res. Vet. Sci. 2006, 81, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.E.; Shak, J.R.; Canizalez-Roman, A. The CpAL quorum sensing system regulates production of hemolysins CPA and PFO to build Clostridium perfringens biofilms. Infect. Immun. 2015, 83, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Tang, X.X.; Chen, H.; Yu, S.; Zhang, L.; Caplan, M.J.; Chan, H.C. Lymphocytes accelerate epithelial tight junction assembly: Role of AMP-activated protein kinase (AMPK). PLoS ONE 2010, 5, e12343. [Google Scholar] [CrossRef]
- Saitoh, Y.; Suzuki, H.; Tani, K.; Nishikawa, K.; Irie, K.; Ogura, Y.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015, 347, 775–778. [Google Scholar] [CrossRef]
- Robertson, S.L.; Smedley, J.G., III; Singh, U.; Chakrabarti, G.; Van Itallie, C.M.; Anderson, J.M.; McClane, B.A. Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell. Microbiol. 2007, 9, 2734–2755. [Google Scholar] [CrossRef]
- Fujita, K.; Katahira, J.; Horiguchi, Y.; Sonoda, N.; Furuse, M.; Tsukita, S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000, 476, 258–261. [Google Scholar] [CrossRef]
- Smedley, J.G., III; Uzal, F.A.; McClane, B.A. Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin. Infect. Immun. 2007, 75, 2381–2390. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Koval, M. Specificity of interaction between Clostridium perfringens enterotoxin and claudin-family tight junction proteins. Toxins 2010, 2, 1595–1611. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, G.; McClane, B.A. The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell. Microbiol. 2005, 7, 129–146. [Google Scholar] [PubMed]
- Broussard, C.T.; Hofacre, C.L.; Page, R.K.; Fletcher, O.J. Necrotic enteritis in cage-reared commercial layer pullets. Avian Dis. 1986, 30, 617–619. [Google Scholar] [CrossRef]
- Long, J.R.; Barnum, D.A.; Pettit, J.R. Necrotic enteritis in broiler chickens II. Pathology and proposed pathogenesis. Can. J. Comp. Med. 1974, 38, 467. [Google Scholar] [PubMed]
- Løvland, A.; Kaldhusdal, M. Liver lesions seen at slaughter as an indicator of necrotic enteritis in broiler flocks. FEMS Immunol. Med. Microbiol. 1999, 24, 345–351. [Google Scholar] [CrossRef]
- Onderka, D.K.; Langevin, C.C.; Hanson, J.A. Fibrosing cholehepatitis in broiler chickens induced by bile duct ligations or inoculation of Clostridium perfringens. Can. J. Vet. Res. 1990, 54, 285. [Google Scholar]
- Park, M.; Rafii, F. Effects of bile acids and nisin on the production of enterotoxin by Clostridium perfringens in a nutrient-rich medium. Int. J. Microbiol. 2018, 2018, 7276523. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Sharif, S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008, 9, 101–110. [Google Scholar] [CrossRef]
- Liebler-Tenorio, E.M.; Pabst, R. MALT structure and function in farm animals. Vet. Res. 2006, 37, 257–280. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Harari, Y.; Chen, C.; Castro, G.A. The Gut. In Advances in Lactoferrin Research; Springer: Cham, Switzerland, 1998; pp. 167–173. [Google Scholar]
- Befus, A.D.; Johnston, N.; Leslie, G.A.; Bienenstock, J. Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer’s patches. J. Immunol. 1980, 125, 2626–2632. [Google Scholar]
- Mowat, A.M.; Viney, J.L. The anatomical basis of intestinal immunity. Immunol. Rev. 1997, 156, 145–166. [Google Scholar] [CrossRef]
- Yun, C.H.; Lillehoj, H.S.; Lillehoj, E.P. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000, 24, 303–324. [Google Scholar] [CrossRef]
- Tagliabue, A.; Befus, A.D.; Clark, D.A.; Bienenstock, J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J. Exp. Med. 1982, 155, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.L.; Jones, A.L. Epithelial cell specialization within human Peyer’s patches: An ultrastructural study of intestinal lymphoid follicles. Gastroenterology 1974, 66, 189–203. [Google Scholar] [CrossRef]
- Jones, B.D.; Ghori, N.; Falkow, S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp. Med. 1994, 180, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, C.; Melgar, S.; Yeung, M.M.; Hammarström, S.; Hammarström, M. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J. Immunol. 1996, 157, 1926–1934. [Google Scholar] [PubMed]
- McGee, D.W.; Beagley, K.W.; Aicher, W.K.; McGhee, J.R. Transforming growth factor-beta and IL-1 beta act in synergy to enhance IL-6 secretion by the intestinal epithelial cell line, IEC-6. J. Immunol. 1993, 151, 970–978. [Google Scholar]
- Reinecker, H.; MacDermott, R.P.; Mirau, S.; Dignass, A.; Podolsky, D.K. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology 1996, 111, 1706–1713. [Google Scholar] [CrossRef]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Temperley, N.D.; Berlin, S.; Paton, I.R.; Griffin, D.K.; Burt, D.W. Evolution of the chicken Toll-like receptor gene family: A story of gene gain and gene loss. BMC Genom. 2008, 9, 62. [Google Scholar] [CrossRef]
- Kannaki, T.R.; Reddy, M.R.; Shanmugam, M.; Verma, P.C.; Sharma, R.P. Chicken toll-like receptors and their role in immunity. Worlds Poult. Sci. J. 2010, 66, 727–738. [Google Scholar] [CrossRef]
- Warren, H.S. Toll-like receptors. Crit. Care Med. 2005, 33, S457–S459. [Google Scholar] [CrossRef]
- Kogut, M.H.; Iqbal, M.; He, H.; Philbin, V.; Kaiser, P.; Smith, A. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005, 29, 791–807. [Google Scholar] [CrossRef]
- Iqbal, M.; Philbin, V.J.; Smith, A.L. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 2005, 104, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Leveque, G.; Forgetta, V.; Morroll, S.; Smith, A.L.; Bumstead, N.; Barrow, P.; Loredo-Osti, J.C.; Morgan, K.; Malo, D. Allelic variation in TLR4 is linked to susceptibility to Salmonella enterica serovar Typhimurium infection in chickens. Infect. Immun. 2003, 71, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Philbin, V.J.; Iqbal, M.; Boyd, Y.; Goodchild, M.J.; Beal, R.K.; Bumstead, N.; Young, J.; Smith, A.L. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology 2005, 114, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.; Cormican, P.; Cahalane, S.; Allan, B.; Lloyd, A.T.; Meade, K.; James, T.; Lynn, D.J.; Babiuk, L.A.; O’farrelly, C. Induction of a novel chicken Toll-like receptor following Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006, 74, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C.A., Jr. How does the immune system distinguish self from nonself? In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 12, pp. 185–188. [Google Scholar]
- Akira, S. Toll receptor families: Structure and function. Semin. Immunol. 2004, 16, 1–2. [Google Scholar] [CrossRef]
- Karpala, A.J.; Lowenthal, J.W.; Bean, A.G. Activation of the TLR3 pathway regulates IFNβ production in chickens. Dev. Comp. Immunol. 2008, 32, 435–444. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Birchler, T.; Seibl, R.; Büchner, K.; Loeliger, S.; Seger, R.; Hossle, J.P.; Aguzzi, A.; Lauener, R.P. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur. J. Immunol. 2001, 31, 3131–3137. [Google Scholar] [CrossRef]
- Iqbal, M.; Philbin, V.J.; Withanage, G.; Wigley, P.; Beal, R.K.; Goodchild, M.J.; Barrow, P.; McConnell, I.; Maskell, D.J.; Young, J. Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar typhimurium. Infect. Immun. 2005, 73, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sarson, A.J.; Gong, J.; Zhou, H.; Zhu, W.; Kang, Z.; Yu, H.; Sharif, S.; Han, Y. Expression profiles of genes in Toll-like receptor-mediated signaling of broilers infected with Clostridium perfringens. Clin. Vaccine Immunol. 2009, 16, 1639–1647. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Li, C.; Liu, D.; Guo, Y. Inflammatory responses to a Clostridium perfringens type A strain and α-toxin in primary intestinal epithelial cells of chicken embryos. Avian Pathol. 2015, 44, 81–91. [Google Scholar] [CrossRef]
- Oh, S.T.; Lillehoj, H.S. The role of host genetic factors and host immunity in necrotic enteritis. Avian Pathol. 2016, 45, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Scott, P. IL-12: Initiation cytokine for cell-mediated immunity. Science 1993, 260, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Höfer, T.; Krichevsky, O.; Altan-Bonnet, G. Competition for IL-2 between regulatory and effector T cells to chisel immune responses. Front. Immunol. 2012, 3, 268. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G.; Pflanz, S.; Kastelein, R.A. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity 2003, 19, 641–644. [Google Scholar] [CrossRef]
- King, A.; Loke, Y.W. Effect of IFN-γ and IFN-α on killing of human trophoblast by decidual LAK cells. J. Reprod. Immunol. 1993, 23, 51–62. [Google Scholar] [CrossRef]
- Kolls, J.K.; Khader, S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010, 21, 443–448. [Google Scholar] [CrossRef]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, I.; Andronicos, N.M.; Swick, R.A.; Hine, B.; Sharma, N.; Kheravii, S.K.; Wu, S.; Hunt, P. Immune responses following experimental infection with Ascaridia galli and necrotic enteritis in broiler chickens. Avian Pathol. 2017, 46, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lillehoj, H.S.; Allen, P.C.; Park, D.W.; FitzCoy, S.; Bautista, D.A.; Lillehoj, E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008, 52, 14–22. [Google Scholar] [CrossRef]
- Yeh, H.; Winslow, B.J.; Junker, D.E.; Sharma, J.M. In vitro effects of recombinant chicken interferon-gamma on immune cells. J. Interferon Cytokine Res. 1999, 19, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Parreira, V.R.; Sharif, S.; Prescott, J.F. Clostridium perfringens antigens recognized by broiler chickens immune to necrotic enteritis. Clin. Vaccine Immunol. 2006, 13, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Chasser, K.M.; Duff, A.F.; Briggs, W.N.; Latorre, J.D.; Barta, J.R.; Bielke, L.R. Comparison of multiple methods for induction of necrotic enteritis in broilers. I. J. Appl. Poult. Res. 2018, 27, 577–589. [Google Scholar] [CrossRef]
- Shojadoost, B.; Vince, A.R.; Prescott, J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: A critical review. Vet. Res. 2012, 43, 1–12. [Google Scholar] [CrossRef]
- Akerele, G.; Al Hakeem, W.G.; Lourenco, J.; Selvaraj, R.K. The Effect of Necrotic Enteritis Challenge on Production Performance, Cecal Microbiome, and Cecal Tonsil Transcriptome in Broilers. Pathogens 2022, 11, 839. [Google Scholar] [CrossRef]
- Gaboriau-Routhiau, V.; Rakotobe, S.; Lécuyer, E.; Mulder, I.; Lan, A.; Bridonneau, C.; Rochet, V.; Pisi, A.; De Paepe, M.; Brandi, G. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009, 31, 677–689. [Google Scholar] [CrossRef]
- Lee, Y.K.; Salminen, S. Handbook of Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Talarico, T.L.; Casas, I.A.; Chung, T.C.; Dobrogosz, W.J. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1988, 32, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Cleusix, V.; Lacroix, C.; Vollenweider, S.; Duboux, M.; Le Blay, G. Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol. 2007, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE 2017, 12, e0188634. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Van Immerseel, F.; Croubels, S.; De Baere, S.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb. Biotechnol. 2011, 4, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furness, J.B.; Rivera, L.R.; Cho, H.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Pontin, K.P.; Hernandez-Velasco, X.; Merino-Guzman, R.; Adhikari, B.; López-Arellano, R.; Kwon, Y.M.; Hargis, B.M.; Arreguin-Nava, M.A. Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions, and ileal microbiota in broiler chickens using a laboratory challenge model. Front. Vet. Sci. 2019, 6, 108. [Google Scholar] [CrossRef]
- Food and Drug Administration Guidance for Industry# 213: New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated feed or Drinking Water of Food Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI# 209. Center for Veterinary Medicine. US Department of Health and Human Services, Washington, DC. Available online: https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM299624.pdf2013 (accessed on 30 May 2022).
- Kaldhusdal, M.; Lovland, A. The economical impact of Clostridium perfringens is greater than anticipated. World Poult. 2000, 16, 50–51. [Google Scholar]
- Craven, S.E.; Stern, N.J.; Bailey, J.S.; Cox, N.A. Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing. Avian Dis. 2001, 45, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins. Gram-Positive Bacteria, 2nd ed.; Lampel, K.; Al-Khaldi, S.; Cahill, S. (Eds.) Center for Food Safety and Applied Nutrition of the Food and Drug Administration (FDA), US Department of Health and Human Services: Silver Spring, MA, USA, 2012. [Google Scholar]
- Wahl, E.; Rømma, S.; Granum, P.E. A Clostridium perfringens outbreak traced to temperature-abused beef stew, Norway, 2012. Eurosurveillance 2013, 18, 20408. [Google Scholar] [CrossRef] [PubMed]
- Labbé, R.G. Clostridium perfringens. J. Assoc. Off. Anal. Chem. 1991, 74, 711–714. [Google Scholar] [CrossRef]
- Teuber, M. Spread of antibiotic resistance with food-borne pathogens. Cell. Mol. Life Sci. CMLS 1999, 56, 755–763. [Google Scholar] [CrossRef]
- Mwangi, S.; Timmons, J.; Fitz-Coy, S.; Parveen, S. Characterization of Clostridium perfringens recovered from broiler chicken affected by necrotic enteritis. Poult. Sci. 2019, 98, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Salvarani, F.M.; Assis, R.A.; Martins, N.; Pires, P.S.; Lobato, F. Antimicrobial susceptibility of Clostridium perfringens strains isolated from broiler chickens. Braz. J. Microbiol. 2009, 40, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Anju, K.; Karthik, K.; Divya, V.; Priyadharshini, M.L.M.; Sharma, R.K.; Manoharan, S. Toxinotyping and molecular characterization of antimicrobial resistance in Clostridium perfringens isolated from different sources of livestock and poultry. Anaerobe 2021, 67, 102298. [Google Scholar] [CrossRef] [PubMed]
- Lyras, D.; Adams, V.; Ballard, S.A.; Teng, W.L.; Howarth, P.M.; Crellin, P.K.; Bannam, T.L.; Songer, J.G.; Rood, J.I. tIS Cpe8, an IS 1595-family lincomycin resistance element located on a conjugative plasmid in Clostridium perfringens. J. Bacteriol. 2009, 191, 6345–6351. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Han, X.; Lyras, D.; Rood, J.I. Antibiotic resistance plasmids and mobile genetic elements of Clostridium perfringens. Plasmid 2018, 99, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Du, X.; Southey, L.; Bulach, D.M.; Seemann, T.; Yan, X.; Bannam, T.L.; Rood, J.I. Functional analysis of a bacitracin resistance determinant located on ICE Cp1, a novel Tn 916-like element from a conjugative plasmid in Clostridium perfringens. Antimicrob. Agents Chemother. 2015, 59, 6855–6865. [Google Scholar] [CrossRef] [PubMed]
- Marchand-Austin, A.; Rawte, P.; Toye, B.; Jamieson, F.B.; Farrell, D.J.; Patel, S.N. Antimicrobial susceptibility of clinical isolates of anaerobic bacteria in Ontario, 2010–2011. Anaerobe 2014, 28, 120–125. [Google Scholar] [CrossRef]
- Tansuphasiri, U.; Matra, W.; Sangsuk, L. Antimicrobial resistance among Clostridium perfringens isolated from various sources in Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 63, 954–961. [Google Scholar]
- Zhou, Y.; Li, J.; Schwarz, S.; Zhang, S.; Tao, J.; Fan, R.; Walsh, T.R.; Wu, C.; Wang, Y. Mobile oxazolidinone/phenicol resistance gene optrA in chicken Clostridium perfringens. J. Antimicrob. Chemother. 2020, 75, 3067–3069. [Google Scholar] [CrossRef]
- Wei, B.; Cha, S.; Zhang, J.; Shang, K.; Park, H.; Kang, J.; Lee, K.; Kang, M.; Jang, H. Antimicrobial susceptibility and association with toxin determinants in Clostridium perfringens isolates from chickens. Microorganisms 2020, 8, 1825. [Google Scholar] [CrossRef]
- Carlson, M.S.; Fangman, T.J. Swine Antibiotics and Feed Additives: Food Safety Considerations; University of Missouri: Colombia, MO, USA, 2018. [Google Scholar]
- Kim, G.; Seo, Y.M.; Kim, C.H.; Paik, I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011, 90, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Shane, S. Update on the poultry disease situation in the USA-Significant disease outbreaks in recent years. Overall, the health status is good but there is no room for complacency. Poult. Int. 2004, 43, 10–15. [Google Scholar]
- Revington, B. Feeding poultry in the post-antibiotic era. In Proceedings of the Multi-State Poultry Meeting, Atlanta, GA, USA, 14–16 May 2002. [Google Scholar]
- Fuller, R. Probiotic in man and animals. J. Appl. Bacteriol. 1989, 66, 131–139. [Google Scholar]
- Fathima, S.; Shanmugasundaram, R.; Adams, D.; Selvaraj, R.K. Gastrointestinal Microbiota and Their Manipulation for Improved Growth and Performance in Chickens. Foods 2022, 11, 1401. [Google Scholar] [CrossRef]
- Ma, T.; Suzuki, Y. Dissect the mode of action of probiotics in affecting host-microbial interactions and immunity in food producing animals. Vet. Immunol. Immunopathol. 2018, 205, 35–48. [Google Scholar] [CrossRef]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–Nomad and Ideal Probiotic. Front. Microbiol. 2021, 2021, 2911. [Google Scholar] [CrossRef]
- Patterson, J.A.; Burkholder, K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003, 82, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Hamidon, F.; Rajangan, C.; Soh, K.P.; Gan, C.Y.; Lim, T.S.; Abdullah, W.N.W.; Liong, M.T. Application of probiotics for the production of safe and high-quality poultry meat. Korean J. Food Sci. Anim. Resour. 2016, 36, 567. [Google Scholar] [CrossRef]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Markazi, A.; Mortada, M.; Ng, T.T.; Applegate, T.J.; Bielke, L.R.; Syed, B.; Pender, C.M.; Curry, S.; Murugesan, G.R. Research Note: Effect of synbiotic supplementation on caecal Clostridium perfringens load in broiler chickens with different necrotic enteritis challenge models. Poult. Sci. 2020, 99, 2452–2458. [Google Scholar] [CrossRef]
- Khalique, A.; Zeng, D.; Shoaib, M.; Wang, H.; Qing, X.; Rajput, D.S.; Pan, K.; Ni, X. Probiotics mitigating subclinical necrotic enteritis (SNE) as potential alternatives to antibiotics in poultry. Amb. Express 2020, 10, 50. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Qing, X.; Liu, L.; Lai, J.; Khalique, A.; Li, G.; Pan, K.; Jing, B.O.; Zeng, D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017, 8, 1592. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Monsan, P.F.; Paul, F. Biotechnology in animal feeds and animal feeding. In Oligosaccharide Feed Additives; CABI: Paris, France, 1995; pp. 233–245. [Google Scholar]
- Awad, W.A.; Ghareeb, K.; Nitsch, S.; Pasteiner, S.; Abdel-Raheem, S.; Böhm, J. Effects of dietary inclusion of prebiotic, probiotic and synbiotic on the intestinal glucose absorption of broiler chickens. Int. J. Poult. Sci. 2008, 7, 686–691. [Google Scholar] [CrossRef]
- Ferket, P.R. Alternatives to antibiotics in poultry production: Responses, practical experience and recommendations. Nutritional biotechnology in the feed and food industries. In Proceedings of the Alltech’s 20th Annual Symposium: Re-imagining the feed industry, Lexington, KY, USA, 23–26 May 2004; Alltech: London, UK, 2004; pp. 57–67. [Google Scholar]
- Nabizadeh, A. The effect of inulin on broiler chicken intestinal microflora, gut morphology, and performance. J. Anim. Feed. Sci. 2012, 21, 725–734. [Google Scholar] [CrossRef]
- Pourabedin, M.; Guan, L.; Zhao, X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome 2015, 3, 1–12. [Google Scholar] [CrossRef]
- Northcote, D.H.; Horne, R.W. The chemical composition and structure of the yeast cell wall. Biochem. J. 1952, 51, 232. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Selvaraj, R.K. Effect of killed whole yeast cell prebiotic supplementation on broiler performance and intestinal immune cell parameters. Poult. Sci. 2012, 91, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hofacre, C.L.; Beacorn, T.; Collett, S.; Mathis, G. Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis. J. Appl. Poult. Res. 2003, 12, 60–64. [Google Scholar] [CrossRef]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Puvača, N.; Ljubojević, D.; Kostadinović, L.J.; Lukač, D.; Lević, J.; Popović, S.; Đuragić, O. Spices and herbs in broilers nutrition: Effects of garlic (Allium sativum L.) on broiler chicken production. Worlds Poult. Sci. J. 2015, 71, 533–538. [Google Scholar] [CrossRef]
- Bravo, D.; Pirgozliev, V.; Rose, S.P. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin improves energy utilization and growth performance of broiler chickens fed maize-based diet. J. Anim. Sci. 2014, 92, 1531–1536. [Google Scholar] [CrossRef]
- Tajodini, M.; Saeedi, H.R.; Moghbeli, P. Use of black pepper, cinnamon and turmeric as feed additives in the poultry industry. Worlds Poult. Sci. J. 2015, 71, 175–183. [Google Scholar] [CrossRef]
- Lewis, M.R.; Rose, S.P.; Mackenzie, A.M.; Tucker, L.A. Effects of dietary inclusion of plant extracts on the growth performance of male broiler chickens. Br. Poult. Sci. 2003, 44, 43–44. [Google Scholar] [CrossRef]
- Naidoo, V.; McGaw, L.J.; Bisschop, S.; Duncan, N.; Eloff, J.N. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008, 153, 214–219. [Google Scholar] [CrossRef]
- Khadem, A.; Soler, L.; Everaert, N.; Niewold, T.A. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Br. J. Nutr. 2014, 112, 1110–1118. [Google Scholar] [CrossRef]
- Guo, F.C.; Kwakkel, R.P.; Williams, B.A.; Parmentier, H.K.; Li, W.K.; Yang, Z.Q.; Verstegen, M.W. Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of Eimeria tenella-infected chickens. Poult. Sci. 2004, 83, 1124–1132. [Google Scholar] [CrossRef]
- Stephen, K.; Ajanusi, O.J.; Suleiman, M.M.; Orakpoghenor, O.; Ogwiji, M. In vitro and in vivo anthelmintic effects of Sterospermum kunthianum (Cham-Holl) leaf extract against Ascaridia galli in experimentally infected broiler chickens. J. Parasit. Dis. 2022, 46, 152–158. [Google Scholar] [CrossRef]
- Wenk, C. Why all the discussion about herbs? In Proceedings of the Alltech’s 16th Annual Symposium of Biotechnology in the Feeding Industry, Nicholasvile, KY, USA, 28 February 2000; Nottingham University Press: Nottingham, UK, 2000; pp. 79–96. [Google Scholar]
- Hernandez, F.; Madrid, J.; Garcia, V.; Orengo, J.; Megias, M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004, 83, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Reansoi, A.; Ruangpanit, Y.; Attamangkune, S. Effect of quaternary benzophenantridine and protopine alkaloids on growth response and gut health of broiler under hot climate management. In Proceedings of the 53rd Kasetsart University Annual Conference Smart Agriculture” The Future of Thailand”. Plants, Animals, Veterinary Medicine, Fisheries, Agricultural Extension and Home Economics, Kasetsart University, Bangkok, Thailand, 3–6 February 2015; pp. 800–807. [Google Scholar]
- Lee, K.; Kim, J.; Oh, S.; Kang, C.; An, B. Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J. Poult. Sci. 2014, 52, 0140073. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.H.; Jeong, M.S.; Kim, J.B.; Jang, H.H.; Jeong, S.C.; Kim, D.W.; Lillehoj, H.S. In vitro analysis of the immunomodulating effects of Allium hookeri on lymphocytes, macrophages, and tumour cells. J. Poult. Sci. 2016, 54, 0160108. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.; Gadde, U.D.; Oh, S.T.; Lee, S.J.; Lillehoj, H.S. Allium hookeri supplementation improves intestinal immune response against necrotic enteritis in young broiler chickens. Poult. Sci. 2018, 97, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Selvaraj, R.K. Application of Nutritional Immunology in the Mitigation of Economic and Production losses in the Poultry Industry Associated with Food-borne Pathogens, Coccidiosis, and Necrotic Enteritis. In Proceedings of the Arkansas Nutrition Conference, Rogers, AR, USA, 13–15 September 2022; Volume 2022, p. 5. [Google Scholar]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S. Organic acid blends improve intestinal integrity, modulate short-chain fatty acids profiles and alter microbiota of broilers under necrotic enteritis challenge. Anim. Nutr. 2022, 8, 82–90. [Google Scholar] [CrossRef]
- Dibner, J.J.; Buttin, P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Kim, S.; Kang, G.; Kang, H.; Lee, S.; Kim, S. A study on the efficacy of dietary supplementation of organic acid mixture in broiler chicks. J. Anim. Sci. Technol. 2009, 51, 207–216. [Google Scholar] [CrossRef]
- Esmaeilipour, O.; Shivazad, M.; Moravej, H.; Aminzadeh, S.; Rezaian, M.; Van Krimpen, M.M. Effects of xylanase and citric acid on the performance, nutrient retention, and characteristics of gastrointestinal tract of broilers fed low-phosphorus wheat-based diets. Poult. Sci. 2011, 90, 1975–1982. [Google Scholar] [CrossRef]
- Hasan, A.; Mehmet, L.O.; Aylin, A.O.; Fisun, K.; Hasan, E.S. The effects of supplementing an organic acid blend and/or microbial phytase to a corn-soybean based diet fed to broiler chickens. Afr. J. Agric. Res. 2011, 6, 642–649. [Google Scholar]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S. Potential of blended organic acids to improve performance and health of broilers infected with necrotic enteritis. Anim. Nutr. 2021, 7, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Tini, M.; Jewell, U.R.; Camenisch, G.; Chilov, D.; Gassmann, M. Generation and application of chicken egg-yolk antibodies. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 569–574. [Google Scholar] [CrossRef]
- Chalghoumi, R.; Beckers, Y.; Portetelle, D.; Théwis, A. Hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: A review. Biotechnol. Agron. Société Environ. 2009, 13, 295–308. [Google Scholar]
- Abadeen, Z.U.; Javed, M.T.; Rizvi, F.; Rahman, S.U. Salutary effects of anti-Clostridium perfringens type A egg yolk antibodies (IgY) on growth performance and hemato-biochemical parameters in experimentally infected broiler chicken. Pak. Vet. J. 2021, 41, 562–566. [Google Scholar] [CrossRef]
- Cook, S.R.; Maiti, P.K.; DeVinney, R.; Allen-Vercoe, E.; Bach, S.J.; McAllister, T.A. Avian-and mammalian-derived antibodies against adherence-associated proteins inhibit host cell colonization by Escherichia coli O157: H7. J. Appl. Microbiol. 2007, 103, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Chalghoumi, R.; Marcq, C.; Thewis, A.; Portetelle, D.; Beckers, Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler chickens. Poult. Sci. 2009, 88, 2081–2092. [Google Scholar] [CrossRef]
- Al-Adwani, S.R.; Crespo, R.; Shah, D.H. Production and evaluation of chicken egg-yolk-derived antibodies against Campylobacter jejuni colonization-associated proteins. Foodborne Pathog. Dis. 2013, 10, 624–631. [Google Scholar] [CrossRef]
- Malik, M.W.; Ayub, N.; Qureshi, I.Z. Passive immunization using purified IgYs against infectious bursal disease of chickens in Pakistan. J. Vet. Sci. 2006, 7, 43–46. [Google Scholar] [CrossRef]
- Voyles, B.A. The Biology of Viruses; Mosby-Year Book Inc.: St. Louis, MO, USA, 1993. [Google Scholar]
- Iqbal, A.; Hasni, S.; Rahman, S. Preparation and evaluation of bacteriophage lysate specific for Salmonella typhimurium. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M. Why microbial predators and parasites do not eliminate their prey and hosts. Annu. Rev. Biol. 1981, 35, 113–133. [Google Scholar] [CrossRef]
- Higgins, J.P.; Higgins, S.E.; Guenther, K.L.; Huff, W.; Donoghue, A.M.; Donoghue, D.J.; Hargis, B.M. Use of a specific bacteriophage treatment to reduce Salmonella in poultry products. Poult. Sci. 2005, 84, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.; Lee, J.; Chae, J.; Kim, J.; Eun, J.; Lee, K.; Seo, K. Characterization of a novel bacteriophage φCJ22 and its prophylactic and inhibitory effects on necrotic enteritis and Clostridium perfringens in broilers. Poult. Sci. 2021, 100, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W.; Skinner, J.; Sulakvelidze, A.; Mathis, G.F.; Hofacre, C.L. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 2010, 54, 33–40. [Google Scholar] [CrossRef]
- Zimmer, M.; Vukov, N.; Scherer, S.; Loessner, M.J. The murein hydrolase of the bacteriophage φ3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl. Environ. Microbiol. 2002, 68, 5311–5317. [Google Scholar] [CrossRef]
- Hosny, R.A.; Gaber, A.F.; Sorour, H.K. Bacteriophage mediated control of necrotic enteritis caused by C. perfringens in broiler chickens. Vet. Res. Commun. 2021, 45, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.D. Bacterial vaccines: Old and new, veterinary and medical. Vaccine 1992, 10, 977–990. [Google Scholar] [CrossRef]
- Thompson, D.R.; Parreira, V.R.; Kulkarni, R.R.; Prescott, J.F. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 2006, 113, 25–34. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Portela, R.W.; Sproat, K.; Ford, M.E.; Bannam, T.L.; Yan, X.; Rood, J.I.; Moore, R.J. Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Vet. Res. 2013, 44, 54. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Portela, R.W.; Ford, M.E.; Bannam, T.L.; Yan, X.X.; Rood, J.I.; Moore, R.J. Maternal immunization with vaccines containing recombinant NetB toxin partially protects progeny chickens from necrotic enteritis. Vet. Res. 2013, 44, 108. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.E.; Anderson, D.M.; Hsiao, H.Y.; Mathis, G.F.; Fodge, D.W. Beneficial effect of β-mannanase feed enzyme on performance of chicks challenged with Eimeria sp. and Clostridium perfringens. Avian Dis. 2003, 47, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Kaldhusdal, M.; Skjerve, E. Association between cereal contents in the diet and incidence of necrotic enteritis in broiler chickens in Norway. Prev. Vet. Med. 1996, 28, 1–16. [Google Scholar] [CrossRef]
- Dahiya, J.P.; Wilkie, D.C.; Van Kessel, A.G.; Drew, M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006, 129, 60–88. [Google Scholar] [CrossRef]



| Toxinotype | Alpha (α) | Beta (β) | Epsilon (ε) | Iota (ι) | CPE | NetB |
|---|---|---|---|---|---|---|
| Type A | + | − | − | − | − | − |
| Type B | + | + | + | − | − | − |
| Type C | + | + | − | − | +/− | − |
| Type D | + | − | + | − | +/− | − |
| Type E | + | − | − | + | +/− | − |
| Type F | + | − | − | − | + | − |
| Type G | + | − | − | − | − | + |
| Toxin/Enzyme | Gene Location | Biological Activity | Diseases and Affected Species |
|---|---|---|---|
| Alpha toxin (CPA) | Chromosome | Phospholipase C and sphingomyelinase | Gas gangrene in humans and different animals [18]. |
| Beta toxin (CPB) | Plasmid | Pore-forming toxin | Enterocolitis and Enterotoxaemia in neonatal animals Lamb dysentery struck [19] |
| Beta2 toxin (CPB2) | Plasmid | Putative pore-forming toxin | Enteric disease in horses [20], pigs [21] |
| Delta-toxin | Plasmid | Pore-forming toxin | Damages epithelial cells [22] |
| Enterotoxin (CPE) | Plasmid/ chromosome | Pore-forming toxin | Food poising in humans [23]. |
| Epsilon toxin (ETX) | Plasmid | Pore-forming toxin | Clostridial enterotoxaemia in sheep and goats [24] |
| NetB (netB) | Plasmid | Pore-forming toxin | Necrotic enteritis [25] |
| NetE | Plasmid | Putative pore-forming toxin | Acute hemorrhagic diarrhea syndrome in dogs [26] |
| NetF | Plasmid | Pore-forming toxin | Acute hemorrhagic diarrhea syndrome in dogs [26] |
| NetG | Plasmid | Putative pore-forming toxin | Acute hemorrhagic diarrhea syndrome in dogs [26] |
| Theta-toxin/perfringolysin O | Chromosome | Pore-forming toxin | Gas gangrene in humans [27] |
| Iota toxin (Iap/ibp) | Plasmid | Actin-specific ADP-ribosyl transferase | Enterotoxaemia in rabbits [28] |
| TepL (TepL) | Plasmid | Ras-specific mono-glucosyltransferase | Necrotic enteritis in chicken [29] |
| BecA, | Plasmid | Actin-specific ADP-ribosyl transferase | Acute gastroenteritis in humans [30] |
| BecB | Plasmid | Actin-specific ADP-ribosyl transferase | Acute gastroenteritis in humans [30] |
| NanH | Chromosome | Sialidase | C. perfringens-mediated tissue infection [31] |
| NanI | Chromosome | Sialidase | C. perfringens-mediated tissue infection [31] |
| NanJ | Chromosome | Sialidase | C. perfringens-mediated tissue infection [31] |
| Kappa-toxin (colA) | Chromosome | Collagenase | |
| Mu-toxin | Chromosome | Hyaluronidase | |
| Lambda-toxin | Plasmid | Protease | |
| Alpha-clostripain | Chromosome | Cysteine protease |
| Name of Test | Result |
|---|---|
| Aerotolerant growth | Negative |
| Gram staining | Gram positive rods |
| Motility | Negative |
| Sheep blood agar (SBA) | Double zone of β-hemolysis |
| TSC agar | Black color colonies |
| Hydrogen sulphide | Positive |
| Nitrate reduction | Reduce nitrate to nitrite |
| Lecithinase activity | Positive |
| Gelatin hydrolysis | Positive |
| Carbohydrate fermentation | Produce acid and gas by the fermentation of glucose, maltose, sucrose, and lactose. |
| Indole test | Negative |
| Methyl Red test | Positive |
| Vogus Proskauer | Negative |
| Toll-Like Receptor Class | Tissue |
|---|---|
| TLR1/6/10, TLR2 types 1 and 2, TLR3, TLR4, TLR5, and TLR7 [122] | Heterophils |
| TLR4, TLR9 [120] | Macrophages |
| TLR1, TLR3, and TLR6 [123] | Blood, spleen, tonsils, bursa of Fabricius, thymus, liver, kidney, oviduct, lungs, small intestine, and large intestine |
| TLR2 [123] | Tonsils, blood, spleen, liver, bursa of Fabricius, oviduct, and intestine |
| TLR4 [123] | Spleen, liver, and tonsils |
| TLR5 [124] | Tonsils, spleen, liver, lungs, heart, intestine, immune cells, and testis |
| TLR7 [125] | Spleen, tonsils, bursa |
| TLR15 [126] | Spleen, liver, bursa, intestine, and tongue |
| TLR21 [126] | Heterophils |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms 2022, 10, 1958. https://doi.org/10.3390/microorganisms10101958
Fathima S, Hakeem WGA, Shanmugasundaram R, Selvaraj RK. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms. 2022; 10(10):1958. https://doi.org/10.3390/microorganisms10101958
Chicago/Turabian StyleFathima, Shahna, Walid Ghazi Al Hakeem, Revathi Shanmugasundaram, and Ramesh K. Selvaraj. 2022. "Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention" Microorganisms 10, no. 10: 1958. https://doi.org/10.3390/microorganisms10101958





