Partial Replacement of Oat Hay with Whole-Plant Hydroponic Barley Seedlings Modulates Ruminal Microbiota and Affects Growth Performance of Holstein Heifers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydroponic Barley Seedling Production
2.2. Nutritional Compositions of Hydroponic Barley Seedlings
2.3. Animals and Experimental Design
2.4. Sample Collection and Analysis
2.5. Statistical Analysis
3. Results
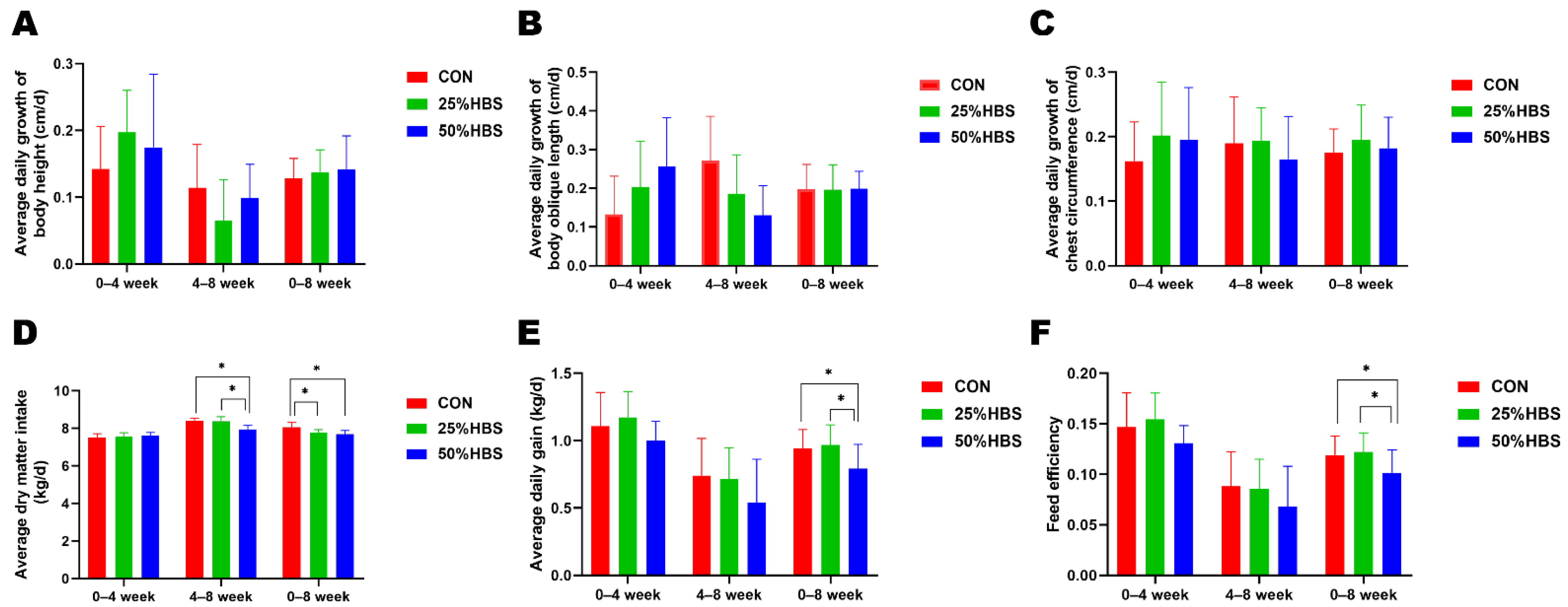
3.1. Feed intake and Growth Performance
3.2. Nutrient Digestibility and Nitrogen Recycling
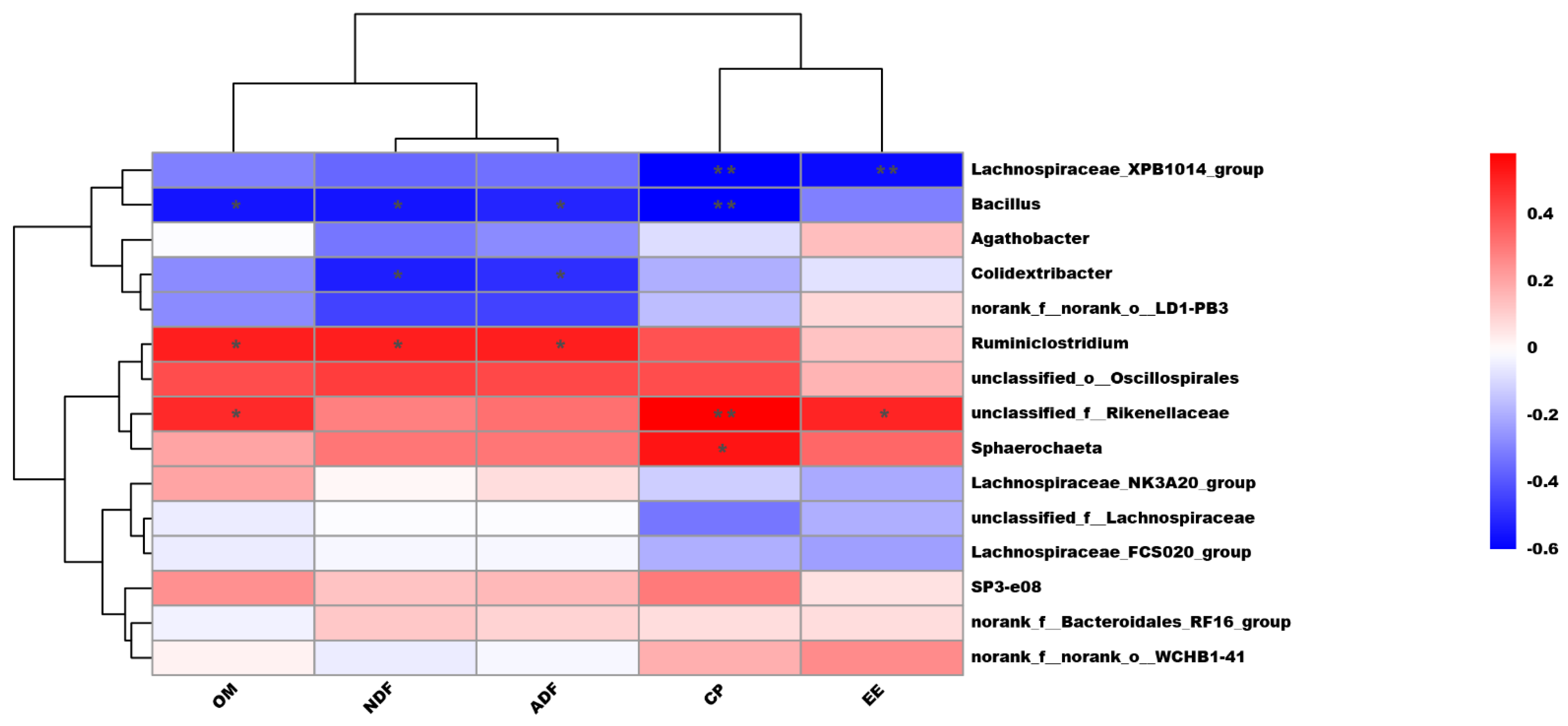
3.3. Microbial Population and Its Association with Nutrient Digestibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaziev, D.; Gadiev, R.; Yusupova, C.; Kazanina, M.; Kopylova, S. Effect of hydroponic green herbage on the productive qualities of parent flock geese. Vet. World 2021, 14, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Girma, F.; Gebremariam, B. Review on hydroponic feed value to livestock production. JSIR 2018, 7, 106–109. [Google Scholar] [CrossRef]
- Gebremedhin, W.K. Nutritional benefit economic value of feeding hydroponically grown maize and barley fodder for Konkan Kanyal goats. IOSR-JAVS 2015, 8, 24–30. [Google Scholar]
- Abouelezz, K.F.M.; Sayed, M.A.M.; Abdelnabi, M.A. Evaluation of hydroponic barley sprouts as a feed supplement for laying Japanese quail: Effect on egg production, egg quality, fertility, blood constituents, and internal organs. Anim. Feed Sci. Tech. 2019, 252, 126–135. [Google Scholar] [CrossRef]
- Dung, D.D.; Godwin, I.R.; Nolan, J.V. Nutrient content and in sacco digestibility of barley grain and sprouted barley. J. Anim. Vet. Adv. 2010, 9, 2485–2492. [Google Scholar] [CrossRef]
- Naik, P.K.; Dhuri, R.B.; Karunakaran, M.; Swain, B.K.; Singh, N.P. Effect of feeding hydroponics maize fodder on digestibility of nutrients and milk production in lactating cows. Indian J. Anim. Sci. 2014, 84, 880–883. [Google Scholar]
- Verma, S.; Sing, A.; Kalra, A.; Saxena, M.J. Effect of feeding hydroponics barley (Hordeum vulgare) fodder on nutrient utilization, growth, blood metabolites and cost effectiveness in Hariana male calves. Indian J. Anim. Nutr. 2015, 32, 10–14. [Google Scholar]
- Chethan, K.P.; Gowda, N.K.S.; Prabhu, T.M.; Krishnamoorthy, P.; Dey, D.K.; Giridhar, K.; Anandan, S. Nutritional evaluation of hydroponic maize (Zea mays) grain sprouts as a newer green feed resource in lambs. Indian J. Anim. Res. 2022, 56, 434–443. [Google Scholar] [CrossRef]
- Farghaly, M.M.; Abdullah, M.A.M.; Youssef, I.M.I.; Abdel-Rahim, I.R.; Abouelezz, K. Effect of feeding hydroponic barley sprouts to sheep on feed intake, nutrient digestibility, nitrogen retention, rumen fermentation and ruminal enzymes activity. Livest. Sci. 2019, 228, 31–37. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Li, J.G. Modern Dairy Production; China Agricultural University Press: Beijing, China, 2007. [Google Scholar]
- Li, Y.; Wang, Y.Q.; Lv, J.Y.; Dou, X.J.; Zhang, Y.G. Effects of dietary supplementation with Clostridium butyricum on the amelioration of growth performance, rumen fermentation, and rumen microbiota of Holstein heifers. Front. Nutr. 2021, 8, 921. [Google Scholar] [CrossRef] [PubMed]
- Van Keulen, J.; Young, B.A. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Wang, Z.S.; Cao, G.; Hu, R.; Wang, X.Y.; Zou, H.W.; Kang, K.; Peng, Q.H.; Xue, B.; et al. Active dry yeast supplementation improves the growth performance, rumen fermentation, and immune response of weaned beef calves. Anim. Nutr. 2021, 7, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Valadares, R.F.; Broderick, G.A.; Valadares Filho, S.C.; Clayton, M.K. Effect of replacing alfalfa silage with high moisture core on ruminal protein synthesis estimated from excretion of total purine derivatives. J. Dairy Sci. 1999, 82, 2686. [Google Scholar] [CrossRef]
- Shen, J.S.; Chai, Z.; Song, L.J.; Liu, J.X.; Wu, Y.M. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 2012, 95, 5978–5984. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Sys. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Fazaeli, H.; Golmohammadi, H.A.; Shoayee, A.A.; Montajebi, N.; Mosharraf, S. Performance of feedlot calves fed hydroponics fodder barley. J. Agric. Sci. Tech. 2011, 13, 367–375. [Google Scholar]
- Reddy, G.V.N.; Reddy, M.R.; Reddy, K.K. Nutrient utilization by milch cattle fed on rations containing artificially grown fodder. Indian J. Anim. Nutr. 1988, 5, 19–22. [Google Scholar]
- Devendar, R.; Kumari, N.N.; Reddy, Y.R.; Rao, K.S.; Reddy, K.K.; Raju, J.; Sridhar, K. Growth performance, nutrient utilization and carcass characteristics of sheep fed hydroponic barley fodder. Anim. Nutr. Feed Technol. 2020, 20, 321–331. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Kim, T.I.; Mayakrishnan, V.; Lim, D.H.; Lee, H.J.; Son, J.K.; Kim, Y.J.; Choi, H.C.; Shin, J.H.; Park, J.H.; Kim, S.C.; et al. Evaluation of feed value of barley fodder as an alternative feed ingredient. J. Korean Soc. Grassl. Forage Sci. 2020, 40, 161–166. [Google Scholar] [CrossRef]
- Bickhart, D.M.; Weimer, P.J. Symposium review: Host-rumen microbe interactions may be leveraged to improve the productivity of dairy cows. J. Dairy Sci. 2018, 101, 7680–7689. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Mizrahi, I. Composition and similarity of bovine rumen microbiota across individual animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef] [Green Version]
- Huo, W.J.; Zhu, W.Y.; Mao, S.Y. Impact of subacute ruminal acidosis on the diversity of liquid and solid-associated bacteria in the rumen of goats. World J. Microbiol. Biotechnol. 2014, 30, 669–680. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Wang, Z.S.; Hu, R.; Wang, X.Y.; Li, F.P.; Zhang, X.F.; Zou, H.W.; Peng, Q.H.; Xue, B.; Wang, L.Z. Comparative study of the bacterial communities throughout the gastrointestinal tract in two beef cattle breeds. Appl. Microbiol. Biot. 2021, 105, 313–325. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Y.B.; Du, Y.H.; Chen, M.H.; Guo, L.Q.; Huang, X.W.; Li, T.T.; Chen, S.; Yang, F.; Yu, F.B.; et al. Effects of Konjaku Flour on the gut microbiota of obese patients. Front. Cell Infect. Microbiol. 2022, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.F.; Lv, L.X.; Liu, B.Q.; Wang, S.T.; Zhang, S.T.; Wu, Z.J.; Yang, L.Y.; Bian, X.Y.; Wang, Q.Q.; Wang, K.C.; et al. Akkermansia muciniphila ameliorates acetaminophen-induced liver injury by regulating gut microbial composition and metabolism. Microbiol. Spectr. 2022, 10, e0159621. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.R.; Zhou, J.; Xiong, X.; Zou, L.J.; Kong, X.F.; Tan, B.; Yin, Y.L. Difference in gut microbial and serum biochemical indices between sows with different productive capacities during perinatal period. Front. Microbiol. 2020, 10, 3047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uryu, H.; Tsukahara, T.; Ishikawa, H.; Oi, M.; Otake, S.; Yamane, I.; Inoue, R. Comparison of productivity and fecal microbiotas of sows in commercial farms. Microorganisms 2020, 8, 1469. [Google Scholar] [CrossRef]
- Wu, J.J.; Xu, Y.B.; Su, J.; Zhu, B.; Wang, S.Q.; Liu, K.H.; Wang, H.J.; Shi, S.S.; Zhang, Q.Y.; Qin, L.P.; et al. Roles of gut microbiota and metabolites in a homogalacturonan-type pectic polysaccharide from Ficus pumila Linn. fruits mediated amelioration of obesity. Carbohyd. Polym. 2020, 248, 116780. [Google Scholar] [CrossRef]

| Items | Treatment | ||
|---|---|---|---|
| CON | 25% HBS | 50% HBS | |
| Ingredients, % DM | |||
| Corn | 2.59 | 2.59 | 2.59 |
| Soybean meal | 12.58 | 12.58 | 12.58 |
| Wheat | 2.96 | 2.96 | 2.96 |
| DDGS 1 | 11.1 | 11.1 | 11.1 |
| Wheat bran | 5.55 | 5.55 | 5.55 |
| Corn silage | 20 | 20 | 20 |
| Wheat stalk | 27 | 27 | 27 |
| Hydroponic barley seedlings | 0 | 4 | 8 |
| Oat hay | 16 | 12 | 8 |
| Calcium bicarbonate | 0.37 | 0.37 | 0.37 |
| Salt | 0.37 | 0.37 | 0.37 |
| Limestone powder | 0.74 | 0.74 | 0.74 |
| 2% Premix 2 | 0.74 | 0.74 | 0.74 |
| Total | 100 | 100 | 100 |
| Nutrient composition | |||
| DM, % | 47.27 | 45.95 | 45.06 |
| CP, %DM | 13.51 | 13.87 | 14.02 |
| EE, %DM | 2.13 | 2.25 | 2.41 |
| NDF, %DM | 45.99 | 43.97 | 43.48 |
| ADF, %DM | 27.26 | 25.30 | 25.13 |
| Ash, %DM | 13.09 | 13.40 | 14.11 |
| NEL 3, MJ/kg DM | 3.98 | 4.10 | 4.08 |
| Items | Treatment | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | 25% HBS | 50% HBS | Treat | Week | Treat × Week | ||
| Dry matter intake, kg/d | 7.99 a | 7.90 ab | 7.74 b | 0.06 | 0.04 | <0.01 | <0.01 |
| Body height | 120.43 | 121.72 | 121.26 | 0.50 | 0.17 | <0.01 | 0.36 |
| Body oblique length | 129.93 | 131.80 | 132.96 | 0.91 | 0.12 | <0.01 | 0.09 |
| Chest circumference | 155.49 | 157.22 | 156.72 | 0.50 | 0.05 | <0.01 | 0.73 |
| Body weight | 333.12 a | 335.14 a | 326.88 b | 1.70 | <0.01 | <0.01 | 0.22 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | 25% HBS | 50% HBS | |||
| CP | 62.81 a | 60.26 a | 54.84 b | 0.77 | <0.01 |
| EE | 75.20 | 72.19 | 71.41 | 0.94 | 0.22 |
| NDF | 51.95 a | 44.22 b | 36.84 c | 1.34 | <0.01 |
| ADF | 49.74 a | 40.62 b | 32.58 c | 1.43 | <0.01 |
| OM | 63.64 a | 60.60 a | 56.19 b | 0.78 | <0.01 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | 25% HBS | 50% HBS | |||
| N intake, g/d | 172.80 | 175.36 | 173.75 | 0.78 | 0.42 |
| Urine volume, L/d | 15.34 | 15.66 | 18.16 | 0.57 | 0.09 |
| N output, g/d | |||||
| Feces | 63.72 c | 68.99 b | 78.15 a | 1.35 | <0.01 |
| Urine | 60.42 | 59.29 | 58.65 | 1.39 | 0.88 |
| Retention 1 | 48.66 a | 47.08 a | 36.94 b | 1.73 | 0.01 |
| N output, % of N intake | |||||
| Feces | 37.17 b | 39.78b | 45.16 a | 0.77 | <0.01 |
| Urine | 34.84 | 33.64 | 33.74 | 0.81 | 0.81 |
| Retention | 27.99 a | 26.58 a | 21.10 b | 1.01 | 0.01 |
| Items | Treatment | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | 25% HBS | 50% HBS | |||
| Lachnospiraceae_NK3A20_group | 0.86 b | 1.09 ab | 1.58 a | 0.12 | 0.02 |
| Lachnospiraceae_XPB1014_group | 0.73 b | 1.09 a | 1.25 a | 0.08 | 0.01 |
| norank_f_Bacteroidales_RF16_group | 1.24 a | 0.79 b | 0.75 b | 0.09 | 0.03 |
| unclassified_f_Lachnospiraceae | 0.83 b | 0.81 b | 1.12 a | 0.06 | 0.04 |
| SP3-e08 | 0.40 a | 0.71 a | 0.22 b | 0.06 | <0.01 |
| unclassified_f_Rikenellaceae | 0.38 a | 0.31 a | 0.14 b | 0.04 | <0.01 |
| norank_f_norank_o_WCHB1-41 | 0.35 a | 0.24 ab | 0.17 b | 0.03 | 0.03 |
| Sphaerochaeta | 0.24 a | 0.20 ab | 0.09 b | 0.02 | <0.01 |
| Ruminiclostridium | 0.14 a | 0.13 a | 0.08 b | 0.01 | <0.01 |
| Lachnospiraceae_FCS020_group | 0.05 ab | 0.04 b | 0.08 a | 0.01 | 0.04 |
| unclassified_o_Oscillospirales | 0.06 a | 0.04 ab | 0.03 b | <0.01 | 0.03 |
| Colidextribacter | 0.01 b | 0.04 a | 0.08 a | 0.01 | 0.02 |
| Bacillus | 0.02 b | 0.04 b | 0.06 a | 0.01 | <0.01 |
| norank_f_norank_o_LD1-PB3 | 0.01 b | 0.04 a | 0.02 ab | 0.01 | 0.03 |
| Agathobacter | 0.01 b | 0.02 ab | 0.02 a | <0.01 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, P.; Deng, M.; Feng, J.; Li, R.; Ma, X.; Liu, J.; Wang, D. Partial Replacement of Oat Hay with Whole-Plant Hydroponic Barley Seedlings Modulates Ruminal Microbiota and Affects Growth Performance of Holstein Heifers. Microorganisms 2022, 10, 2000. https://doi.org/10.3390/microorganisms10102000
Ren P, Deng M, Feng J, Li R, Ma X, Liu J, Wang D. Partial Replacement of Oat Hay with Whole-Plant Hydroponic Barley Seedlings Modulates Ruminal Microbiota and Affects Growth Performance of Holstein Heifers. Microorganisms. 2022; 10(10):2000. https://doi.org/10.3390/microorganisms10102000
Chicago/Turabian StyleRen, Peng, Mengmeng Deng, Juan Feng, Ruocheng Li, Xiaojiao Ma, Jianxin Liu, and Diming Wang. 2022. "Partial Replacement of Oat Hay with Whole-Plant Hydroponic Barley Seedlings Modulates Ruminal Microbiota and Affects Growth Performance of Holstein Heifers" Microorganisms 10, no. 10: 2000. https://doi.org/10.3390/microorganisms10102000





