Effect of Sodium Nitrate and Cysteamine on In Vitro Ruminal Fermentation, Amino Acid Metabolism and Microbiota in Buffalo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Treatments
2.2. In Vitro Batch Culture
2.3. Determination of Total Gas, Methane (CH4) Production and Hydrogen Balance
2.4. Determination of Rumen Fermentation Parameters
2.5. Determination of Amino Acid Concentration
2.6. DNA Extraction and Determination of Microbial Population
2.7. 16 S rDNA Gene Sequencing and Bioinformatic Analysis
2.8. Statistical Analysis
3. Results
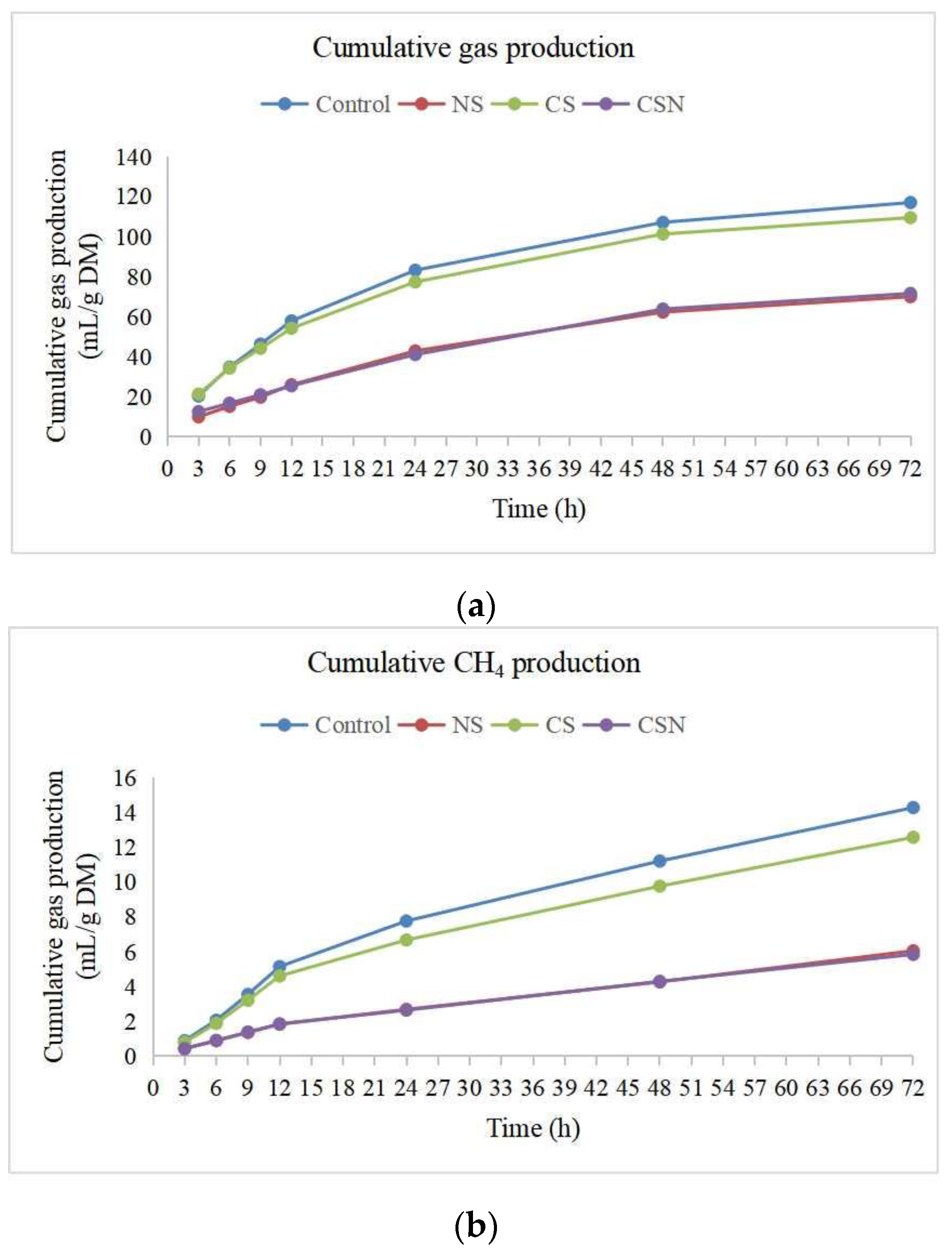
3.1. Total Gas Production, CH4 Production and Hydrogen Balance
3.2. Rumen Fermentation Parameters
3.3. Ruminal Amino Acids
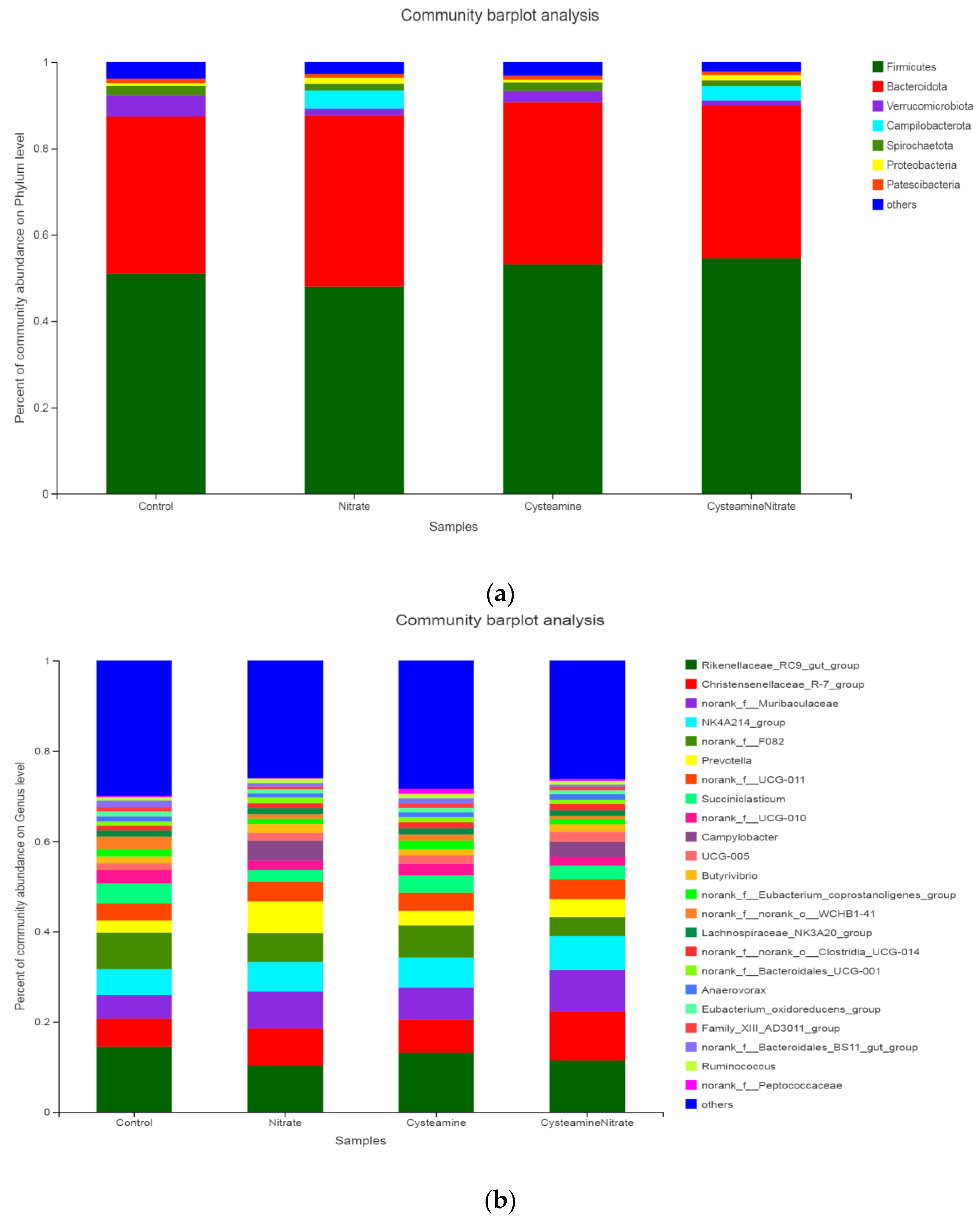
3.4. Rumen Microbial Populations
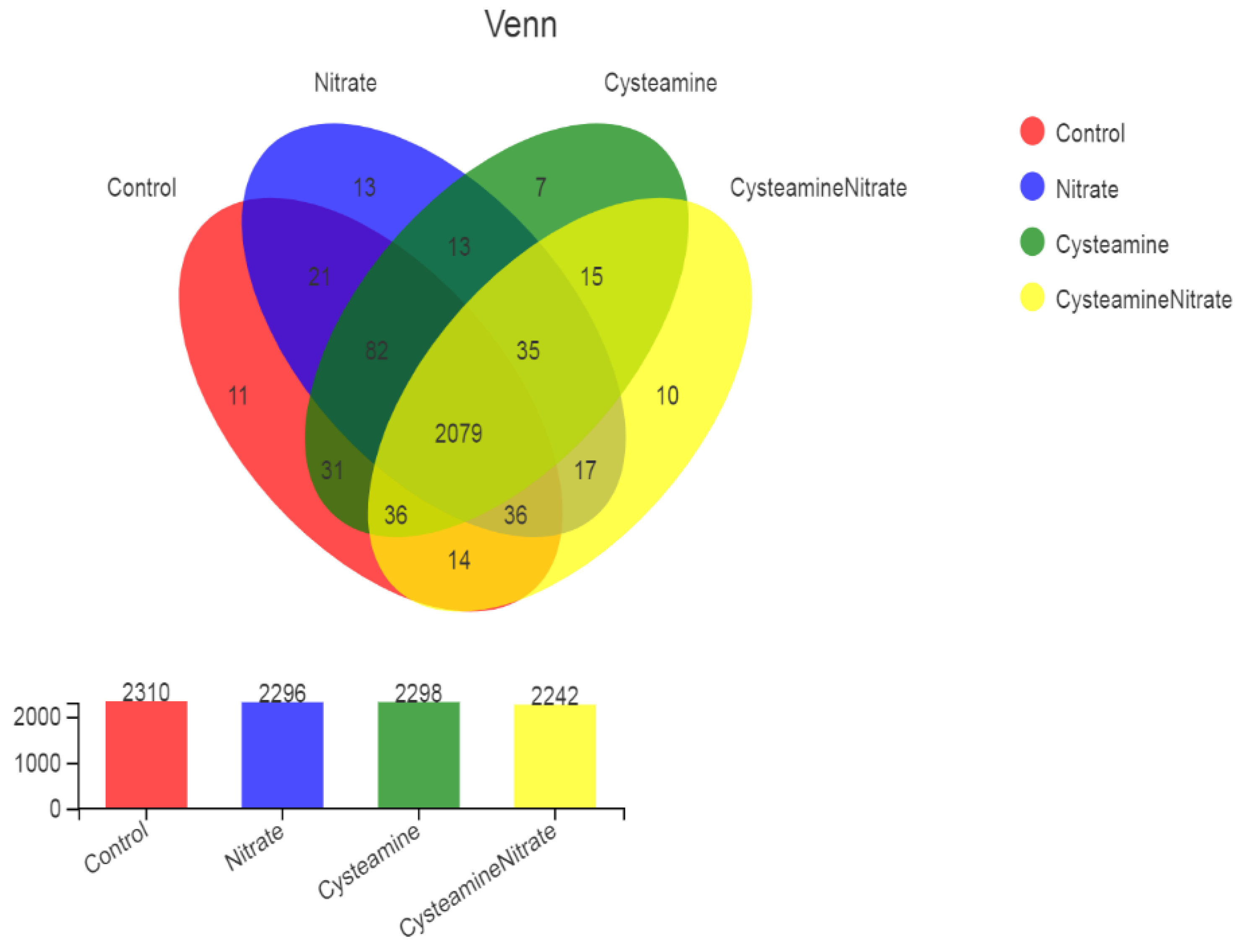
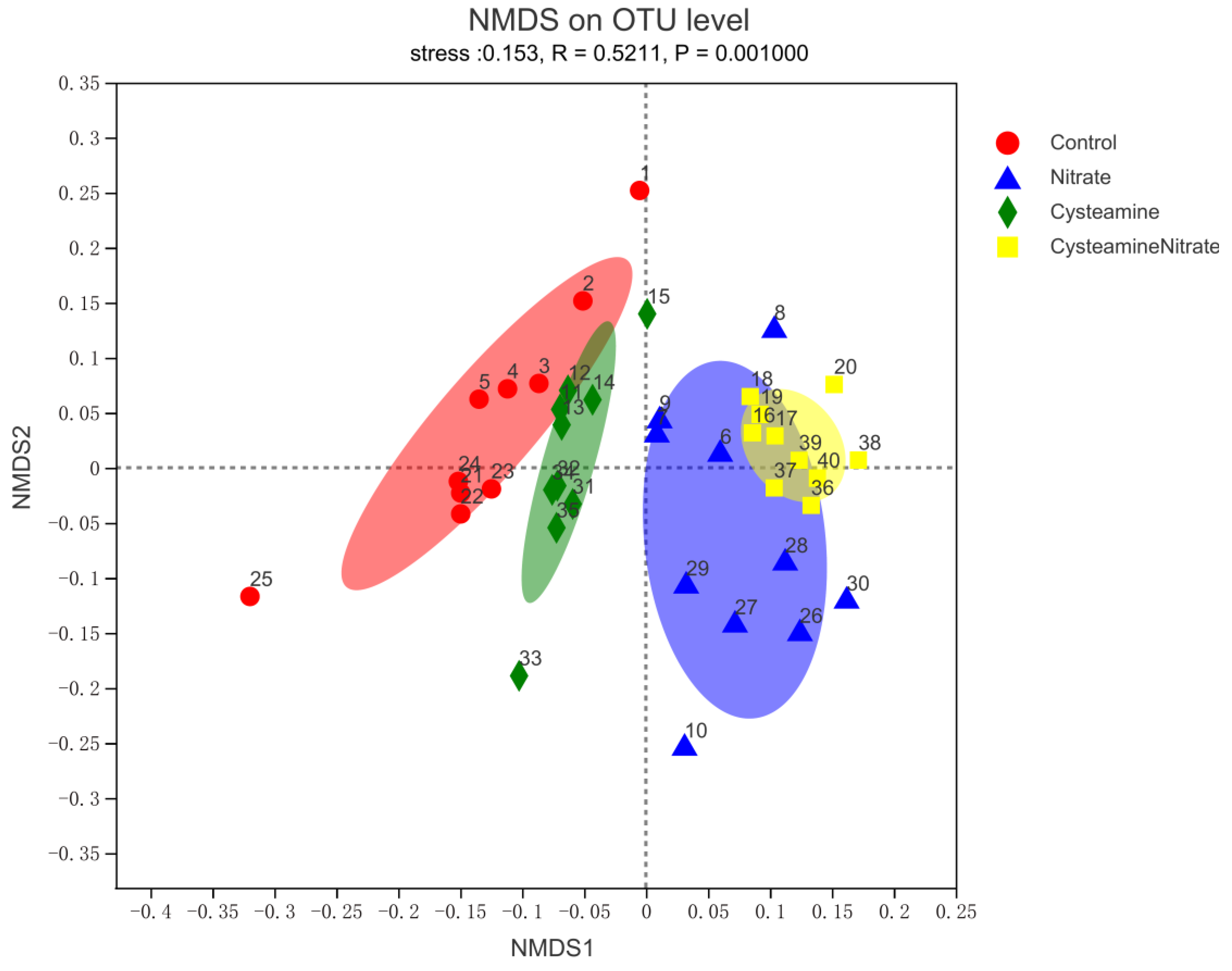
3.5. Rumen Bacterial Diversity
3.5.1. Alpha and Beta Diversity Analysis
3.5.2. Relative Abundance of Bacterial Populations
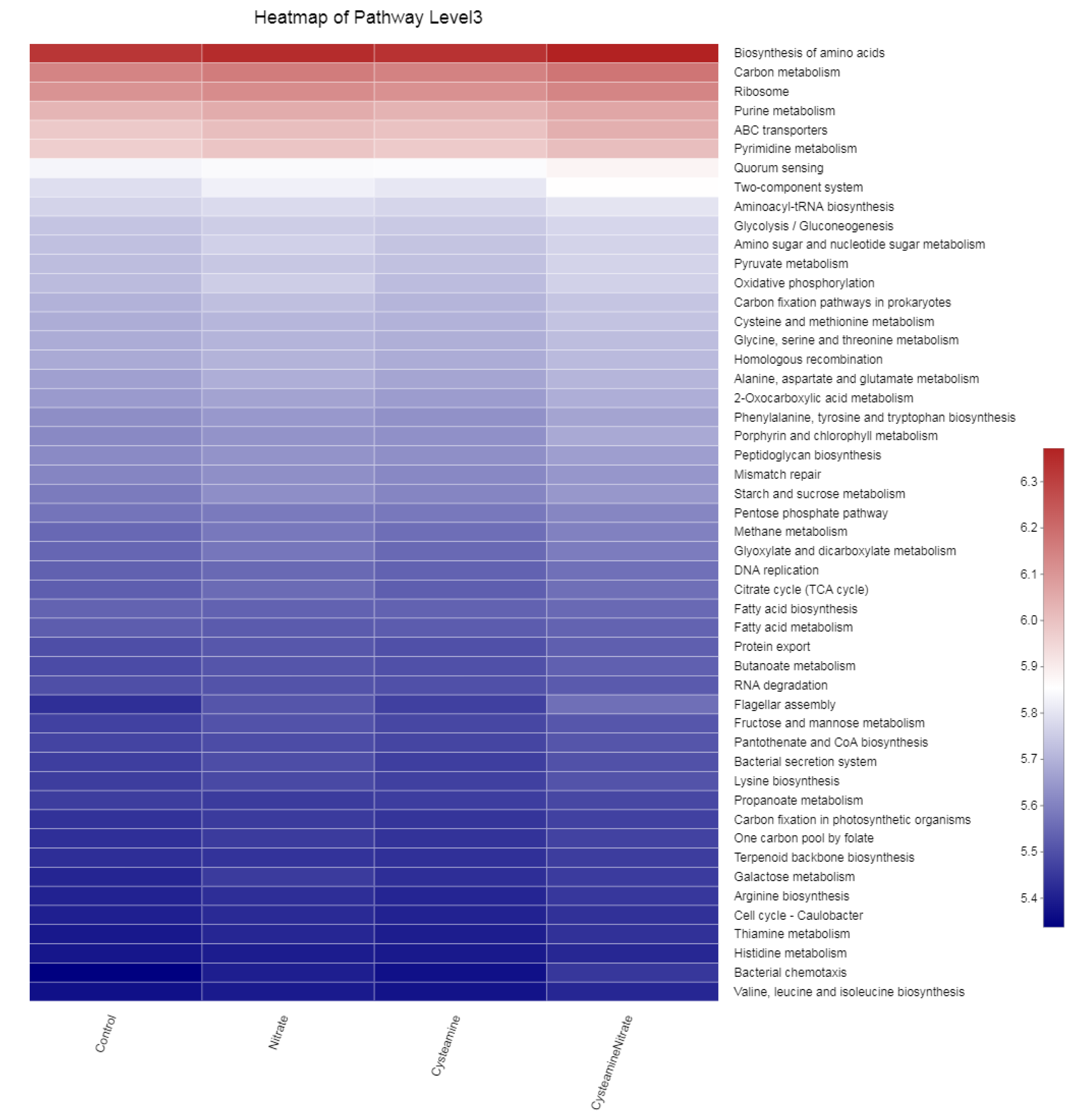
3.5.3. Biomarker Bacteria Taxa and Metagenomic Functional Profile
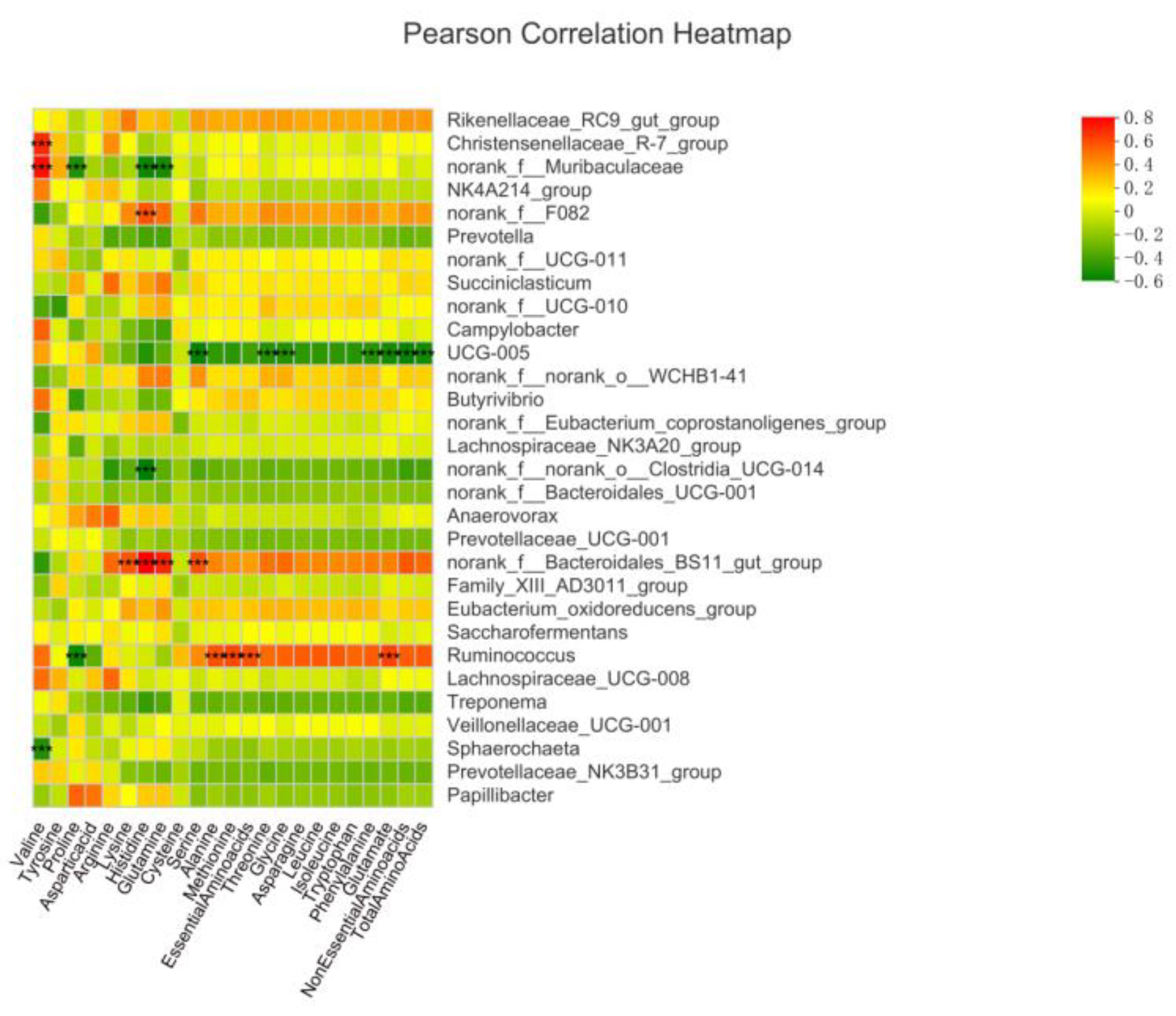
3.5.4. Association of Rumen Bacteria with Ruminal Gas, Fermentation Parameters and Amino Acid Contents
4. Discussion
4.1. Total Gas Production, CH4 Production and Hydrogen Balance
4.2. Rumen Fermentation Parameters
4.3. Ruminal Amino Acids
4.4. Rumen Microbial Populations
4.5. Rumen Bacterial Diversity
4.6. Biomarker Bacteria Taxa and Metagenomic Functional Profile
4.7. Association of Rumen Bacteria with Ruminal Gas, Fermentation Parameters and Amino Acid Contents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Appuhamy, J.; France, J.; Kebreab, E. Models for predicting enteric methane emissions from dairy cow s in North America, Europe, and Australia and New Zealand. Glob. Chang. Biol. 2016, 22, 3039–3056. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Xu, J.; Cong, Y. Reducing rumen methane emission through regulating rumen microorganisms by adding hydrogen-consuming compounds. Chin. J. Anim. Sci. 2019, 31, 4967–4972. [Google Scholar]
- Hulshof, R.B.; Berndt, A.; Gerrits, W.J.; Dijkstra, J.; van Zijderveld, S.M.; Newbold, J.R.; Perdok, H.B. Dietary nitrary supplementation reduces methane emission in beef cattle fed sugarcane-based dieds. J. Anim. Sci. 2012, 90, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Huyen, T.; Do, H.Q.; Preston, T.R.; Leng, R.A. Nitrate as fermentable nitrogen supplement to reduce rumen methane production. Livest. Res. Rural Develop. 2010, 22, 146. [Google Scholar]
- Li, L.; Davis, J.; Nolan, J.; Hegarty, R. An initial investigation on rumen fermentation pattern and methane emission of sheep offered diets containing urea or nitrate as the nitrogen source. Anim. Prod. Sci. 2012, 52, 653–658. [Google Scholar] [CrossRef]
- Schwab, C.G.; Broderick, G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017, 100, 10094–10112. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, M.; Tang, Z.; Liang, X.; Peng, L.; Peng, K.; Wang, X. Effects of Cysteamine on lactation performance, antioxidant performance and rumen microbial diversity of Buffalo in summer. Chin. Anim. Husband. Vet. Med. 2021, 48, 901–915. [Google Scholar]
- Huang, Y.; Zou, C.; Wei, S.; Laing, X.; Li, S.; Lu, T.; Yang, B.; Laing, X. Effects of cysteamine on ruminal fermentation parameters and methane production of water buffalo by in vitro gas production method. Chin. J. Anim. Sci. 2014, 26, 125–133. [Google Scholar]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 177–222. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Li, M.; Tang, Z.; Peng, L.; Peng, K.; Laing, X.; Xie, F.; Yang, C. Effects of disodium fumarate on fermentation parameters, fatty acid composition and the number of key rumen microorganisms of buffalo in vitro. Chin. Anim. Husband. Vet. Med. 2021, 53, 24–31. [Google Scholar]
- Demeyer, D.I. Quantitative aspects of microbial metabolism in the rumen and hindgut. In Rumen Microbial Metabolism and Ruminant Digestion; Jouany, J.P., Ed.; INRA Editions: Versailles, France, 1991; pp. 217–237. [Google Scholar]
- Weatherburn, M. Phenol-hypochlorite reaction for determination of ammonia. Analyt. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Joyce, R.; Kuziene, V.; Zou, X.; Wang, X.; Pullen, F.; Loo, R.L. Development and validation of an ultra-performance liquid chromatography quadrupole time of flight mass spectrometry method for rapid quantification of free amino acids in human urine. Amino Acids. 2016, 48, 219–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, H.M.; Mengwei, L.; Kholif, A.E.; Hassan, F.U.; Lijuan, P.; Xin, L.; Chengjian, Y. Moringa oleifera oil modulates rumen microflora to mediate in vitro fermentation kinetics and methanogenesis in total mix rations. Curr. Microbiol. 2020, 77, 1271–1282. [Google Scholar] [CrossRef]
- Maeda, H.; Fujimoto, C.; Haruki, Y.; Maeda, T.; Kokeguchi, S.; Petelin, M.; Arai, H.; Tanimoto, I.; Nishimura, F.; Takashiba, S. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol. Med. Microbiol. 2003, 39, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Denman, S.E.; Martinez, F.G.; Shinkai, T.; Mitsumori, M.; McSweeney, C.S. Metagenomic analysis of the rumen microbial community following inhibition of methane formation by a halogenated methane analog. Front. Microbiol. 2015, 6, 1087. [Google Scholar] [CrossRef] [Green Version]
- El-Zaiat, H.; Araujo, R.; Soltan, Y.; Morsy, A.; Louvandini, H.; Pires, A.; Patino, H.O.; Correa, P.S.; Abdalla, A. Encapsulated nitrate and cashew nut shell liquid on blood and rumen constituents, methane emission, and growth performance of lambs. J. Anim. Sci. 2014, 92, 2214–2224. [Google Scholar] [CrossRef]
- Lin, M.; Schaefer, D.; Zhao, G.; Meng, Q. Effects of nitrate adaptation by rumen inocula donors and substrate fiber proportion on in vitro nitrate disappearance, methanogenesis, and rumen fermentation acid. Animal 2013, 7, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Xu, X.; Cao, Y.; Cai, C.; Cui, H.; Yao, Y. Nitrate decreases methane production also by increasing methane oxidation through stimulating NC10 population in ruminal culture. Amb. Express 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pal, K.; Patra, A.; Sahoo, A.; Mandal, G. Effect of nitrate and fumarate in Prosopis cineraria and Ailanthus excelsa leaves-based diets on methane production and rumen fermentation. Small Rumin. Res. 2014, 121, 168–174. [Google Scholar] [CrossRef]
- Shi, C.; Meng, Q.; Hou, X.; Ren, L.; Zhou, Z. Response of ruminal fermentation, methane production and dry matter digestibility to microbial source and nitrate addition level in an in vitro incubation with rumen microbes obtained from wethers. J. Anim. Vet. Adv. 2012, 11, 3334–3341. [Google Scholar]
- Zhou, Z.; Yu, Z.; Meng, Q. Effects of nitrate on methane production, fermentation, and microbial populations in in vitro ruminal cultures. Biores. Technol. 2012, 103, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Granja-Salcedo, Y.T.; Fernandes, R.M.; Araujo, R.C.D.; Kishi, L.T.; Berchielli, T.T.; Resende, F.D.D.; Berndt, A.; Siqueir, G.R. Long-term encapsulated nitrate supplementation modulates rumen microbial diversity and rumen fermentation to reduce methane emission in grazing steers. Front. Microbiol. 2019, 10, 614. [Google Scholar] [CrossRef]
- Russell, J.; Wallace, R. Energy-yielding and energy-consuming reactions. In The Rumen Microbial Ecosystem; Springer: Berlin/Heidelberg, Germany, 1997; pp. 246–282. [Google Scholar]
- Zhou, Z.; Meng, Q.; Yu, Z. Effects of methanogenic inhibitors on methane production and abundances of methanogens and cellulolytic bacteria in in vitro ruminal cultures. Appl. Environ. Microbiol. 2011, 77, 2634–2639. [Google Scholar] [CrossRef] [Green Version]
- Hassan, F.U.; Guo, Y.; Li, M.; Tang, Z.; Peng, L.; Liang, X.; Yang, C. Effect of methionine supplementation on rumen microbiota, fermentation, and amino acid metabolism in in vitro cultures containing nitrate. Microorga 2021, 9, 1717. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Peng, L.; Peng, K.; Tang, Z.; Liang, X.; Xie, F.; Yang, C. Effects of Sodium Nitrate on Methane Production and Fatty Acid Hydrogenation Process of Buffalo in vitro Fermentation. Chin. Anim. Husband. Vet. Med. 2020, 47, 2071–2080. [Google Scholar]
- Patra, A.K.; Yu, Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of rumen microbial populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.K.; Yan, X.G.; Ban, Z.B.; Yang, H.M.; Zhao, Y.M. Effect of nitrate on the methane production and productivity of cattle. J. Chin. Agri. Univ. 2017, 22, 54–60. [Google Scholar]
- Guyader, J.; Ungerfeld, E.M.; Beauchemin, K.A. Redirection of metabolic hydrogen by inhibiting methanogenesis in the rumen simulation technique (RUSITEC). Front. Microbiol. 2017, 8, 393. [Google Scholar] [CrossRef] [Green Version]
- Lund, P.; Dahl, R.; Yang, H.; Hellwing, A.; Cao, B.; Weisbjerg, M. The acute effect of addition of nitrate on in vitro and in vivo methane emission in dairy cows. Anim. Prod. Sci. 2014, 54, 1432–1435. [Google Scholar] [CrossRef]
- Lin, M.; Troy, S.; Duthie, C.; Hyslop, J.; Roehe, R.; Ross, D.; Wallace, R.; Waterhouse, A.; Rooke, J. Effectiveness of nitrate addition and increased oil content as methane mitigation strategies for beef cattle fed two contrasting basal diets. J. Anim. Sci. 2015, 93, 1815–1823. [Google Scholar]
- Ungerfeld, E.M.; Kohn, R.A. The role of thermodynamics in the control of ruminal fermentation. In Ruminant Physiology; Sejrsen, K., Hvelplund, T., Nielsen, M.O., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 55–85. [Google Scholar]
- Van Zijderveld, S.; Gerrits, W.; Apajalahti, J.; Newbold, J.; Dijkstra, J.; Leng, R.; Perdok, H. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J. Dairy Sci. 2010, 93, 5856–5866. [Google Scholar] [CrossRef] [Green Version]
- Silivong, P.; Preston, T.; Van Man, N. Effect of supplements of potassium nitrate or urea as sources of NPN on methane production in an in vitro system using molasses and Paper mulberry or Muntingia foliages as the substrate. Livest. Res. Rural Develop. 2012, 24, 12–14. [Google Scholar]
- Sophea, I.; Preston, T. Effect of different levels of supplementary potassium nitrate replacing urea on growth rates and methane production in goats fed rice straw, mimosa foliage and water spinach. Livest. Res. Rural Develop. 2011, 23, 71. [Google Scholar]
- Thanh, V.D.; Thu, N.; Preston, T. Effect of potassium nitrate or urea as NPN source and levels of Mangosteen peel on in vitro gas and methane production using molasses, Operculina turpethum and Brachiaria mutica as substrate. Livest. Res. Rural Dev. 2012, 24, 63. [Google Scholar]
- Asanuma, N.; Iwamoto, M.; Kawato, M. Numbers of nitrate-reducing bacteria in the rumen as estimated by competitive polymerase chain reaction. Anim. Sci. J. 2002, 73, 199–205. [Google Scholar] [CrossRef]
- Xu, J.; Yu, C.; Sun, Y. Effects of cysteamine on rumen and cecum environment of Liaoning cashmere goats. China Feed. 2010, 7, 26–28. [Google Scholar]
- Sun, G.; Zhang, C.; Teng, L. Effects of dietary cysteamine and yeast culture on ruminal microbial protein production and nutrient digestibility of dairy cows. Chin. J. Anim. Sci. 2017, 53, 79–83. [Google Scholar]
- Leng, R.A. The Potential of Feeding Nitrate to Reduce Enteric Methane Production in Ruminants; Report to Department of Climate Change; Commonwealth Government: Canberra, Australia, 2008; p. 82. [Google Scholar]
- Zhao, L.P. Effects of Nitrate on Rumen Fermentation, Microbe Diversity, Blood Biochemical and Antioxidant Ability. Master’s Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- Barnett, M.; Hegarty, R.S. Cysteamine: A human health dietary additive with potential to improve livestock growth rate and efficiency. Anim. Prod. Sci. 2016, 56, 1330–1338. [Google Scholar] [CrossRef]
- Fan, Z.X. Effects of Sodium Nitrate and Methionine on Lactation Performance, Dietary Digestibility, Rumen Fermentation, Bacterial Diversity and Key Microbial Numbers of Buffalo. Master’s Thesis, Shihezi University, Shihezi, Xinjiang, China, 2021. [Google Scholar]
- Ungerfeld, E.M.; Aedo, M.F.; Martínez, E.D.; Saldivia, M. Inhibiting methanogenesis in rumen batch cultures did not increase the recovery of metabolic hydrogen in microbial amino acids. Microorgan 2019, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.Z.; Wang, Y.; Liu, G.; Zhou, A.G. Effects of dietary supplementation with cysteamine on performance, carcass characteristics, meat quality and antioxidant status in finishing pigs. J. Agr. Sci. Technol.-Iran. 2011, 5, 735–740. [Google Scholar]
- Guo, Y.; Li, M.; Tang, Z.; Peng, L.; Peng, K.; Xie, F.; Xie, H.; Yang, C. Effects of different dosages of sodium nitrate on fatty acid composition and microbial population of buffalo rumen fermentation in vitro under the condition of linoleic acid. Acta Pratacul. Sinica. 2021, 30, 159–167. [Google Scholar]
- Guo, Y.; Hassan, F.; Li, M.; Tang, Z.; Peng, L.; Peng, K.; Yang, C. Effect of hydrogen-consuming compounds on in vitro ruminal fermentation, fatty acids profile, and microbial community in water buffalo. Curr. Microbiol. 2022, 79, 220. [Google Scholar] [CrossRef] [PubMed]
- Leng, R.A. Interactions between microbial consortia in biofilms: A paradigm shift in rumen microbial ecology and enteric methane mitigation. Anim. Prod. Sci. 2014, 54, 519–543. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q. Effects of cysteamine on in vitro fermentation by rumen microbes from goats. J. Huazhong Agr. 2002, 6, 535–539. [Google Scholar]
- Sun, Y.K.; Ban, A.B.; Yang, H.M.; Hegarty, R.S.; Zhao, Y.M. The effect of cysteamine hydrochloride and nitrate supplementation on in-vitro and in-vivo methane production and productivity of cattle. Anim. Feed Sci. Technol. 2017, 232, 49–56. [Google Scholar] [CrossRef]
- Min-Oo, G.; Ayi, K.; Bongfen, S.E.; Tam, M.; Radovanovic, I.; Gauthier, S.; Santiago, H.; Rothfuchs, A.G.; Roffê, E.; Sher, A.; et al. Cysteamine, the natural metabolite of pantetheinase, shows specific activity against Plasmodium. Exp. Parasitol. 2010, 125, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Schaefer, D.; Guo, X.; Ren, L.; Meng, Q. Use of nitrate-nitrogen as a sole dietary nitrogen source to inhibit ruminal methanogenesis and to improve microbial nitrogen synthesis in vitro. Asian-Austr. J. Anim. Sci. 2009, 22, 542–549. [Google Scholar] [CrossRef]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tracttreponemaisolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Ren, D.; Liu, J. Diversity of rumen microflora in dairy buffaloes at different ages. Chin. J. Anim. Nutr. 2019, 31, 5053–5064. [Google Scholar]
- Zhang, H.; Li, M.; Tang, Z.; Liang, X.; Peng, L.; Peng, K.; Wang, X.; Yang, C. Effects of supplemental feeding of cysteamine and chromium nicotinate on lactation performance, antioxidant performance, rumen fermentation parameters and microbial diversity of buffalo in summer. Chin. J. Anim. Nutr. 2020, 32, 5760–5777. [Google Scholar]
- Marais, J.P.; Therion, J.J.; Mackie, R.I.; Kistner, A.; Dennison, C. Effect of nitrate and its reduction products on the growth and activity of the rumen microbial population. Br. J. Nutr. 1988, 59, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Q.; Jia, J.L.; Chen, Q. Effects of dietary protein level on rumen morphology, microbial community structure and function of weaned lambs. Chin. J. Anim. Nutr. 2020, 32, 1–10. [Google Scholar]
- Zeng, Y.; Gao, Y.; Peng, Z. Effects of yeast culture adding in diet on rumen fermentation parameters and microflora of house-feeding yak. Chin. J. Anim. Nutr. 2020, 32, 1721–1733. [Google Scholar]
- Miller, W.G.; On, S.L.; Wang, G.; Fontanoz, S.; Lastovica, A.J.; Mandrell, R.E. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J. Clinic. Microbiol. 2005, 43, 2315–2329. [Google Scholar] [CrossRef] [Green Version]
- Derakhshani, H.; Tun, H.M.; Cardoso, F.C.; Plaizier, J.C.; Khafipour., E.; Loor, J.J. Linking peripartal dynamics of ruminal microbiota to dietary changes and production parameters. Front. Microbiol. 2016, 7, 2143. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Fitzsimmons, C.; Plastow, G.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wright, A.D.; Liu, H.; Fan, Z.; Yang, F.; Zhang, Z.; Li, G. Response of the rumen microbiota of sika deer (cervus nippon) fed different concentrations of tannin rich plants. PLoS ONE 2015, 10, e0123481. [Google Scholar] [CrossRef] [Green Version]
- Gylswyk, N. Succiniclasticum ruminis gen. nov. sp. nov. a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 1995, 45, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Palevich, N.; Kelly, W.J.; Leahy, S.C.; Denman, S.; Altermann, E.; Rakonjac, J.; Attwood, G.T. Comparative genomics of rumen Butyrivibrio uncovers a continuum of polysaccharide-degrading capabilities. Appl. Environ. Microbiol. 2019, 86, e01993-19. [Google Scholar] [CrossRef]
- Paillard, D.; McKain, N.; Chaudhary, L.C.; Walker, N.D.; Pizette, F.; Koppova, I.; McEwan, N.R.; Kopecný, J.; Vercoe, P.E.; Louis, P.; et al. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio-like bacteria from the rumen. Antonie. Van. Leeuwenhoek. 2007, 91, 417–422. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Efimov, B.A.; Kondova, I.; Ouwerling, B.; Chaplin, A.V.; Shcherbakova, V.A.; Langermans, J. Peptococcus simiae sp nov. isolated from rhesus macaque faeces and emended description of the genus Peptococcus. Int. J. Syst. Evol. Microbiol. 2016, 66, 5187–5191. [Google Scholar] [CrossRef]
- Clarissa, S.; Hans-Joachim, R.; Vera, B.; Pham, V.T.; Beerenwinkel, N.; Lacroix, C. Trophic Interactions of Infant Bifidobacteria and Eubacterium hallii during L-Fucose and Fucosyllactose Degradation. Front. Microbiol. 2017, 8, 95. [Google Scholar]
- Yu, J.K. Study of Rumen Microbial Composition and Functional Profiles Using Metagenomics and the Regulation of Rumen Microbial Fermentation with Exogenous Additives. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Yang, L. Study on the Differences of Intestinal Microorganisms of Kele Pigs and Hybrid Pigs and Their Effects on Rough Feeding. Master’s Thesis, Guizhou university, Guizhou, China, 2021. [Google Scholar]
- Martínez-Álvaro, M.; Auffret, M.D.; Stewart, R.D.; Dewhurst, R.J.; Duthie, C.-A.; Rooke, J.A.; Wallace, R.J.; Shih, B.; Freeman, T.C.; Watson, M. Identification of complex rumen microbiome interaction within diverse functional niches as mechanisms affecting the variation of methane emissions in bovine. Front. Microbiol. 2020, 11, 659. [Google Scholar] [CrossRef]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.G.; Baba, Y.; Asano, R.; Fukuda, Y.; Tada, C.; Nakai, Y. Identification of bacteria involved in the decomposition of lignocellulosic biomass treated with cow rumen fluid by metagenomic analysis. J. Biosci. Bioeng. 2020, 130, 137–141. [Google Scholar] [CrossRef]


| Ingredient | Content |
|---|---|
| Elephant grass (%) | 90.0 |
| Concentrate Mixture (%) * | 10.0 |
| Chemical composition | |
| Dry matter (%) | 20.0 |
| Crude protein (%) | 9.07 |
| Neutral detergent fiber (%) | 75.3 |
| Acid detergent fiber (%) | 46.0 |
| Ash (%) | 9.72 |
| Gross Energy (kcal/kg DM) | 4.69 |
| Items | Control | SN | CS | CSN | SEM | p Value |
|---|---|---|---|---|---|---|
| Total gas production (mL/gDM) | 117 a | 69.9 b | 120 a | 71.5 b | 1.85 | 0.001 |
| Methane production (CH4, mL/gDM) | 14.3 a | 6.05 b | 13.8 a | 5.84 b | 0.23 | 0.001 |
| Reductive hydrogen | ||||||
| H2 Produced (mmol) | 9.89 a | 8.46 b | 9.98 a | 8.49 b | 0.11 | 0.001 |
| H2 Utilized (mmol) | 8.10 a | 5.51 b | 8.06 a | 5.38 b | 0.08 | 0.001 |
| H2 Recovery (%) | 81.9 a | 65.1 b | 80.8 a | 63.3 b | 0.29 | 0.002 |
| Items | Control | SN | CS | CSN | SEM | p Value |
|---|---|---|---|---|---|---|
| pH | 6.83 b | 6.90 a | 6.81 b | 6.89 a | 0.01 | 0.001 |
| Acetate (mmol/L) | 38.3 b | 42.0 a | 39.2 b | 42.9 a | 0.46 | 0.001 |
| Propionate (mmol/L) | 21.4 a | 19.1 b | 21.1 a | 18.6 b | 0.24 | 0.001 |
| Butyrate (mmol/L) | 13.4 a | 6.94 b | 13.45 a | 6.75 b | 0.13 | 0.001 |
| Isobutyrate (mmol/L) | 2.05 a | 1.60 b | 1.99 a | 1.53 b | 0.03 | 0.001 |
| Valerate (mmol/L) | 2.13 a | 1.61 b | 2.16 a | 1.62 b | 0.03 | 0.001 |
| Isovalerate (mmol/L) | 4.47 a | 3.54 b | 4.36 a | 3.43 b | 0.06 | 0.001 |
| Total volatile fatty acid (TVFA, mmol/L) | 81.7 a | 74.8 b | 82.3 a | 74.9 b | 0.99 | 0.001 |
| Acetate to propionate ratio (A/P) | 1.79 d | 2.20 b | 1.86 c | 2.31 a | 0.01 | 0.001 |
| Ammonia nitrogen (NH3-N, mg/100 mL) | 18.7 | 18.1 | 18.8 | 18.5 | 0.19 | 0.109 |
| Microbial protein (MCP, mg/mL) | 4.01 c | 4.82 b | 4.06 c | 5.65 a | 0.15 | 0.001 |
| Dry matter digestibility (DMD, %) | 43.0 | 39.8 | 42.0 | 37.0 | 1.92 | 0.173 |
| Items | Control | SN | CS | CSN | SEM | p Value |
|---|---|---|---|---|---|---|
| Alanine | 131 c | 486 a | 321 b | 149 c | 38.3 | 0.001 |
| Valine | 236 d | 384 b | 286 c | 460 a | 23.2 | 0.001 |
| Histidine | 1119 a | 759 b | 639 c | 378 d | 69.3 | 0.001 |
| Arginine | 289 a | 197 b | 126 c | 223 b | 16.2 | 0.001 |
| Glycine | 102 c | 226 a | 161 b | 98.2 c | 14.2 | 0.001 |
| Glutamine | 57.4 a | 16.7 b | 18.9 b | 10.1 b | 4.84 | 0.001 |
| Glutamate | 1690 c | 3851 a | 3160 b | 1849 c | 233 | 0.001 |
| Proline | 95.7 a | 29.4 c | 44.6 bc | 49.2 b | 6.55 | 0.001 |
| Leucine | 45.7 c | 214 a | 145 b | 60.2 c | 18.2 | 0.001 |
| Lysine | 40.9 a | 28.4 ab | 13.4 b | 11.6 b | 3.83 | 0.006 |
| Methionine | 22.5 c | 148 a | 101 b | 44.2 c | 13.0 | 0.001 |
| Tryptophan | 26.9 c | 105 a | 60.6 b | 25.4 c | 8.56 | 0.001 |
| Phenylalanine | 68.3 c | 314 a | 195 b | 69.5 c | 27.0 | 0.001 |
| Threonine | 125 c | 281 a | 205 b | 107 c | 19.1 | 0.001 |
| Isoleucine | 40.7 c | 209 a | 140 b | 55.2 c | 18.2 | 0.001 |
| Tyrosine | 35.0 | 36.1 | 36.2 | 40.9 | 1.51 | 0.519 |
| Serine | 291 b | 436 a | 295 b | 204 c | 24.2 | 0.001 |
| Asparagine | 44.0 c | 204 a | 141 b | 59.7 c | 7.05 | 0.001 |
| Aspartic acid | 20.9 a | 11.8 b | 8.91 b | 12.5 b | 1.44 | 0.001 |
| Cysteine | 4.00 | 4.38 | 4.96 | 5.40 | 0.47 | 0.757 |
| Essential Amino acids 1 | 607 c | 1685 a | 1146 b | 833 c | 108. | 0.001 |
| Non-Essential Amino acids 2 | 3880 c | 6258 a | 4955 b | 3079 d | 318 | 0.001 |
| Total Amino Acids | 4487 c | 7943 a | 6101 b | 3912 c | 418 | 0.001 |
| Items | Control | SN | CS | CSN | SEM | p Value |
|---|---|---|---|---|---|---|
| Bacteria | 11.9 c | 12.1 ab | 12.0 bc | 12.3 a | 0.03 | 0.001 |
| Fungi | 9.97 b | 10.2 ab | 10.1 b | 10.4 a | 0.04 | 0.009 |
| Protozoa | 8.13 | 8.55 | 8.54 | 8.52 | 0.08 | 0.168 |
| Methanogens | 10.0 b | 10.1 b | 10.1 b | 10.3 a | 0.03 | 0.001 |
| Items | Control | SN | CS | CSN | SEM | p Value |
|---|---|---|---|---|---|---|
| Shannon index | 6.07 a | 5.85 b | 6.01 a | 5.73 b | 0.03 | 0.001 |
| Shimposon index (×10−2) | 0.70 b | 1.05 a | 0.77 b | 1.11 a | 0.05 | 0.001 |
| Ace index (×103) | 1.90 a | 1.90 a | 1.90 a | 1.82 b | 0.01 | 0.001 |
| Chao index (×103) | 1.92 a | 1.93 a | 1.93 a | 1.84 b | 0.01 | 0.031 |
| Coverage (%) | 98.8 | 98.7 | 98.7 | 98.8 | 0.02 | 0.941 |
| Taxonomic Level | Microbes | Control | SN | CS | CSN | SEM | p Value |
|---|---|---|---|---|---|---|---|
| Phylum | Firmicutes | 50.9 a | 46.8 b | 52.5 a | 54.3 a | 4.02 | 0.001 |
| Bacteroidota | 36.2 | 40.0 | 37.8 | 35.8 | 3.73 | 0.379 | |
| Verrucomicrobiota | 5.13 a | 2.16 b | 2.72 b | 1.03 c | 1.34 | 0.001 | |
| Campilobacterota | 0.02 b | 4.33 a | 0.01 b | 3.18 a | 0.96 | 0.001 | |
| Spirochaetota | 2.02 a | 1.59 b | 2.24 a | 1.49 b | 0.64 | 0.021 | |
| Proteobacteria | 0.69 b | 1.34 a | 0.70 b | 1.21 a | 0.35 | 0.001 | |
| Patescibacteria | 1.01 a | 0.90 ab | 0.86 ab | 0.76 b | 0.21 | 0.031 | |
| Genus | Rikenellaceae_RC9_gut_group | 13.4 a | 11.3 b | 12.6 a | 11.2 b | 1.95 | 0.005 |
| Christensenellaceae_R-7_group | 6.94 c | 7.61 b | 7.18 c | 10.57 a | 1.64 | 0.001 | |
| norank_f__Muribaculaceae | 4.75 c | 7.71 b | 7.18 b | 9.04 a | 1.56 | 0.001 | |
| NK4A214_group | 6.21 bc | 5.94 c | 6.61 b | 7.44 a | 1.04 | 0.005 | |
| norank_f__F082 | 7.54 a | 6.07 ab | 6.51 ab | 4.15 b | 0.85 | 0.001 | |
| Prevotella | 2.62 d | 6.63 a | 3.31 c | 4.40 b | 2.10 | 0.019 | |
| norank_f__UCG-011 | 4.04 | 4.16 | 4.41 | 4.34 | 1.07 | 0.540 | |
| Succiniclasticum | 4.65 a | 3.00 b | 3.16 b | 3.40 b | 1.92 | 0.027 | |
| norank_f__UCG-010 | 2.84 a | 1.98 b | 2.75 a | 1.93 b | 0.38 | 0.001 | |
| Campylobacter | 0.01 b | 4.32 a | 0.01 b | 3.15 a | 0.95 | 0.001 | |
| UCG-005 | 1.68 b | 1.63 b | 1.75 b | 2.12 a | 0.37 | 0.031 | |
| norank_f__norank_o__WCHB1-41 | 3.21 a | 1.46 b | 1.40 b | 0.61 c | 1.13 | 0.001 | |
| Butyrivibrio | 1.24 b | 1.99 a | 1.45 ab | 1.73 a | 0.33 | 0.001 | |
| norank_f__Eubacterium_coprostanoligenes_group | 1.68 a | 1.08 b | 1.89 a | 1.21 b | 0.36 | 0.001 | |
| Lachnospiraceae_NK3A20_group | 1.23 ab | 1.37 ab | 1.56 a | 1.20 b | 0.31 | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Hassan, F.-u.; Li, M.; Xie, H.; Peng, L.; Tang, Z.; Yang, C. Effect of Sodium Nitrate and Cysteamine on In Vitro Ruminal Fermentation, Amino Acid Metabolism and Microbiota in Buffalo. Microorganisms 2022, 10, 2038. https://doi.org/10.3390/microorganisms10102038
Guo Y, Hassan F-u, Li M, Xie H, Peng L, Tang Z, Yang C. Effect of Sodium Nitrate and Cysteamine on In Vitro Ruminal Fermentation, Amino Acid Metabolism and Microbiota in Buffalo. Microorganisms. 2022; 10(10):2038. https://doi.org/10.3390/microorganisms10102038
Chicago/Turabian StyleGuo, Yanxia, Faiz-ul Hassan, Mengwei Li, Huade Xie, Lijuan Peng, Zhenhua Tang, and Chengjian Yang. 2022. "Effect of Sodium Nitrate and Cysteamine on In Vitro Ruminal Fermentation, Amino Acid Metabolism and Microbiota in Buffalo" Microorganisms 10, no. 10: 2038. https://doi.org/10.3390/microorganisms10102038
APA StyleGuo, Y., Hassan, F.-u., Li, M., Xie, H., Peng, L., Tang, Z., & Yang, C. (2022). Effect of Sodium Nitrate and Cysteamine on In Vitro Ruminal Fermentation, Amino Acid Metabolism and Microbiota in Buffalo. Microorganisms, 10(10), 2038. https://doi.org/10.3390/microorganisms10102038







