Abstract
The tick-transmitted disease bovine babesiosis causes significant economic losses in many countries around the world. Current control methods include modified live-attenuated vaccines that have limited efficacy. Recombinant proteins could provide effective, safe, and low-cost alternative vaccines. We compared the expression of the Babesia bovis thrombospondin-related anonymous protein (TRAP) family from parasites in bovine blood, in vitro induced sexual stages, and kinetes from tick hemolymph. Quantitative PCR showed that in blood and sexual stages, TRAP3 was highly transcribed as compared to the other TRAPs. In contrast, the TRAP1 gene was highly transcribed in kinetes as compared to the other TRAPs. Fixed immunofluorescence assays showed that TRAP2, 3, and 4 proteins were expressed by both blood and sexual stages. Conversely, TRAP1 protein, undetected on blood and induced sexual stages, was the only family member expressed by kinetes. Live IFA revealed that TRAP2, 3, and 4 proteins were expressed on the surface of both B. bovis blood and sexual stages. Modeling of B. bovis TRAP1 and TRAP4 tertiary structure demonstrated both proteins folded the metal-ion-dependent adhesion site (MIDAS) domain structure of Plasmodium TRAP. In conclusion, TRAP proteins may serve as potential vaccine targets to prevent infection of bovine and ticks with B. bovis essential for controlling the spread of bovine babesiosis.
1. Introduction
Bovine babesiosis is a disease caused by intraerythrocytic parasites, including Babesia bovis, B. bigemina, and B. divergens [1,2,3]. This disease is common in tropical and subtropical climates, causing significant economic losses in many countries [4,5]. Infected bovines show signs of fever, anemia, and, in severe cases, death [3]. Climate change poses a significant threat to the control of bovine babesiosis as it allows the spread of the tick vector into non-endemic areas and evasion of current control strategies [6]. Therefore, there is a need for vaccines that effectively control this disease. Current methods for the control of bovine babesiosis include drugs, acaricide treatment, and live-attenuated vaccines [7,8,9]. Each method comes with its own challenges. Drugs are expensive, unable to prevent severe disease, and may leave chemical residues in milk and meat [3]. The utilization of acaricide treatments can lead to resistant tick populations and contamination of the environment [10]. Live vaccines are expensive, difficult to make, and may have a limited shelf life [11]. Live vaccines present further challenges such as the potential for contamination with other pathogenic organisms and the loss of immunogenicity [12]. It is imperative to develop a subunit vaccine that is less expensive, safer, with a longer shelf life, and more protective than the current live vaccines [13].
The life cycle of Babesia is complex and consists of important stages for parasite development within the mammalian host and tick vectors. Within the tick midgut lumen, sexual stages of the parasite are formed. Babesia bovis sexual stages express HAP2 protein, which plays an important role in fertilization and formation of the zygote [14]. A previous study demonstrated that HAP2 was expressed on the surface of Plasmodium microgametes and was important for the fusion of parasite sexual stages prior to infecting insect gut epithelial cells [15]. Zygotes infect tick midgut epithelial cells and transform into kinetes [16]. The kinetes mature within midgut cells before being released into tick hemolymph, gaining access to, and invading the ovaries. The parasites are transovarially transferred to the next generation of ticks [17]. To date, there are only a few reports regarding protein expression by different stages of Babesia parasites that cause bovine babesiosis [16,18]. These studies identified differentially expressed proteins that may allow the parasite to infect tick ovaries and invade eggs. Within tick larvae, the parasite forms a dense spherical body inside salivary gland cells [17]. When infected larvae feed on a bovine, the spherical bodies divide into sporozoites [19]. Sporozoites are inoculated via tick saliva into the mammalian host and directly infect erythrocytes, transforming into trophozoites and merozoites that lyse the erythrocyte, causing hemolytic anemia, and infecting other erythrocytes resulting in the persistent infection of the mammalian host [17,19]. Understanding protein expression by B. bovis blood and tick stages is critically important for the development of new control strategies.
A previous study suggested that the B. bovis thrombospondin-related anonymous protein (TRAP) family were potential antigen candidates for vaccine development [18]. These proteins are conserved among all Apicomplexan parasites [20,21]. During gliding movement, these proteins are involved in the moving junction, a structure composed of a few rhoptry proteins that form complexes with host surface proteins and parasite surface-exposed integral membrane microneme proteins [22]. The purpose of the vWFA and TSP-1 domains is to form cell-matrix interactions that assist in erythrocyte invasion [20,23,24]. Proteolytic cleavage of TRAP facilitates junction movement, releasing the parasite into the parasitophorous vacuole [22,23]. This vacuole is an invagination of the erythrocytic membrane that engulfs the parasite, allowing it entry to the cell [3]. Once the parasite is inside the cell, the parasitophorous vacuole degrades. Further investigation into the function and expression of TRAP genes is necessary to determine if these proteins are appropriate candidates for vaccine development. In this study, we evaluated the expression of the TRAP family, TRAP1 (BBOV_II002650), TRAP2 (BBOV_II002890), TRAP3 (BBOV_II002630) and TRAP4 (BBOV_II002870), by B. bovis parasite stages during its development within mammalian and tick hosts. We quantified transcript levels expressed by B. bovis blood stages, in vitro induced sexual stages, and kinetes derived from tick hemolymph. We determined protein expression and surface location using fixed and live immunofluorescence assays (IFAs), respectively. We also modeled the tertiary structure of TRAP1 and TRAP4 to characterize structural and functional attributes. Herein, we present the repertoire of TRAP expression by distinct life stages of B. bovis and discuss the importance of TRAP proteins for the parasite’s life cycle and their potential for vaccine development to block parasite transmission by the tick vector and prevent infection of the mammalian host.
2. Materials and Methods
2.1. Cattle, Pathogen, and Tick Vector
Three four-month-old splenectomized calves Babesia-free as determined as previously described [18] were used for the experiment. A B. bovis Texas strain, S74-T3Bo, and Rhipicephalus microplus ticks, La Minita strain, were also used. Briefly, R. microplus larvae were fed under a cloth patch on each calf. When nymphs started to molt to the adult stage, ~107 B. bovis-infected erythrocytes were intravenously inoculated into the calves to synchronize tick feeding with the peak parasitemia [17,18]. Clinical signs of babesiosis were monitored daily, including fever and anemia. Giemsa-stained blood smears were used to determine the presence of parasites in calf blood.
The experiment conducted in this study was in accordance with the institutional guidelines based on the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Guide for the Care and Use of Agricultural Animals in Research and Training. This study was approved by the University of Idaho’s Institutional Animal Care and Use Protocol Committee, Moscow, Idaho, (IACUC #2018-16).
2.2. Babesia bovis Blood Stages
Blood from B. bovis-infected calves during acute parasitemia was collected into flasks containing glass beads and shaken. Babesia bovis-infected erythrocytes were grown in culture medium as previously described [18]. The cultures were incubated at 37 °C with 5% CO2 to allow optimum growth conditions. Infected B. bovis blood smears were generated to detect the expression of TRAP proteins by fixed IFA. Exoerythrocytic B. bovis merozoites were collected by differential centrifugation. Erythrocytes were pelleted at 400× g for 10 min. The supernatant was collected and centrifuged as before. Free merozoites remaining in the supernatant were pelleted from the final differential supernatant at 3000× g for 10 min. Exoerythrocytic parasites were used to determine surface-exposed proteins by a cell surface membrane staining assay of intact parasites. Moreover, B. bovis-infected cultures were centrifugated and the supernatant removed. TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) was added to the infected erythrocyte pellet and stored at −80 °C.
2.3. Babesia bovis Induced Sexual Stages
Induction of B. bovis sexual stages was performed as previously described [14,25]. Microaerophilous stationary phase B. bovis cultures [26] at a parasitemia of 10% were suspended in induction medium for sexual stage development and incubated at 26 °C in air for up to 20 h or at 37 °C for 20 h as previously reported [14]. Induced sexual stages were collected to detect the expression of TRAP proteins by fixed and live IFAs. Additionally, induced sexual stages were collected, centrifuged, the pellet suspended in TRIzol, and stored at −80 °C for assessing TRAP transcription.
2.4. Babesia bovis Kinete Stage Isolation
Isolation of kinetes from tick hemolymph was performed as previously described [16]. Engorged female ticks were collected and incubated at 26 °C and 92% relative humidity to allow kinete accumulation in hemolymph as previously described [27]. Collected kinetes were stored in TRIzol at −80 °C. Kinetes were also collected in Hank’s Balanced Salt Solution to generate smears to detect the expression of TRAP proteins by fixed IFA.
2.5. RNA Isolation
Total RNA was extracted from TRIzol samples and treated with Turbo DNA-Free (Thermo Fisher Scientific) per the manufacture’s protocols. cDNA was synthesized from 100 ng of each total RNA using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific). The number of individual TRAP transcripts expressed was determined using Babesia stage-specific cDNA.
2.6. Quantitative PCR
Triplicate PCR reactions were performed as previously described [18]. Quantitative PCR primers are presented in Table 1. The gene-family expression profile of TRAP was calculated by dividing the mean of each TRAP gene per mean of total TRAP transcripts and multiplied by 100. The expression profile compares gene expression by a single Babesia stage within a gene family without the need to normalize parasite numbers [28], thereby allowing assessment of differential expression by individual gene-family members between stages.

Table 1.
Quantitative PCR primers used to determine the number of transcripts expressed by B. bovis blood stages, in vitro induced sexual stages, and kinetes.
2.7. Fixed Immunofluorescence Assay
To detect stage-specific TRAP protein expression, smears of each stage were made on positively charged slides and stored at −80 °C as previously described [29]. Polyclonal antibodies were produced by immunizing rabbits with TRAP peptides (Table 2) predicted as surface-exposed moieties (GenScript, Piscataway, NJ, USA). Slides were stained for IFA as previously described [16]. A secondary conjugate of goat-anti-rabbit IgG Alexa Fluor 555 (Thermo Fisher Scientific) was used to detect specific antibody reactivity. ProLong Gold antifade reagent with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Thermo Fisher Scientific) was used to stain nuclei. A Leica microscope (Buffalo Grove, IL, USA) was used to examine antibody reactivity. Since HAP2 is expressed by B. bovis sexual stages but not blood stages, we used rabbit anti-HAP2 antibody as controls [14] to distinguish B. bovis sexual stages from blood stages.

Table 2.
TRAP peptides used for rabbit immunization to generate specific antibodies against B. bovis TRAP proteins.
2.8. Live Immunofluorescence Assay
To examine if TRAP proteins were expressed on the surface of live and intact cells, exoerythrocytic B. bovis merozoites from cultures and induced sexual stages [14,30] were washed in 10% BSA–PBS and incubated with 4 µg/mL of individual anti-TRAP primary antibodies for 30 min. The cells were washed in 10% BSA–PBS two times at 2000× g for 2 min to pellet the parasites. Washed cells were incubated with secondary conjugate antibody as above. The cells were washed two times at 2000× g for 2 min and incubated with 10 µg/mL 5(6)-Carboxyfluorescein Diacetate (5(6)-cFDA) (Sigma-Aldrich, St. Louis, MO, USA) and Hoechst 33342, trihydrochloride, trihydrate (Invitrogen, Waltham, MA, USA) in PBS. Washed samples were independently visualized as above. Rabbit anti-HAP2 antibody [14] was used as a control to detect surface exposed protein and to distinguish B. bovis sexual stages from blood stages.
2.9. Bioinformatic Analysis of TRAP Family Members
Babesia bovis TRAP family member polypeptide sequences were submitted for tertiary structure prediction to the Distance-guided Iterative Threading ASSEmbly Refinement (D-I-TASSER) server (https://zhanggroup.org//D-I-TASSER/, accessed on 27 August 2022). The D-I-TASSER relies on multiple deep neural network predictors that generate inter-amino acid residue interactions of contact maps, distance maps and hydrogen bond networks. Five models of tertiary structure prediction for the polypeptide were returned with a ranking of models based on a comparison to randomized D-I-TASSER predictions, and confidence in the models inferred by an eTM-score that was based on the strength of agreement of multiple D-I-TASSER simulations and contact map satisfaction rate. Threading technology was also paired with the distance-guided tertiary prediction in D-I-TASSER using 10 distinct threading technologies to match to structural templates in the Protein Data Bank (PDB: https://www.rcsb.org, accessed on 27 August 2022). A normalized Z-score was associated with the PDB threading hit generated by each threading program, with a Z-score > 1 indicating meaningful agreement in structure between the prediction model and PDB known structure. Model prediction results included a pdb file that contained information to generate a molecular model with contact distances and secondary structures that build the tertiary structure. The pdb file was opened using Chimera (http://www.cgl.ucsf.edu/chimera/, accessed on 27 August 2022) to view tertiary structures, and the Chimera MatchMaker feature used to overlay pdb files from TRAP predictions with those of PDB solved structures identified by threading.
2.10. Statistical Analysis
For within B. bovis development stage analysis, a full mixed linear model was evaluated including fixed effects of gene, plate, and technical replicate with random effect of the individual sample (tick or calf) in SAS 9.4 (SAS Inst. Inc., Cary, NC, USA). Plate and technical replicates were not significant and therefore removed from the final model. Pair-wise differences between genes within each of the three life stages was determined with a Tukey–Kramer adjustment. Gene-family profiling [28] was conducted to compare gene expression across tick stages. Differences across tick stages were determined with a full mixed linear module with fixed effects of life stage and gene and random effect of the individual sample in SAS 9.4. Pair-wise differences between genes across each of the life stages were determined with a Tukey–Kramer adjustment.
3. Results
3.1. Quantitative PCR
Within an individual B. bovis stage, expression of TRAP genes was significantly different (p < 0.05) (Table 3). In blood stages, there was no pair-wise statistical difference between gene expression of TRAP1 and TRAP4. However, TRAP2 and TRAP3 were statistically different for all pair-wise comparisons. TRAP gene expression by in vitro sexual stages showed there were no differences between TRAP1 and TRAP2; TRAP4 was different from TRAP2 but not different from TRAP1. There was no significant difference between TRAP3 and TRAP4 expression; however, expression of the TRAP3 gene was statistically different for all other pair-wise comparisons. In kinetes, there was no statistical difference between TRAP2, TRAP3, and TRAP4. In contrast, expression of TRAP1 was statistically different for all pair-wise comparisons.

Table 3.
TRAP family transcript expression levels in B. bovis blood, sexual, and kinetes stages.
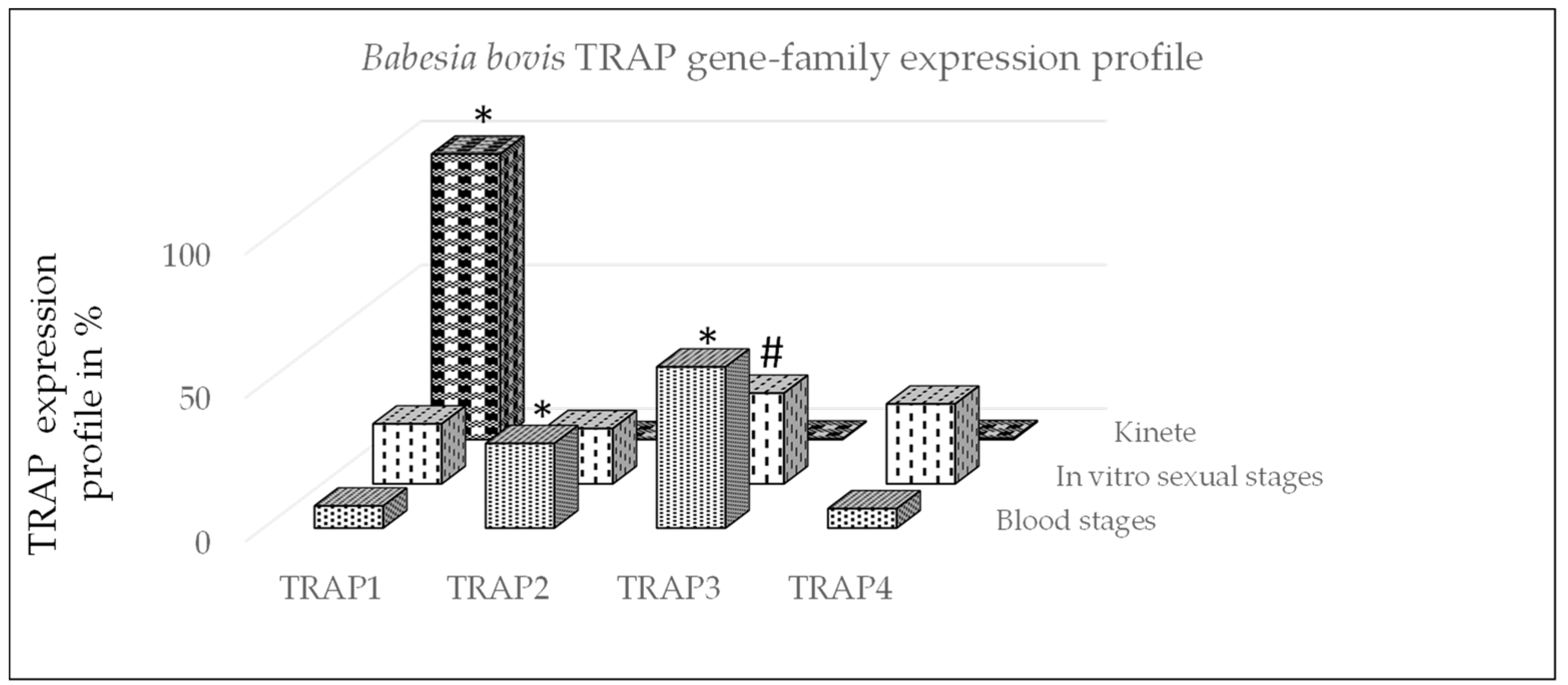
In this study, we used gene-family expression profiles to calculate differences in the expression of TRAP family genes between B. bovis stages (Figure 1). The expression profile demonstrated that TRAP1 was significantly greater expressed by kinetes as compared to blood or sexual stages (p < 0.05). The expression of TRAP2 was significantly greater by B. bovis blood stages as compared to sexual stages or kinetes (p < 0.05). The expression of TRAP3 was significantly greater by B. bovis blood stages as compared to kinetes (p < 0.05) but not sexual stages (p = 0.06). The data suggested that TRAP1 may play an important function for B. bovis kinetes, whereas TRAP2 and 3 appear to be more important for B. bovis blood and sexual stages.
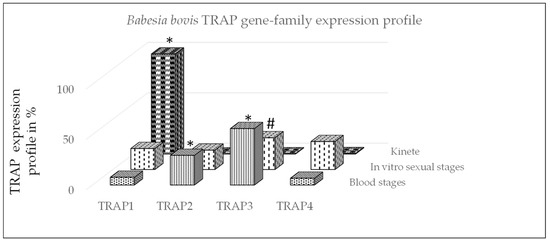
Figure 1.
Babesia bovis gene-family expression profile using qPCR. The profile of TRAP genes was calculated for each stage by dividing each TRAP gene expression value by the total of TRAP transcripts and multiplied by 100 for a percentage and then summing the percentages within life stage per gene. *: significant within gene-family differences between stages (p < 0.05) and #: non-significant difference between blood and sexual stages (p = 0.06).
3.2. Expression of TRAP Proteins by B. bovis Blood Stages, Sexual Stages, and Kinetes
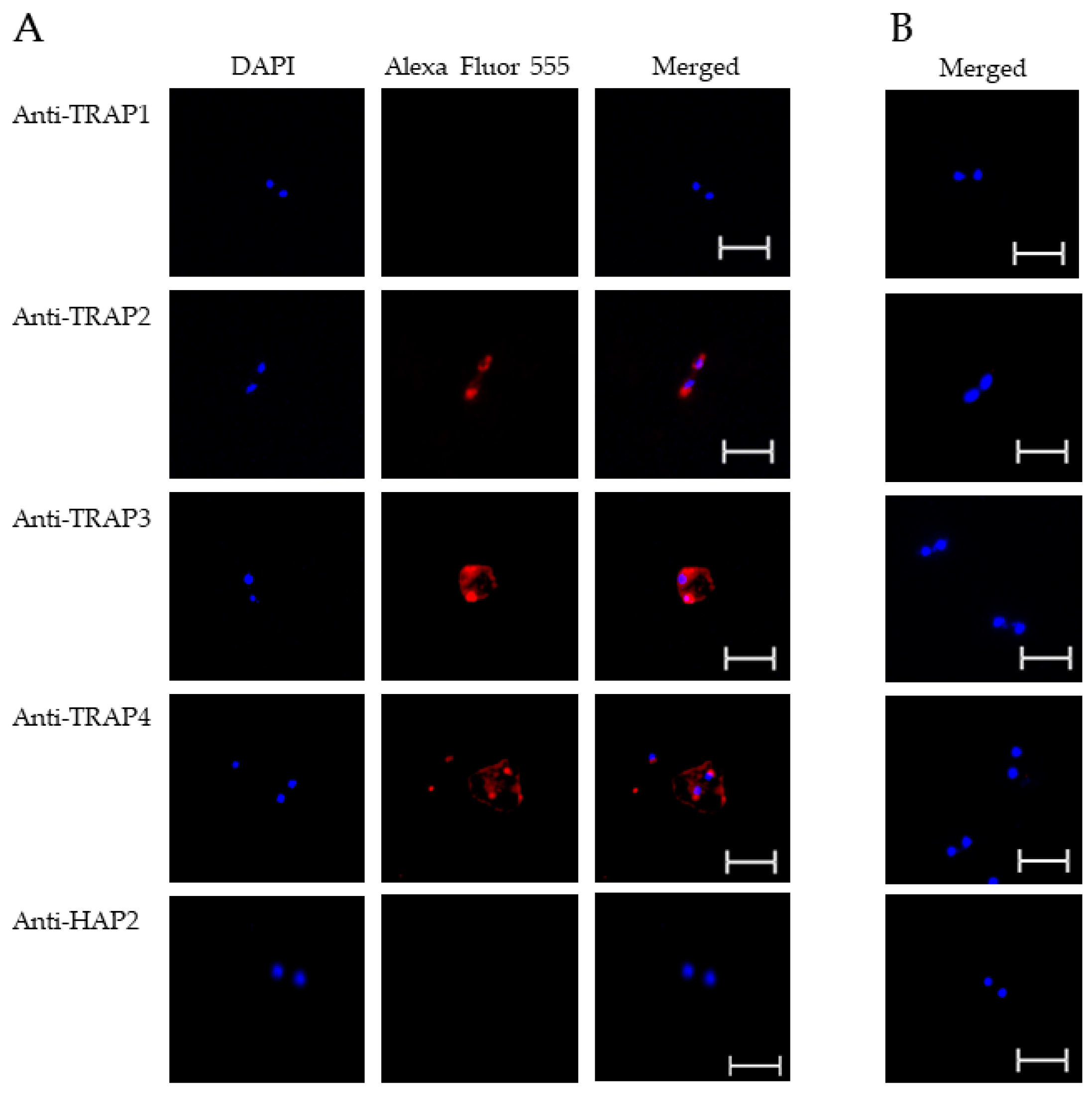
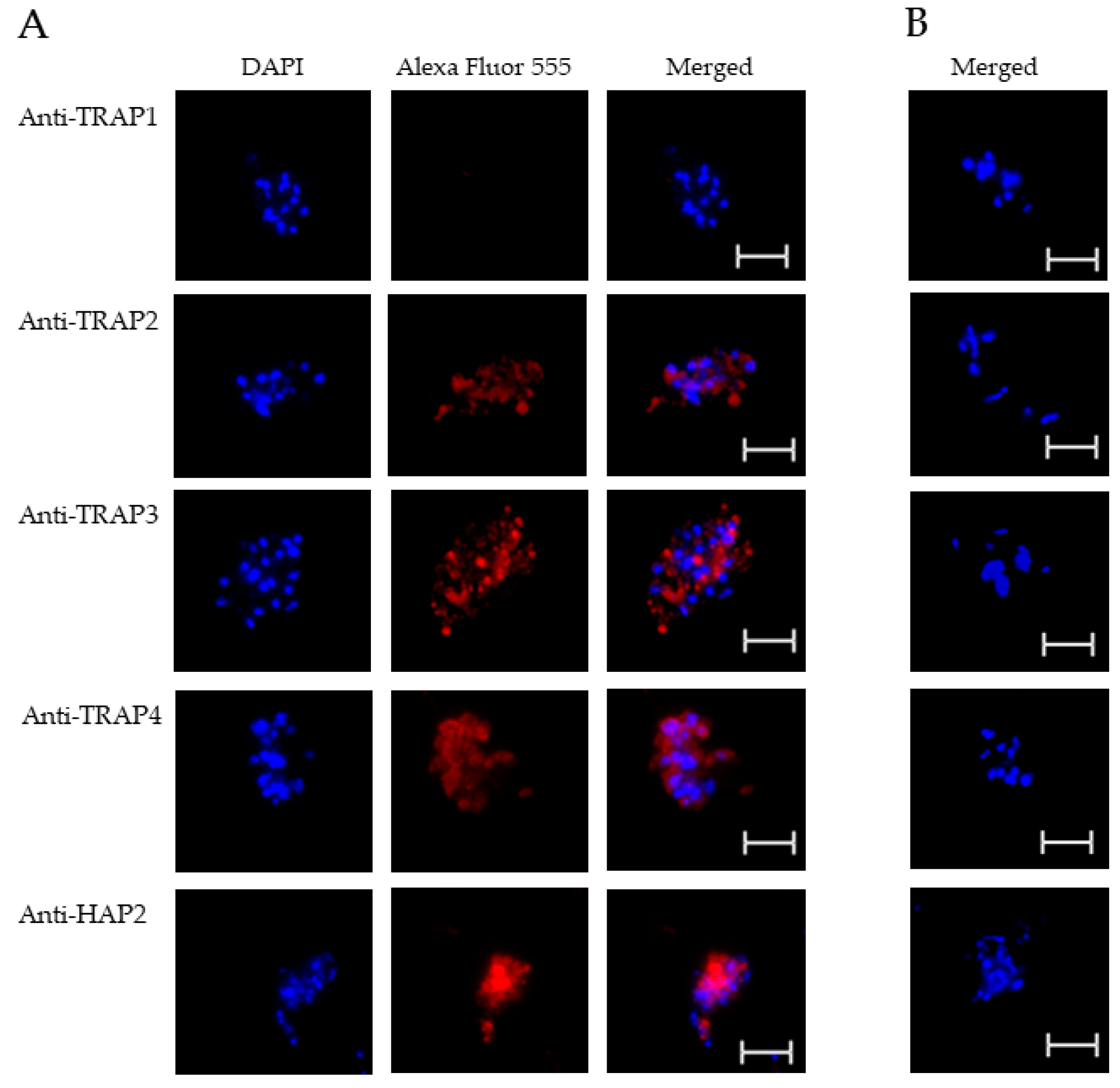
To test TRAP protein expression by B. bovis blood stages, sexual stages, or kinetes, antibodies against TRAP peptides were generated in rabbits and used in fixed or live IFA. Using fixed IFA, we demonstrated anti-TRAP2, 3, and 4 antibodies reacted with both B. bovis blood stages (Figure 2A) and in vitro induced sexual stages (Figure 3A). Matching pre-immune rabbit sera showed no antibody reactivity with B. bovis blood stages (Figure 2B) or in vitro induced sexual stages (Figure 3B).
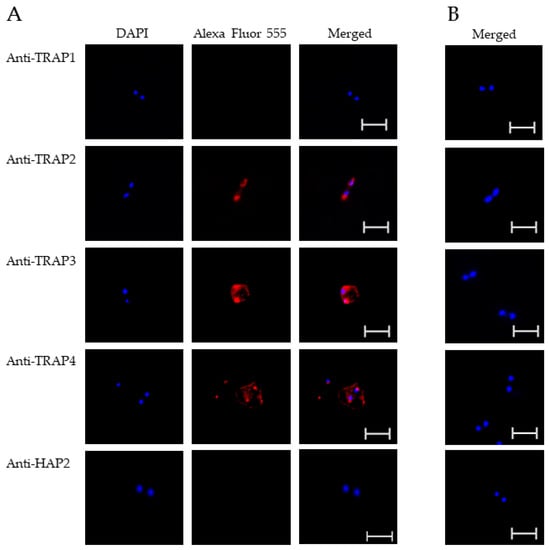
Figure 2.
Evaluation of TRAP protein expression by B. bovis blood stages using a fixed immunofluorescence assay incubated with anti-TRAP 1, 2, 3, 4 or HAP2. Panels are (A) immune and (B) pre-immune rabbit serum. Blue indicates DAPI stained DNA and red indicates anti-rabbit IgG Alexa Fluor 555 reactivity. Scale bar: 5 μm.
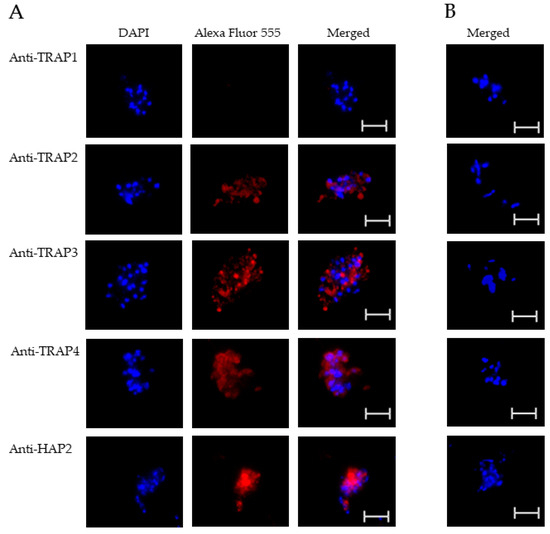
Figure 3.
Evaluation of TRAP protein expression by B. bovis sexual stages using a fixed immunofluorescence assay incubated with anti-TRAP 1, 2, 3, 4, or HAP2. Panels are (A) immune and (B) pre-immune rabbit serum. Blue indicates DAPI stained DNA and red indicates anti-rabbit IgG Alexa Fluor 555 reactivity. Scale bar: 5 μm.
The results indicated that TRAP2, 3, and 4 were expressed by B. bovis blood and sexual stages. Anti-HAP2 antibody, as expected, reacted only with B. bovis sexual stages (Figure 3A). Anti-TRAP1 antibody reactivity was undetectable using either B. bovis blood stages (Figure 2A) or in vitro induced sexual stages (Figure 3A). Matching pre-immune rabbit sera showed no antibody reactivity with B. bovis blood stages (Figure 2B) or in vitro induced sexual stages (Figure 3B).
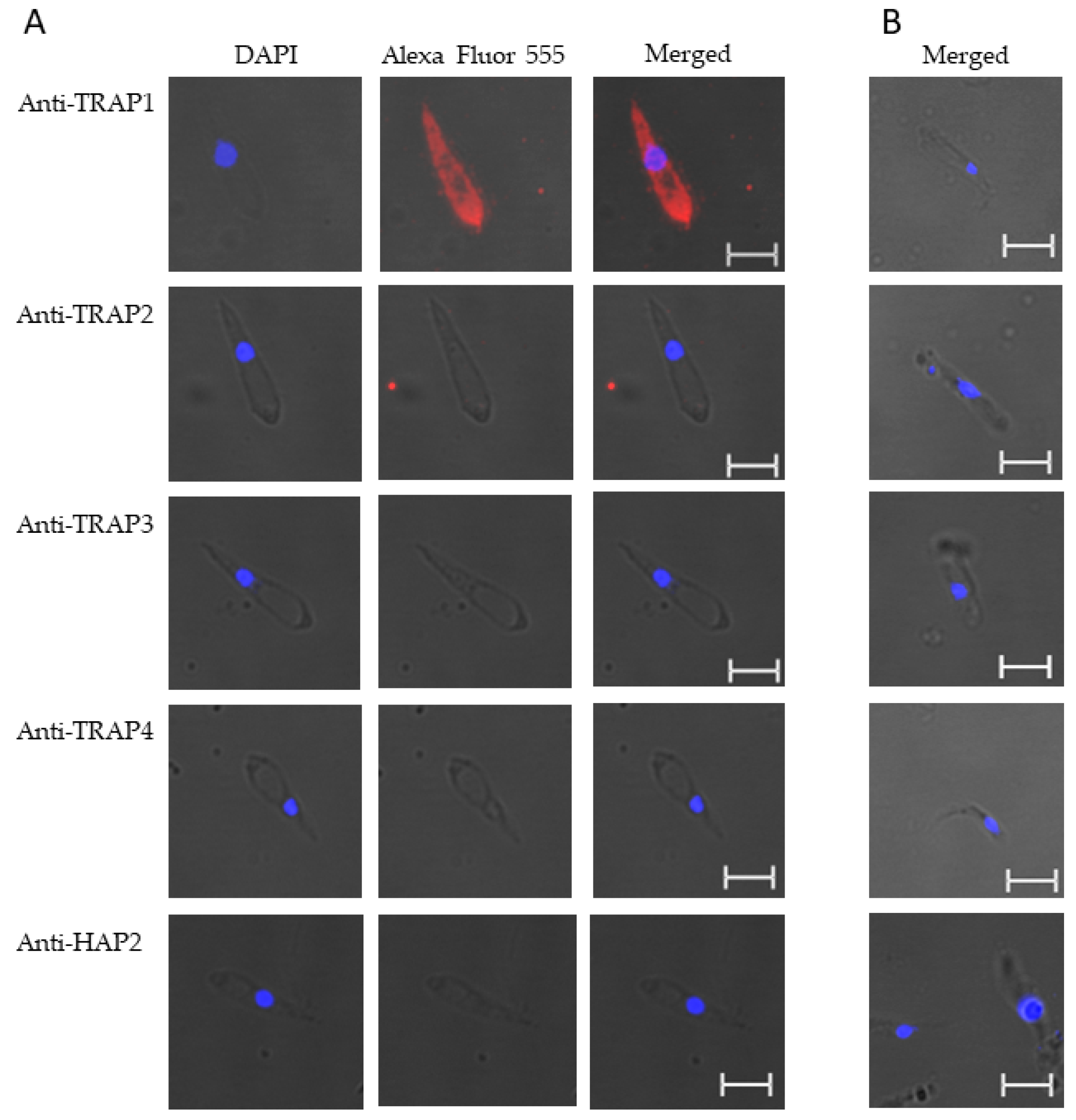
These results suggested that TRAP1 was not expressed by B. bovis blood or sexual stages or was expressed at levels below the limit of detection of our assay. Using kinetes isolated from infected R. microplus females, anti-TRAP1 antibody reacted with fixed kinetes while anti-TRAP2, 3, 4, and HAP2 antibodies failed to react (Figure 4A). Matching pre-immune rabbit sera showed no antibody reactivity with B. bovis kinetes (Figure 4B).
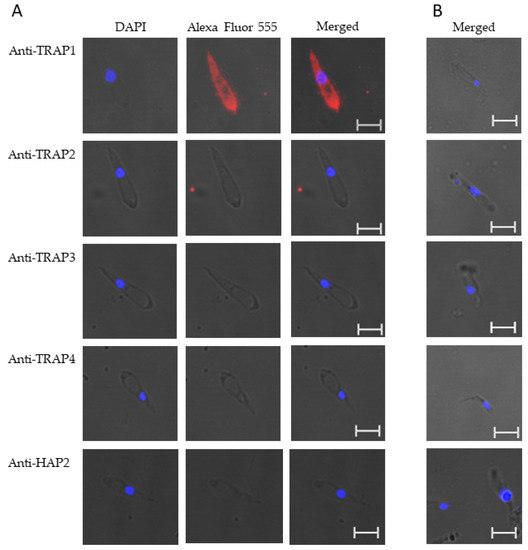
Figure 4.
Babesia bovis kinetes expressed TRAP1 protein as determined by a fixed immunofluorescence assay incubated with anti-TRAP1, 2, 3, 4, or HAP2. Panels are (A) immune and (B) pre-immune rabbit serum. Blue indicates DAPI stained DNA and red indicates anti-rabbit IgG Alexa Fluor 555 reactivity. Scale bar: 5 μm.
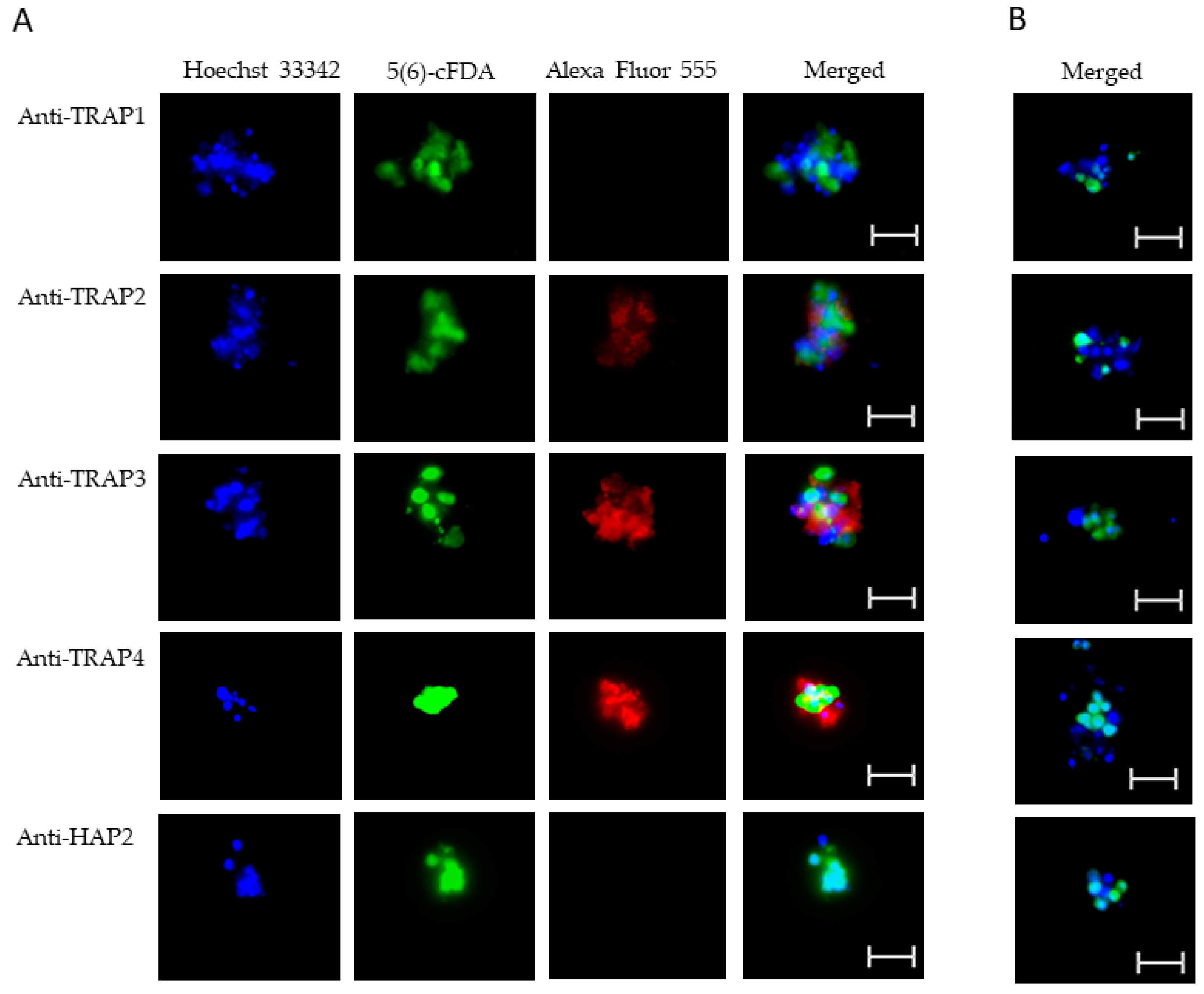
Using live IFA, we demonstrated that TRAP2, 3, and 4 were surface-exposed proteins on both blood (Figure 5A) and in vitro induced sexual stages (Figure 6A). Matching pre-immune rabbit sera showed no antibody reactivity with B. bovis blood (Figure 5B) or in vitro induced sexual stages (Figure 6B). We were unable to determine if TRAP proteins were surface exposed due to the lack of methods to isolate intact live kinetes.
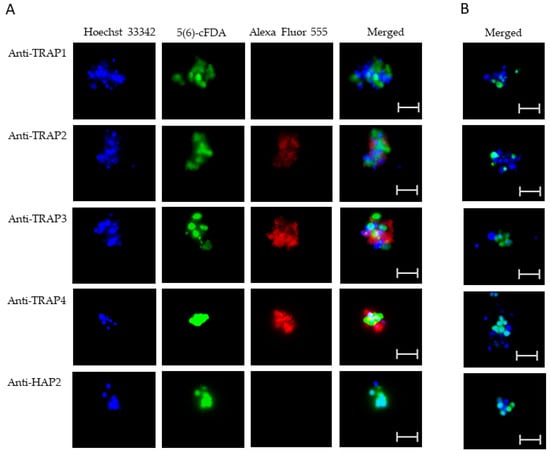
Figure 5.
Evaluation of surface expression of TRAP proteins by B. bovis blood stages using a live immunofluorescence assay incubated with anti-TRAP 1, 2, 3, 4, or HAP2. Panels are (A) immune and (B) pre-immune rabbit serum. Blue indicates Hoechst 33342 stained DNA, green indicates live, intact exoerythrocytic parasites retaining 5(6)-cFDA, and red indicates anti-rabbit IgG Alexa Fluor 555 reactivity. Scale bar: 5 μm.
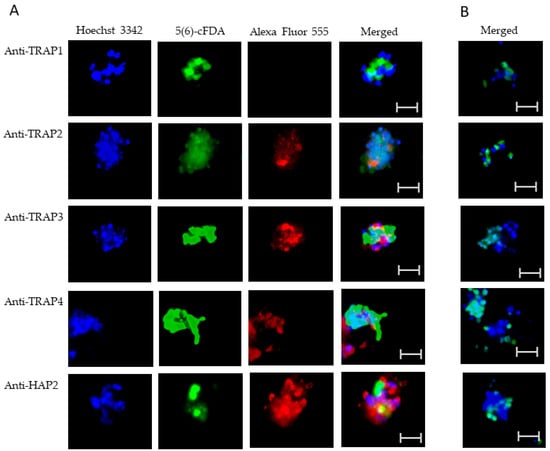
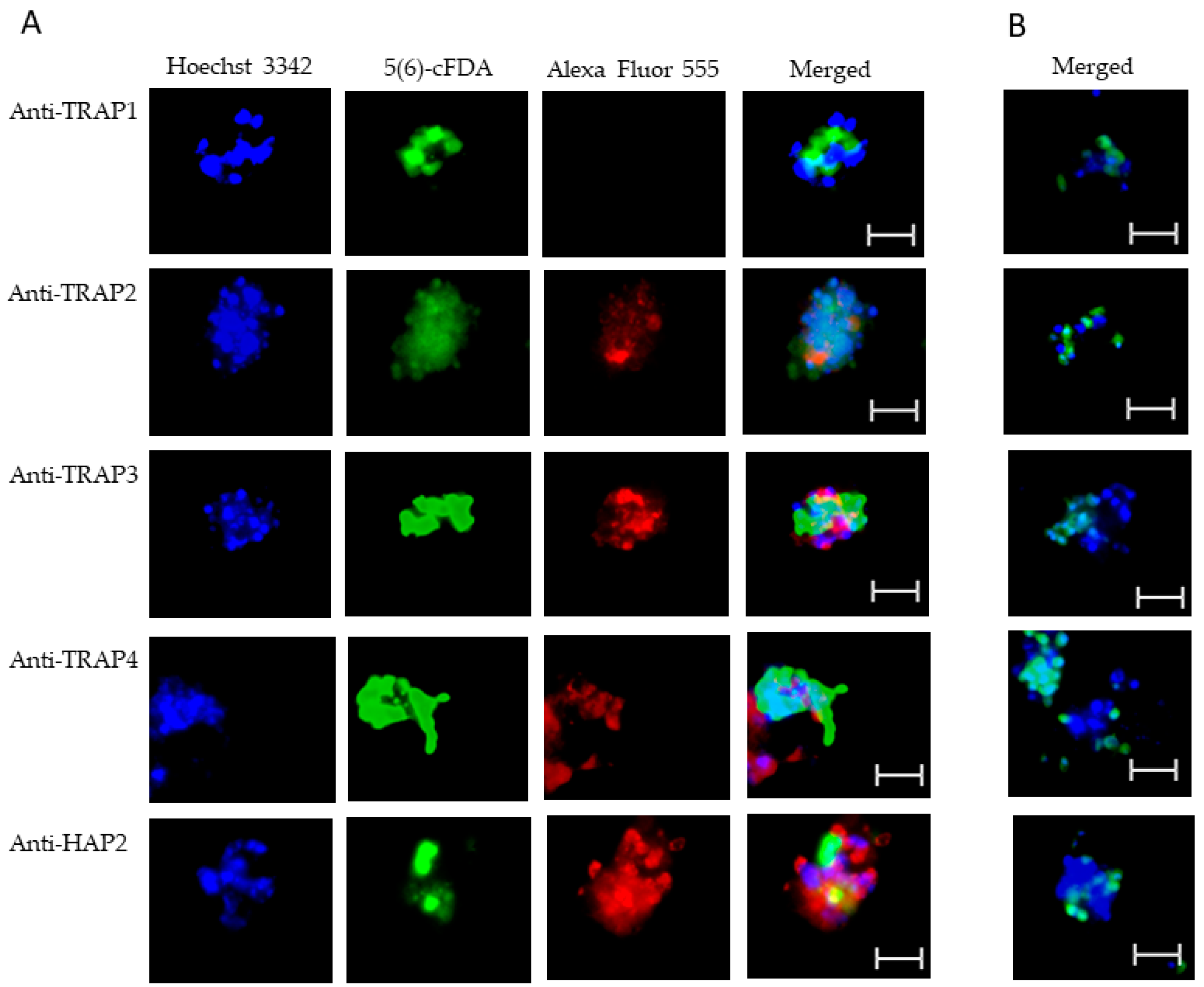
Figure 6.
Evaluation of surface expression of TRAP proteins by B. bovis sexual stages using a live immunofluorescence assay incubated with anti-TRAP 1, 2, 3, 4, or HAP2. Panels are (A) immune and (B) pre-immune rabbit serum. Blue indicates Hoechst 33342 stained DNA, green indicates live, intact exoerythrocytic parasites retaining 5(6)-cFDA, and red indicates anti-rabbit IgG Alexa Fluor 555 reactivity. Scale bar: 5 μm.
3.3. Bioinformatic Analysis of TRAP Family Members
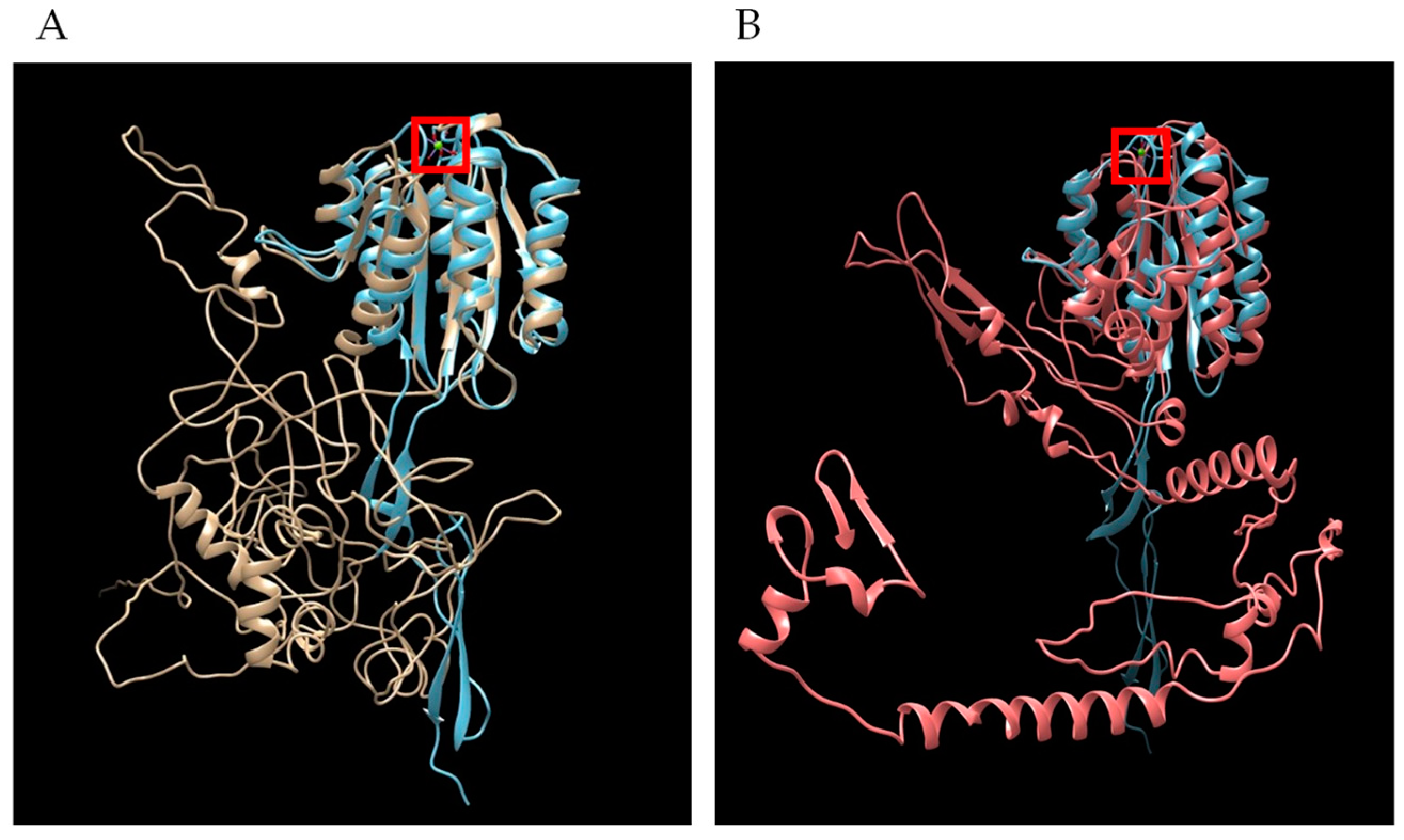
Bioinformatic analysis demonstrated similar features and protein architecture with closely related hemoprotozoan pathogens of human beings that cause malaria. Sequence length restrictions of the D-I-TASSER server limited polypeptide length to 750 amino acid residues. Therefore, full length predictions were possible only for TRAP1 and TRAP4, but not for TRAP2 or TRAP3. For both B. bovis TRAP1 and TRAP4, threading hits with Z-scores greater than one generated by D-I-TASSER found several solved Plasmodium species TRAP structures in PDB, with the highest score of 3.99 to PDB 4hqlA, magnesium-loaded Plasmodium vivax TRAP protein [23]. The resulting pdb files from B. bovis TRAP1 (Figure 7A and Figure S1) and TRAP4 (Figure 7B) were overlayed with the pdb for 4hqlA to find visually appreciable overlap of the Plasmodium MIDAS (metal-ion dependent adhesion site) Mg2+ binding domain. Each helical and sheet structure in the Plasmodium domain was represented by an overlapping structure in the Babesia TRAP molecules.
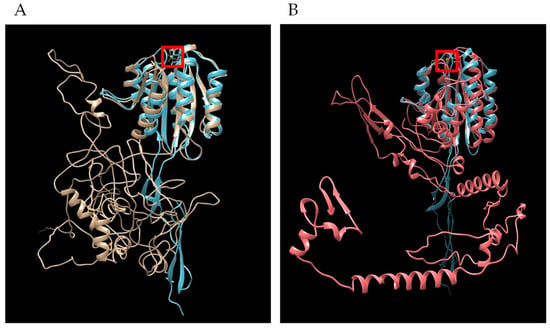
Figure 7.
Overlap of secondary structures shared in tertiary models of Plasmodium TRAP with B. bovis TRAP. (A) Plasmodium vivax TRAP (4hqlA) is blue and B. bovis TRAP1 is gold and (B) Plasmodium vivax TRAP (4hqlA) is blue and B. bovis TRAP4 is red. Image generated by Chimera MatchMaker. Red square is Mg2+ ion bound by P. vivax TRAP.
A movie (Figure S1) showing the rotation of the overlapping molecular models reveals more completely the extent of the MIDAS domain structure similarity, while species-specific structural differences are also evident. The two Plasmodium TRAP molecules with solved structures in the PDB are AAA29775 (P. falciparum) and AAC97484 (P. vivax). The two Plasmodium proteins shared 43% identity, while B. bovis TRAP1 had 23% identity to either of the Plasmodium molecules. Cysteines forming disulfide bonds critical to the tertiary structure of the MIDAS binding domain in the Plasmodium TRAP molecules [23] were likewise conserved in B. bovis TRAP1 (Table 4). Further, key residues that bind the Mg2+ ion in the Plasmodium TRAP molecules were conserved in B. bovis TRAP1. Babesia bovis TRAP4 displayed reduced conservation but consistently formed the MIDAS binding domain and Mg2+ binding residues (Table 3).

Table 4.
Conservation of residues of significance in Plasmodium sp. TRAP and B. bovis TRAP1 orthologs, with comparison to other B. bovis TRAP paralogs.
Babesia bovis TRAP2 and TRAP3 truncated polypeptides were likewise submitted for D-I-TASSER analysis. However, neither TRAP2 or TRAP3 truncated versions generated significant Z-scores and did not return hits to any solved PDB structures related to TRAP, including 4hqlA. While key cysteines that form disulfide bonds of the MIDAS domain are largely conserved in B. bovis TRAP2 and TRAP3, the Mg2+ binding residues were not conserved (Table 3).
4. Discussion
Understanding protein expression by B. bovis life stages within bovine and ticks is a critically important step for developing strategies to protect bovine against babesiosis. Previous studies indicated that B. bovis TRAP proteins are potential candidates for vaccine development [20,21,24]. The structure of TRAP proteins is conserved among Apicomplexan parasites [21,22]. TRAP is defined as a protein that contains a vWFA region and TSP-1 domains [21]. The TRAP ectodomain, VWA domain, MIDAS ligand-binding site, and TSP-1 domain all play a role in Plasmodium motility [23]. In malaria, TRAP proteins are expressed by multiple stages of the parasite during its life cycle within vertebrate and arthropod hosts, including merozoites (MTRAP), ookinetes (CTRAP), and the salivary gland sporozoites (TRAP) [31]. Plasmodium TRAP proteins were used as vaccines against malaria infection of the vertebrate host or to block transmission by the biological vector [32,33]. Previous studies have also demonstrated that Plasmodium TRAP family proteins are responsible for parasite motility and invasion of host cells. In Plasmodium parasites, TRAP mediates gliding motility by attaching and detaching to different substrates along the surface of the parasites [23]. However, it is unknown if B. bovis TRAP proteins play important roles in parasite gliding movement or invasion of vertebrate and invertebrate host cells.
Our results demonstrated significant increased transcription levels of TRAP2 and 3 by B. bovis blood stages as compared to kinetes. However, TRAP3 in blood stages was similar to sexual stages. In contrast, TRAP1 was highly expressed by kinetes as compared to the other TRAP family members and B. bovis stages. It is clear from this and other studies that control of transcription is under the influence of various regulatory systems [18,34]. However, the mechanisms that control transcription in different parasite stages are not completely understood in protozoan parasites [34]. In this study using fixed IFA, we demonstrated that TRAP2, 3, and 4 proteins were expressed by B. bovis blood and sexual stages, whereas TRAP1 protein was only expressed by B. bovis kinetes. Like other Apicomplexa parasites, B. bovis TRAP proteins contain similar features, including a vWFA region. Due to this similarity, we propose that B. bovis TRAP proteins have a parallel function that facilitates parasite infection of bovine and tick vectors. A previous study demonstrated that all four TRAP proteins had signal peptides and transmembrane domains suggesting that they are predicted to be proteins exposed on the parasite’s surface [18]. Our live IFA results, corroborated with bioinformatic predictions, demonstrated that TRAP2, 3, and 4 were surface-exposed proteins on both B. bovis blood and sexual stages. Unfortunately, methods to isolate intact live kinetes are not available. A previous study using differential gel electrophoresis of surface-labeled proteins demonstrated that kinetes did not survive isolation intact and both cell membrane exterior surface and internal proteins were stained [16]. Therefore, we were unable to demonstrate the presence of surface-exposed proteins by kinetes. These collective data support the possibility that TRAP2, 3, and 4 proteins could be appropriate candidates for vaccine development to prevent infection of erythrocytes and to disrupt the formation of infectious B. bovis forms that infect tick midgut. A previous study showed that B. bovis TRAP2 was associated with the microneme at the apical end of the merozoite stage [24]. Zhan et al. found that B. orientalis TRAP2 was localized on the apical end of the parasite [35]. Babesia bovis TRAP2 shares 97% identity with B. orientalis TRAP2, indicating high conservation and a high likelihood of a similar function [35]. Furthermore, Terkawi et al. found that an antibody to rBbTRAP2 partially inhibited B. bovis growth in vitro by interfering with erythrocyte invasion [24]. In comparison to MSA-2c, RAP-1CT, and SBP-1, B. bovis TRAP2 antibodies had the greatest level of parasite growth inhibition [24]. Limited information is available for B. bovis TRAP3 and 4. However, these proteins may have a redundant function to infect bovine erythrocytes or be important for other stages of parasite development. TRAP2, 3, and 4 proteins were also detected in in vitro induced B. bovis sexual stages. Babesia bovis gametes are formed in the tick midgut lumen and fuse to form a zygote. We hypothesize that B. bovis TRAP proteins assist gamete motility through the midgut milieu to find their partners.
In this study, we demonstrated that, similar to Plasmodium, TRAP1 protein was made only by kinetes in the arthropod, suggesting that this protein may be involved in kinete motility and invasion of tick ovaries. However, previous studies have demonstrated that TRAP1 was expressed by B. bovis merozoites [20,36] and by B. bigemina blood stages [37]. The discrepancy between studies could be explained by Babesia species or strain diversity. In the current study, we used the B. bovis S74-T3Bo strain, while previous studies of B. bovis TRAP1 used either an Israel strain, clonal line C61411, or an alternative Texas strain [20,36]. This assumption is also supported by Plasmodium strain diversity that negatively impacted the detection of infection in human beings [38]. In addition, a serological survey using field collected samples from geographically distinct areas showed that TRAP1 induced inconsistent levels of antibodies, suggesting that the protein was either a poor immunogen or not expressed by B. bovis isolates [36]. Nonetheless, B. bovis TRAP1 could be used as an antigen for vaccine development to disrupt parasite transmission by tick vectors and prevent mammalian host infection.
In Plasmodium species, TRAP allows the parasite to invade mosquito tissues and mammalian host cells. Herein, we demonstrated that B. bovis TRAP1 was highly expressed by Babesia kinete parasites. This result is similar to those obtained in a previous study [39]. Even though B. bovis TRAP1 shares only 23% amino acid identity with the Plasmodium sp. TRAP protein, B. bovis TRAP1 appears to fold a similar MIDAS domain suggesting mechanistic conservation. Furthermore, the B. bovis TRAP4 gene was upregulated, and the protein made by both B. bovis blood and sexual stages. The presence of a MIDAS domain suggests that TRAP4 may be important for gliding and invasion of vertebrate and invertebrate host cells by Babesia blood and sexual stages. The location of B. bovis TRAP1 and TRAP4 predicted that rhomboid protease cleavage sites, AGGALLG and VGIICLV, respectively, are each within an alpha helix. Previous studies showed that a Plasmodium rhomboid protease cleaved TRAP proteins as a mechanism of gliding motility [40]. In B. bovis, rhomboid transcripts are differentially expressed between Babesia blood stages and kinetes. In blood stages, two rhomboid genes, BBOV_II005940 and BBOV_II005930, were upregulated, whereas two different rhomboid genes, BBOV_II006070 and BBOV_II006100, were upregulated in kinetes [18]. Therefore, we hypothesize that BBOV_II005940 and BBOV_II005930 cleave TRAP2, 3, or 4 for merozoite gliding and BBOV_II006070 and BBOV_II006100 cleave TRAP1 for kinete gliding in the tick hemolymph.
5. Conclusions
In conclusion, this study is the first to identify the stage-specific expression profile of B. bovis TRAP1, 2, 3, and 4 in blood stages, in vitro induced sexual stages, and kinetes. Babesia bovis TRAP2, 3, and 4 surface proteins may be important for parasite development in the mammalian host and tick midgut lumen, whereas B. bovis TRAP1 may play a key role in the infection of tick ovary cells. Further studies, including gene editing and antibody inhibition approaches, may elucidate the involvement of B. bovis TRAP proteins in parasite motility and invasion within the vertebrate and invertebrate hosts as seen with closely related Apicomplexan parasites that infect human beings. The results herein will contribute to the design of effective strategies to control infection of vertebrate and invertebrate hosts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10112173/s1, Figure S1: B. bovis TRAP1_Pvivax TRAP Chimera movie.
Author Contributions
Conceptualization, H.E.M., W.C.J. and M.W.U.; methodology, H.E.M., L.K., J.C.-P., H.E.H., M.R.M., D.J.H.-S. and J.M.L.; validation, H.E.M., J.C.-P. and J.M.L.; formal analysis, H.E.M., N.S.T., W.C.J., L.K., M.R.M., J.M. and M.W.U.; investigation, H.E.M., M.W.U.; resources, M.W.U.; data curation, H.E.M., W.C.J., L.K.; visualization, H.E.M., N.S.T., W.C.J., L.K., J.C.-P., D.J.H.-S., J.M.L. and M.W.U.; writing—original draft preparation, H.E.M. and M.W.U.; writing—review and editing, H.E.M., N.S.T., W.C.J., L.K., J.C.-P., H.E.H., M.R.M., D.J.H.-S., J.M.L., J.M. and M.W.U.; supervision, M.W.U.; project administration, M.W.U.; funding acquisition, M.W.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the U.S. Department of Agriculture-Agricultural Research Service (Project #2090-32000-040-000-D), and The U.S. Department of Agriculture-Agricultural Research Service Offshore fund.
Institutional Review Board Statement
The experiment conducted in this study was in accordance with institutional guidelines based on the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Guide for the Care and Use of Agricultural Animals in Research and Training. This study was approved by the University of Idaho—the Institutional Animal Care and Use Protocol Committee, Moscow, Idaho, (IACUC #2018-16).
Data Availability Statement
The data presented in this study are available in Thrombospondin-Related Anonymous Protein (TRAP) family expression by Babesia bovis life stages within the mammalian host and tick vector.
Acknowledgments
We express our gratitude to Sara Davis, Paul Lacy, Gavin Scoles, and Megan Jacks for their excellent assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beugnet, F.; Moreau, Y. Babesiosis. Rev. Sci. Tech. 2015, 34, 627–639. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; de Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129 (Suppl. S1), S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.E.; Noh, S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Florin-Christensen, M.; Schnittger, L. Piroplasmids and ticks: A long-lasting intimate relationship. Front. Biosci. (Landmark Ed.) 2009, 14, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef]
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Entomol. 2021, 66, 373–388. [Google Scholar] [CrossRef]
- George, J.E. Present and future technologies for tick control. Ann. N. Y. Acad. Sci. 2000, 916, 583–588. [Google Scholar] [CrossRef]
- Mosqueda, J.; Olvera-Ramirez, A.; Aguilar-Tipacamu, G.; Canto, G.J. Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 2012, 19, 1504–1518. [Google Scholar] [CrossRef]
- Rojas-Martínez, C.; Rodríguez-Vivas, R.I.; Millán, J.V.F.; Bautista-Garfias, C.R.; Castañeda-Arriola, R.O.; Lira-Amaya, J.J.; Urióstegui, P.V.; Carrasco, J.J.O.; Martínez, J. Bovine babesiosis: Cattle protected in the field with a frozen vaccine containing Babesia bovis and Babesia bigemina cultured in vitro with a serum-free medium. Parasitol. Int. 2018, 67, 190–195. [Google Scholar] [CrossRef]
- Guerrero, F.D.; Lovis, L.; Martins, J.R. Acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet. 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Florin-Christensen, M.; Suarez, C.E.; Rodriguez, A.E.; Flores, D.A.; Schnittger, L. Vaccines against bovine babesiosis: Where we are now and possible roads ahead. Parasitology 2014, 141, 1563–1592. [Google Scholar] [CrossRef] [PubMed]
- de Waal, D.T.; Combrink, M.P. Live vaccines against bovine babesiosis. Vet. Parasitol. 2006, 138, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo Ortiz, J.M.; Paoletta, M.S.; Gravisaco, M.J.; López Arias, L.S.; Montenegro, V.N.; de la Fournière, S.A.M.; Valenzano, M.N.; Guillemi, E.C.; Valentini, B.; Echaide, I.; et al. Immunisation of cattle against Babesia bovis combining a multi-epitope modified vaccinia Ankara virus and a recombinant protein induce strong Th1 cell responses but fails to trigger neutralising antibodies required for protection. Ticks. Tick Borne Dis. 2019, 10, 101270. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.E.; Bastos, R.G.; Schneider, D.A.; Johnson, W.C.; Adham, F.K.; Davis, W.C.; Laughery, J.M.; Herndon, D.R.; Alzan, H.F.; Ueti, M.W.; et al. The Babesia bovis hap2 gene is not required for blood stage replication, but expressed upon in vitro sexual stage induction. PLoS Negl. Trop. Dis. 2017, 11, e0005965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tewari, R.; Ning, J.; Blagborough, A.M.; Garbom, S.; Pei, J.; Grishin, N.V.; Steele, R.E.; Sinden, R.E.; Snell, W.J.; et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008, 22, 1051–1068. [Google Scholar] [CrossRef]
- Johnson, W.C.; Taus, N.S.; Reif, K.E.; Bohaliga, G.A.; Kappmeyer, L.S.; Ueti, M.W. Analysis of Stage-Specific Protein Expression during Babesia Bovis Development within Female Rhipicephalus Microplus. J. Proteome Res. 2017, 16, 1327–1338. [Google Scholar] [CrossRef]
- Howell, J.M.; Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Knowles, D.P. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J. Clin. Microbiol. 2007, 45, 426–431. [Google Scholar] [CrossRef]
- Ueti, M.W.; Johnson, W.C.; Kappmeyer, L.S.; Herndon, D.R.; Mousel, M.R.; Reif, K.E.; Taus, N.S.; Ifeonu, O.O.; Silva, J.C.; Suarez, C.E.; et al. Comparative analysis of gene expression between Babesia bovis blood stages and kinetes allowed by improved genome annotation. Int. J. Parasitol. 2021, 51, 123–136. [Google Scholar] [CrossRef]
- Mosqueda, J.; McElwain, T.F.; Stiller, D.; Palmer, G.H. Babesia bovis merozoite surface antigen 1 and rhoptry-associated protein 1 are expressed in sporozoites, and specific antibodies inhibit sporozoite attachment to erythrocytes. Infect. Immun. 2002, 70, 1599–1603. [Google Scholar] [CrossRef]
- Gaffar, F.R.; Yatsuda, A.P.; Franssen, F.F.; de Vries, E. A Babesia bovis merozoite protein with a domain architecture highly similar to the thrombospondin-related anonymous protein (TRAP) present in Plasmodium sporozoites. Mol. Biochem. Parasitol. 2004, 136, 25–34. [Google Scholar] [CrossRef]
- Paoletta, M.S.; Wilkowsky, S.E. Thrombospondin Related Anonymous Protein Superfamily in Vector-Borne Apicomplexans: The Parasite’s Toolkit for Cell Invasion. Front. Cell. Infect. Microbiol. 2022, 12, 831592. [Google Scholar] [CrossRef] [PubMed]
- Morahan, B.J.; Wang, L.; Coppel, R.L. No TRAP, no invasion. Trends Parasitol. 2009, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Koksal, A.C.; Lu, C.; Springer, T.A. Shape change in the receptor for gliding motility in Plasmodium sporozoites. Proc. Natl. Acad. Sci. USA 2012, 109, 21420–21425. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Ratthanophart, J.; Salama, A.; AbouLaila, M.; Asada, M.; Ueno, A.; Alhasan, H.; Guswanto, A.; Masatani, T.; Yokoyama, N.; et al. Molecular characterization of a new Babesia bovis thrombospondin-related anonymous protein (BbTRAP2). PLoS ONE 2013, 8, e83305. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda, J.; Falcon, A.; Antonio Alvarez, J.; Alberto Ramos, J.; Oropeza-Hernandez, L.F.; Figueroa, J.V. Babesia bigemina sexual stages are induced in vitro and are specifically recognized by antibodies in the midgut of infected Boophilus microplus ticks. Int. J. Parasitol. 2004, 34, 1229–1236. [Google Scholar] [CrossRef]
- Levy, M.G.; Ristic, M. Babesia bovis: Continuous cultivation in a microaerophilous stationary phase culture. Science 1980, 207, 1218–1220. [Google Scholar] [CrossRef]
- Bohaliga, G.A.R.; Johnson, W.C.; Taus, N.S.; Hussein, H.E.; Bastos, R.G.; Suarez, C.E.; Scoles, G.A.; Ueti, M.W. Identification of proteins expressed by Babesia bigemina kinetes. Parasit. Vectors 2019, 12, 271. [Google Scholar] [CrossRef]
- Ellefsen, S.; Stensløkken, K.O. Gene-family profiling: A normalization-free real-time RT-PCR approach with increased physiological resolution. Physiol. Genom. 2010, 42, 1–4. [Google Scholar] [CrossRef]
- Goff, W.L.; McElwain, T.F.; Suarez, C.E.; Johnson, W.C.; Brown, W.C.; Norimine, J.; Knowles, D.P. Competitive enzyme-linked immunosorbent assay based on a rhoptry-associated protein 1 epitope specifically identifies Babesia bovis-infected cattle. Clin. Diagn. Lab. Immunol. 2003, 10, 38–43. [Google Scholar] [CrossRef]
- Laughery, J.M.; Knowles, D.P.; Schneider, D.A.; Bastos, R.G.; McElwain, T.F.; Suarez, C.E. Targeted surface expression of an exogenous antigen in stably transfected Babesia bovis. PLoS ONE 2014, 9, e97890. [Google Scholar] [CrossRef]
- Kumar, H.; Tolia, N.H. Getting in: The structural biology of malaria invasion. PLoS Pathog. 2019, 15, e1007943. [Google Scholar] [CrossRef] [PubMed]
- Tiono, A.B.; Nébié, I.; Anagnostou, N.; Coulibaly, A.S.; Bowyer, G.; Lam, E.; Bougouma, E.C.; Ouedraogo, A.; Yaro, J.B.B.; Barry, A.; et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5–17 months old infants and children. PLoS ONE 2018, 13, e0208328. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Song, G.; Beale, K.; Yan, J.; Garst, E.; Feng, J.; Lund, E.; Catteruccia, F.; Springer, T.A. Design and assessment of TRAP-CSP fusion antigens as effective malaria vaccines. PLoS ONE 2020, 15, e0216260. [Google Scholar] [CrossRef] [PubMed]
- Alzan, H.F.; Knowles, D.P.; Suarez, C.E. Comparative Bioinformatics Analysis of Transcription Factor Genes Indicates Conservation of Key Regulatory Domains among Babesia bovis, Babesia microti, and Theileria equi. PLoS Negl. Trop. Dis. 2016, 10, e0004983. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; He, J.; Yu, L.; Liu, Q.; Sun, Y.; Nie, Z.; Guo, J.; Zhao, Y.; Li, M.; Luo, X.; et al. Identification of a novel thrombospondin-related anonymous protein (BoTRAP2) from Babesia orientalis. Parasit. Vectors 2019, 12, 200. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Huyen, N.X.; Wibowo, P.E.; Seuseu, F.J.; Aboulaila, M.; Ueno, A.; Goo, Y.K.; Yokoyama, N.; Xuan, X.; Igarashi, I. Spherical body protein 4 is a new serological antigen for global detection of Babesia bovis infection in cattle. Clin. Vaccine Immunol. 2011, 18, 337–342. [Google Scholar] [CrossRef]
- Montenegro, V.N.; Paoletta, M.S.; Jaramillo Ortiz, J.M.; Suarez, C.E.; Wilkowsky, S.E. Identification and characterization of a Babesia bigemina thrombospondin-related superfamily member, TRAP-1: A novel antigen containing neutralizing epitopes involved in merozoite invasion. Parasit. Vectors 2020, 13, 602. [Google Scholar] [CrossRef]
- Chenet, S.M.; Branch, O.H.; Escalante, A.A.; Lucas, C.M.; Bacon, D.J. Genetic diversity of vaccine candidate antigens in Plasmodium falciparum isolates from the Amazon basin of Peru. Malar. J. 2008, 7, 93. [Google Scholar] [CrossRef]
- Ueti, M.W.; Johnson, W.C.; Kappmeyer, L.S.; Herndon, D.R.; Mousel, M.R.; Reif, K.E.; Taus, N.S.; Ifeonu, O.O.; Silva, J.C.; Suarez, C.E.; et al. Transcriptome dataset of Babesia bovis life stages within vertebrate and invertebrate hosts. Data Brief. 2020, 33, 106533. [Google Scholar] [CrossRef]
- Baker, R.P.; Wijetilaka, R.; Urban, S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2006, 2, e113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).