Unusual Cell Structures and Organelles in Giardia intestinalis and Trichomonas vaginalis Are Potential Drug Targets
Abstract
:1. Introduction
2. Chemotherapy against Parasitic Protozoa
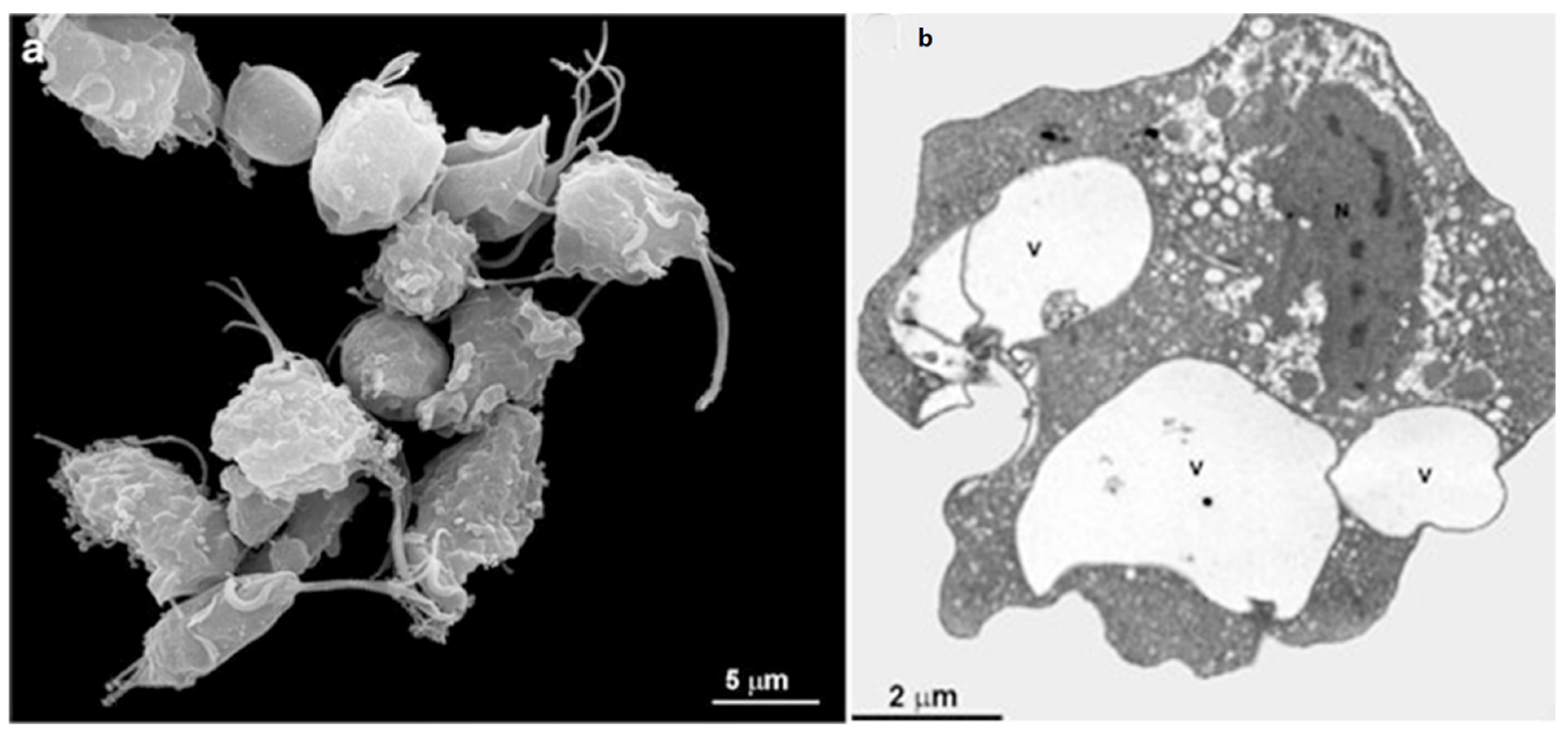
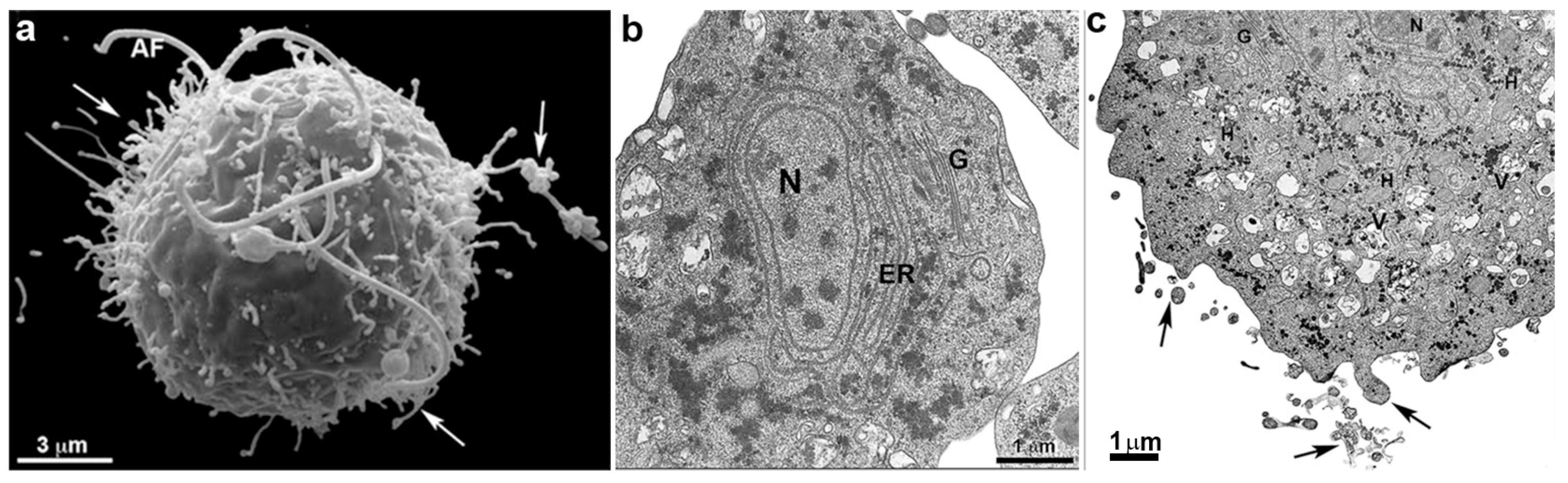
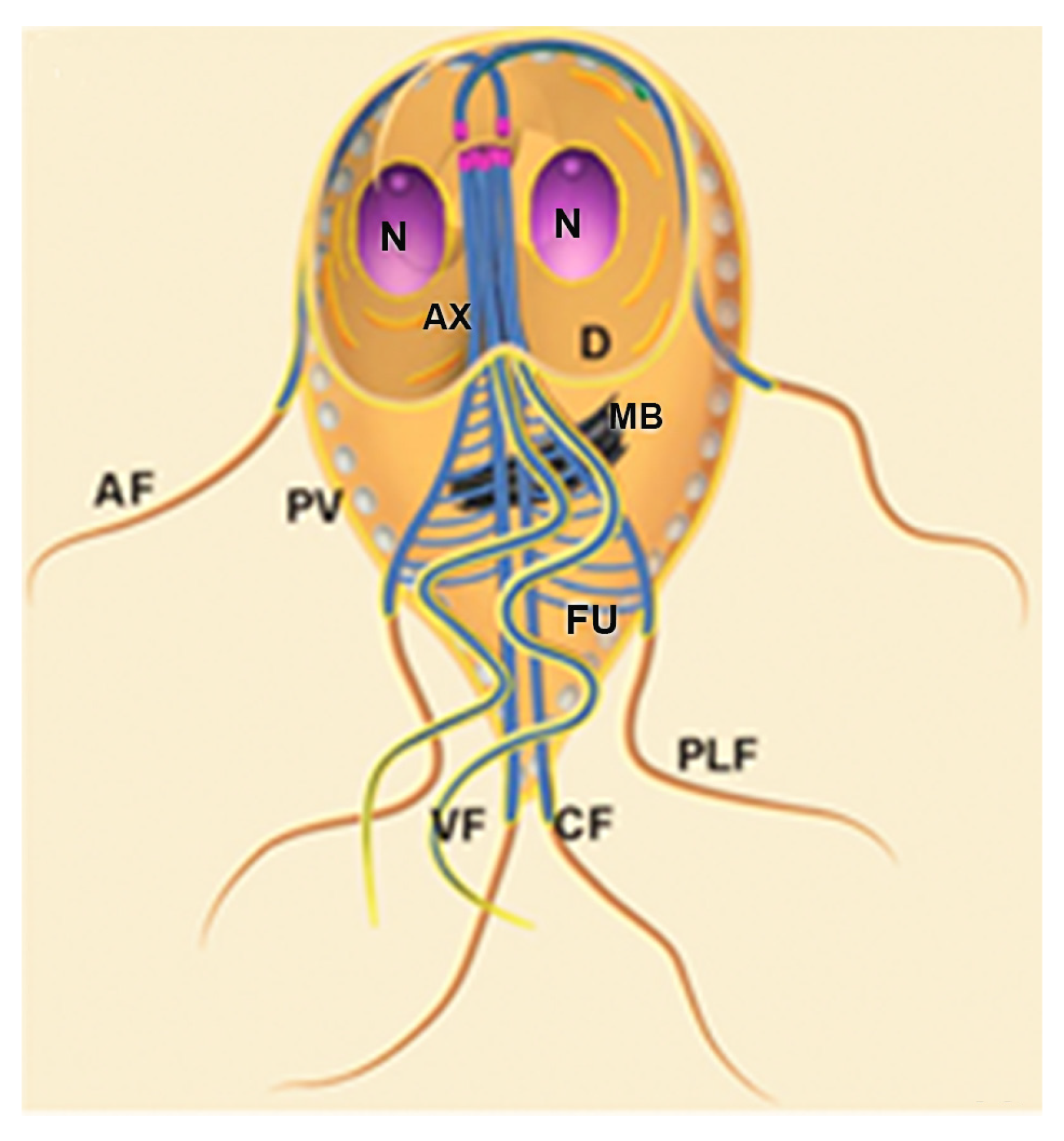
3. Trichomonas vaginalis Features
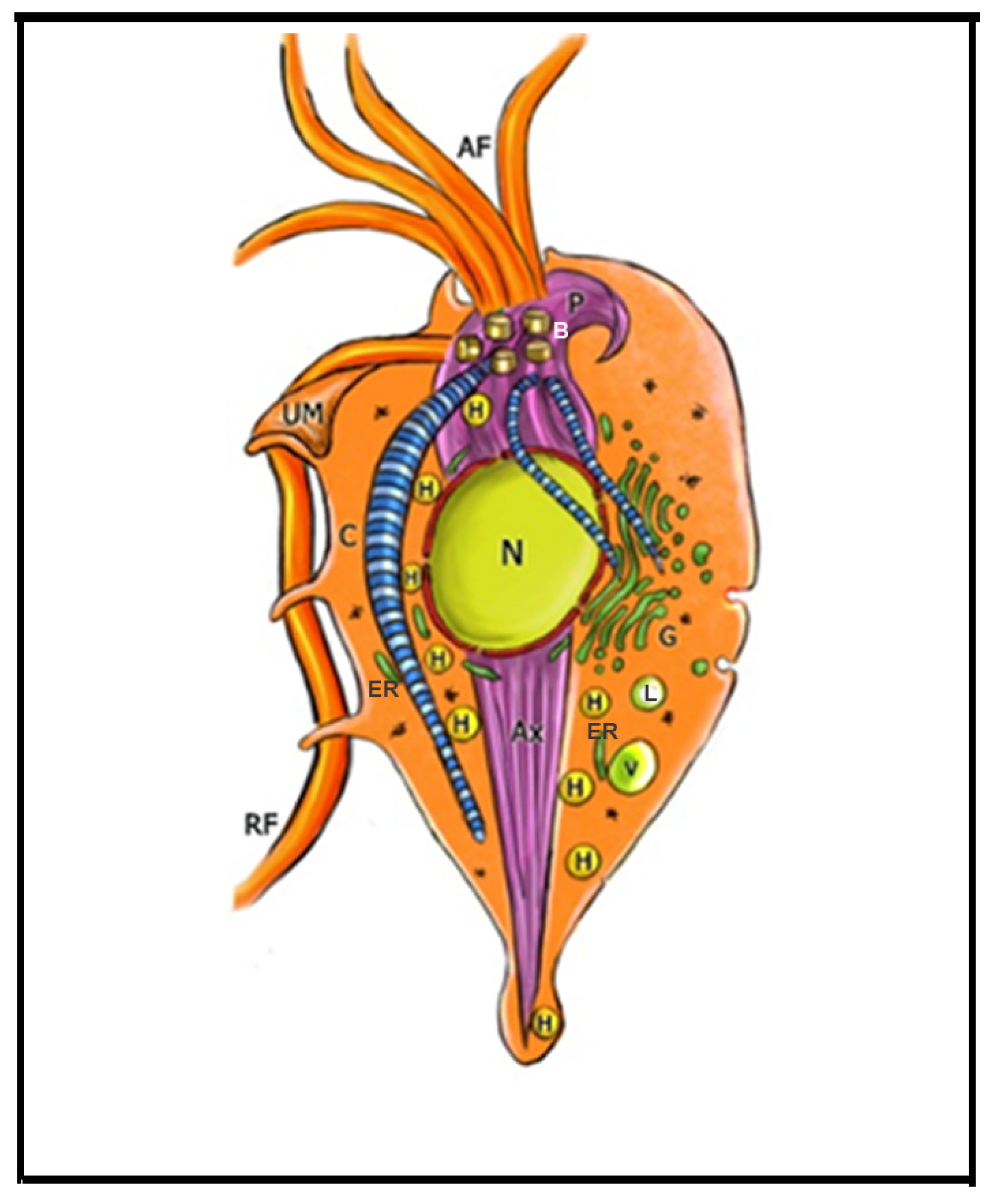
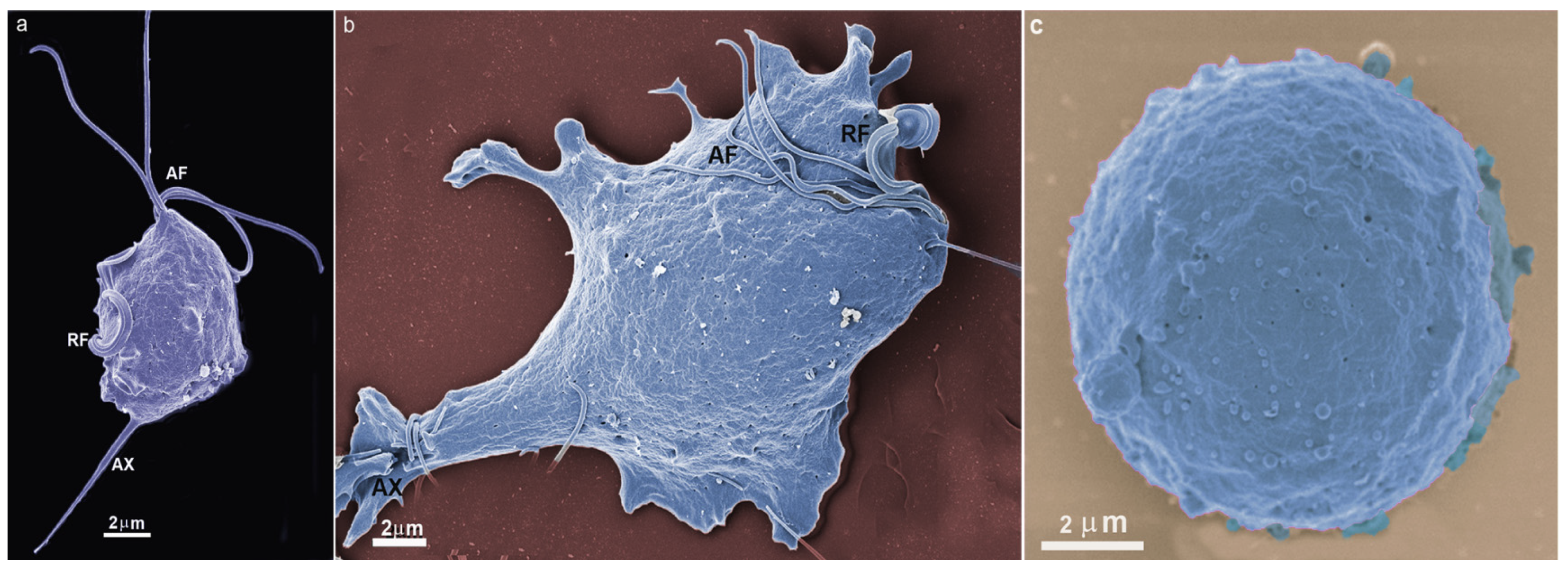
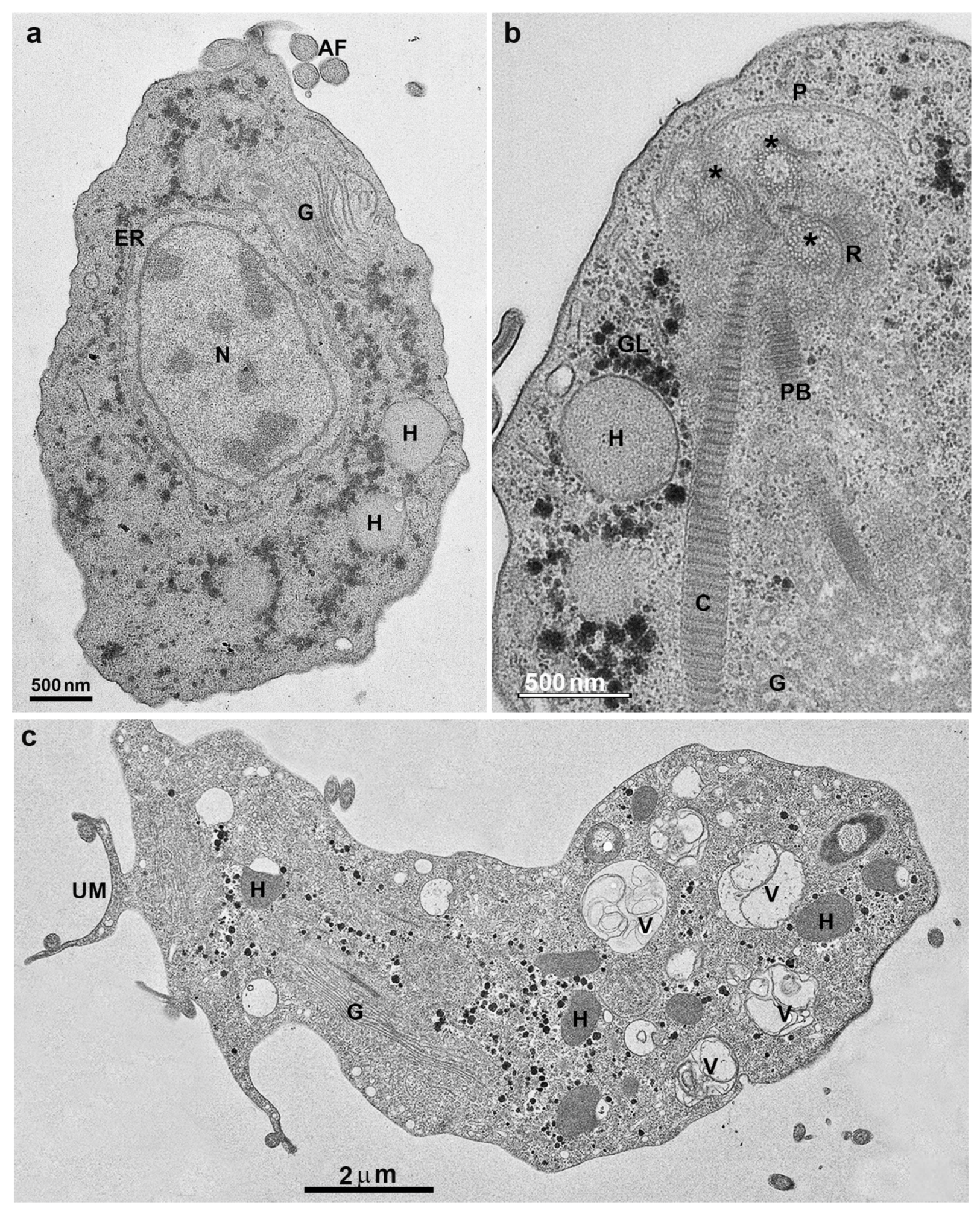
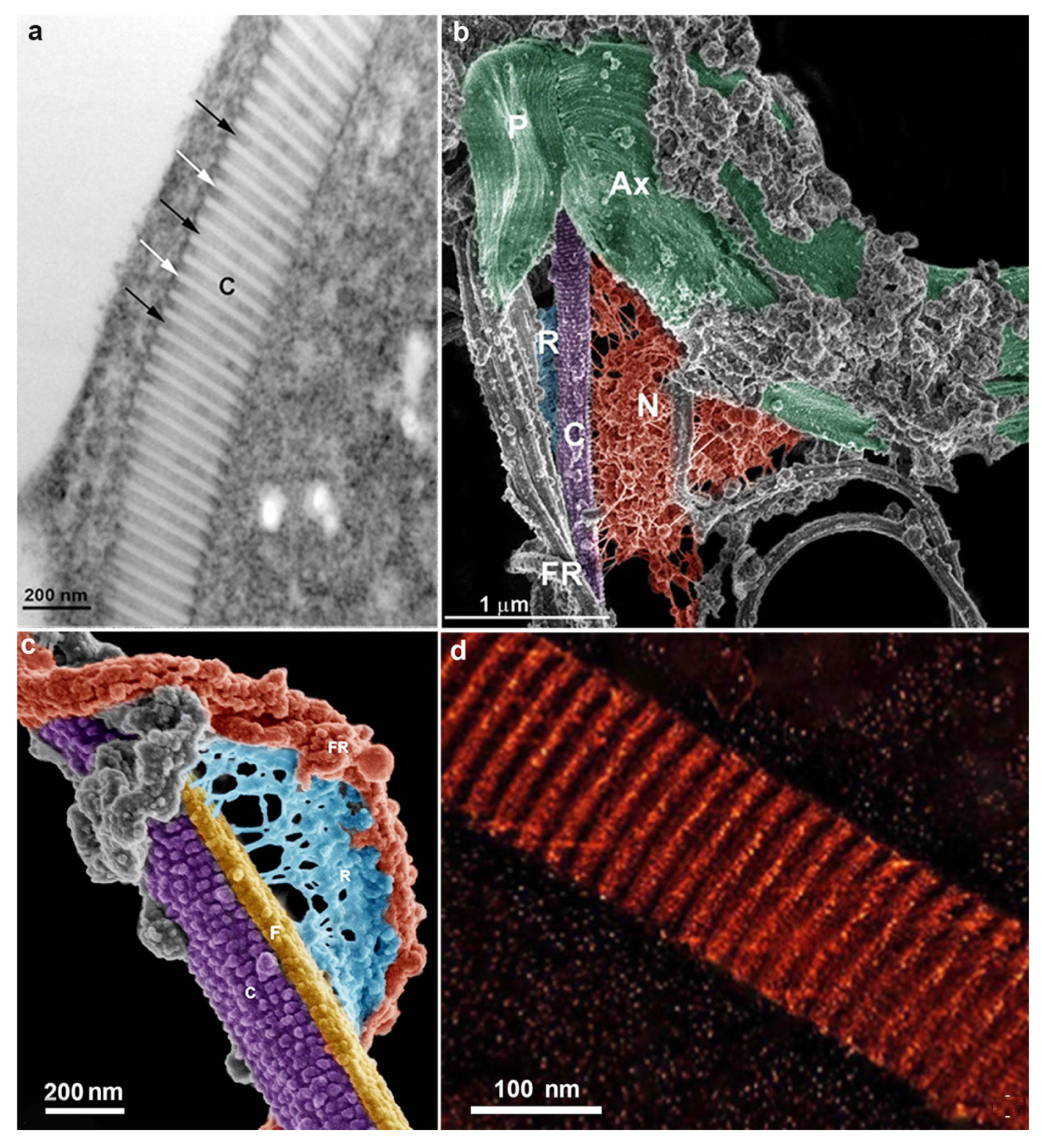
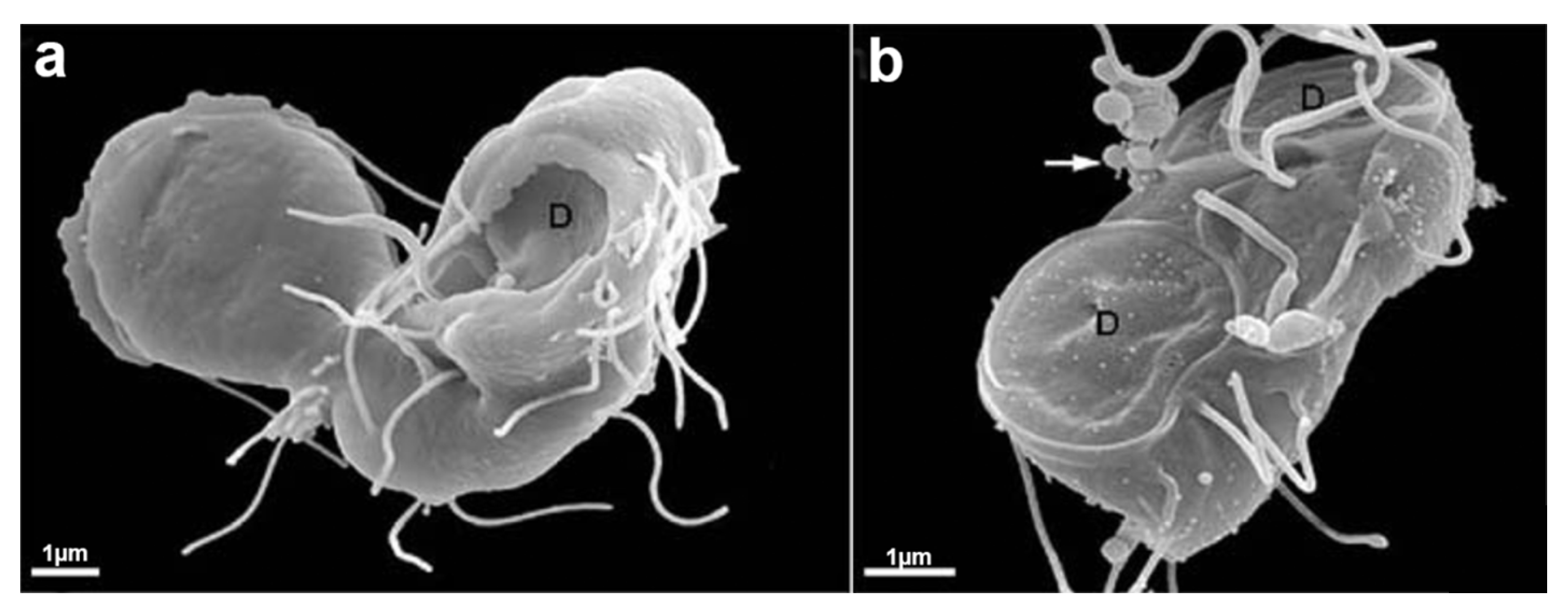
4. Trichomonas and Its Unusual Structures
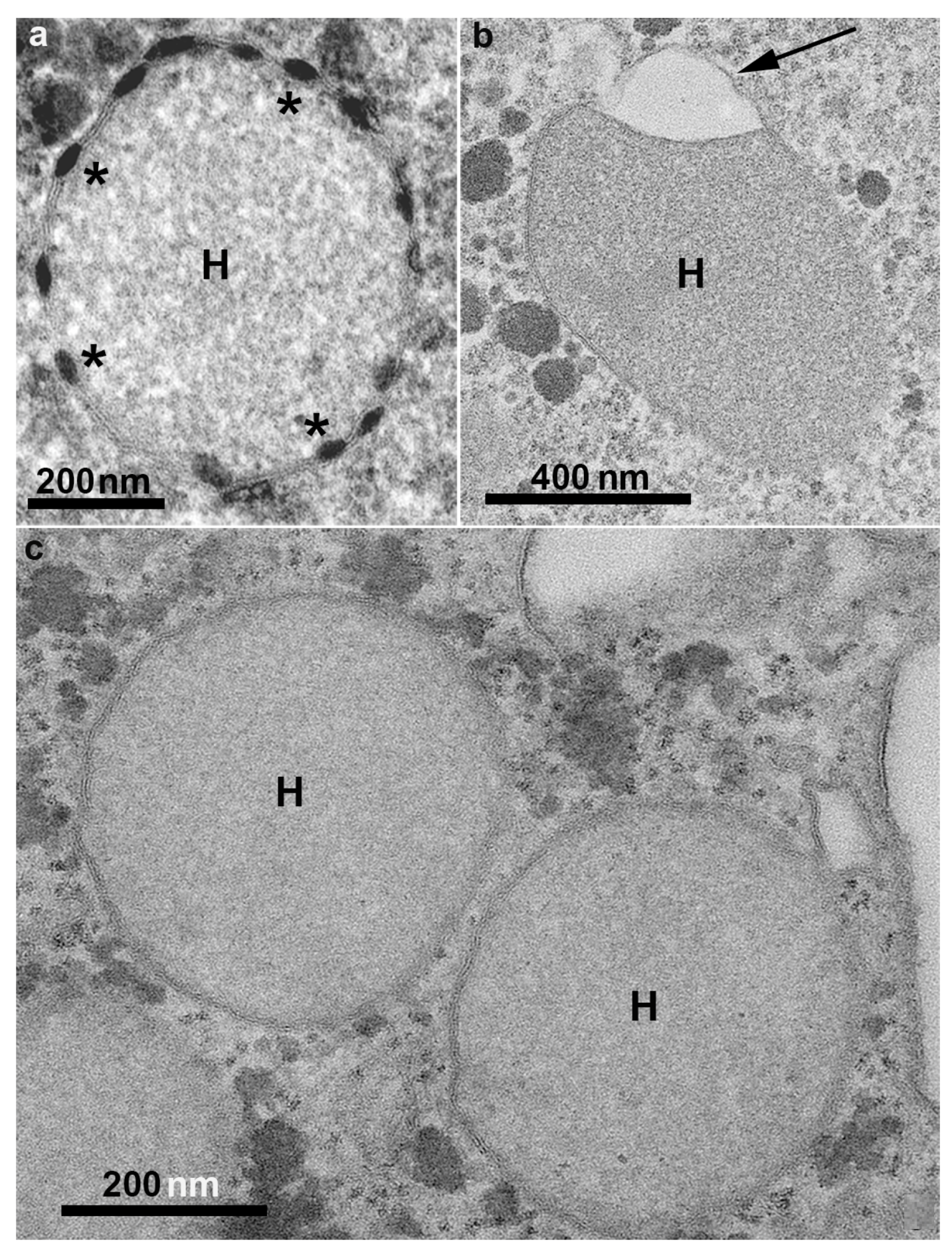
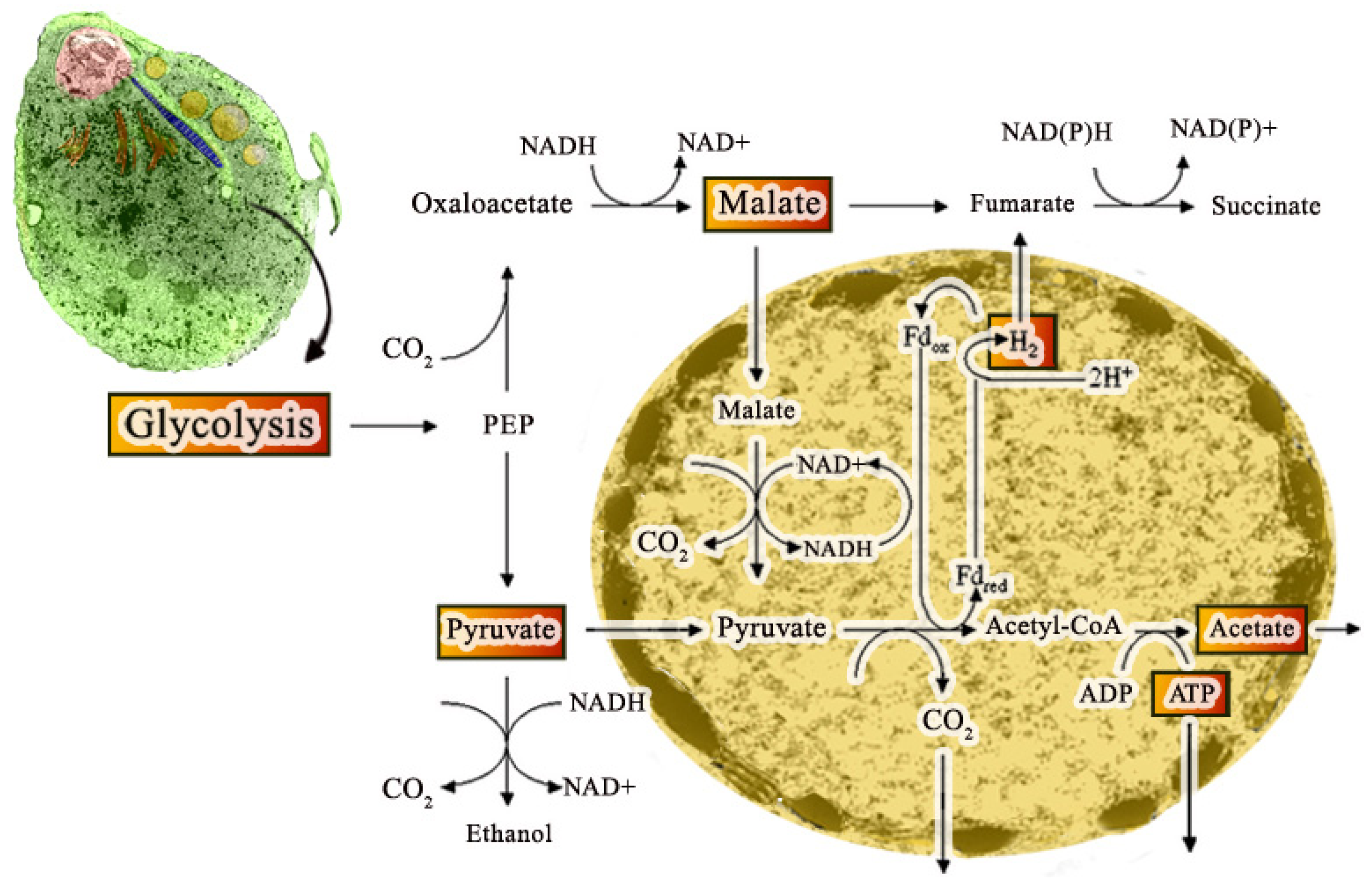
Hydrogenosomes
5. Drugs Affecting Trichomonas
5.1. Metronidazole
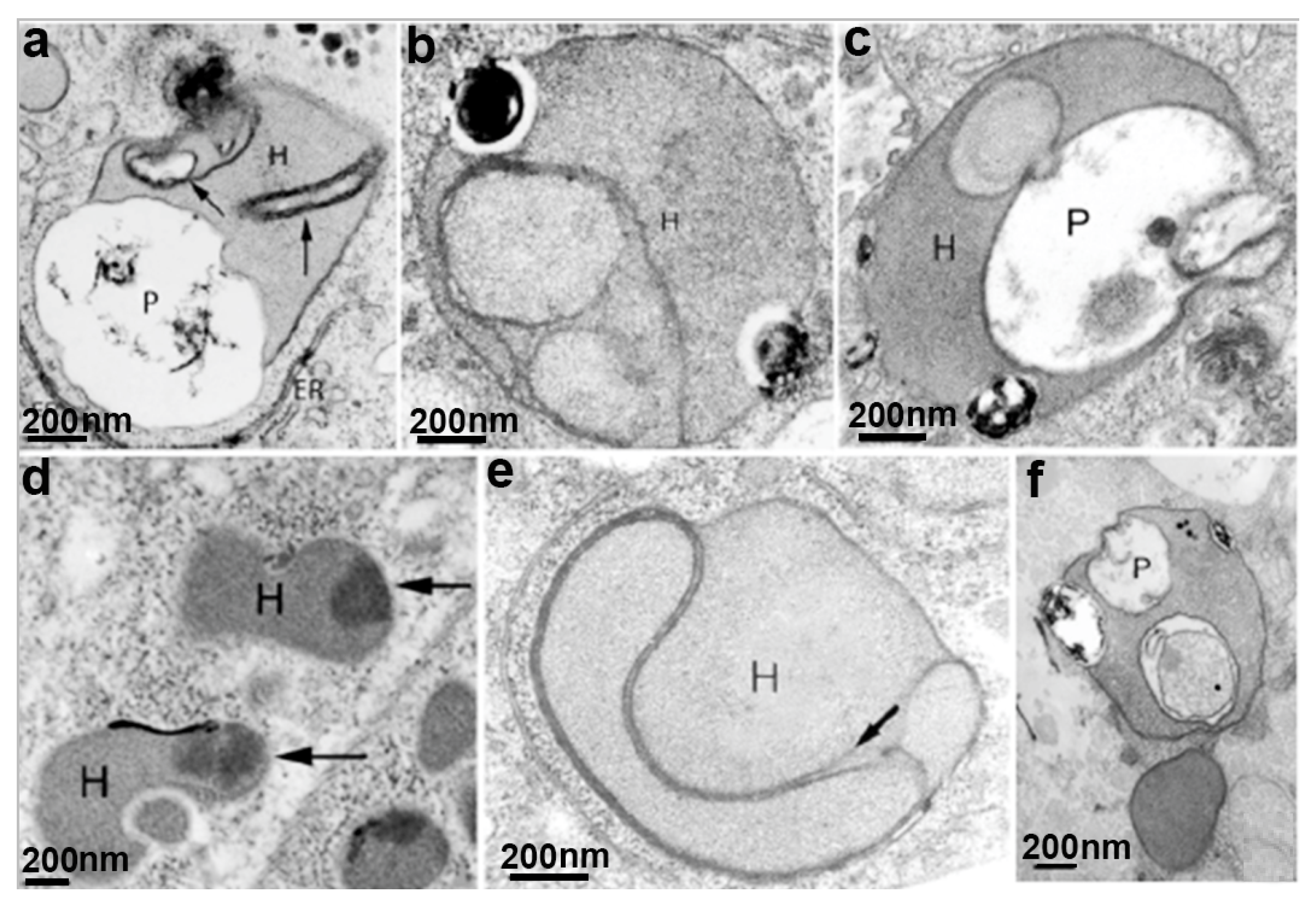
5.2. Effects on Trichomonas Structures by Other Drugs (Table 1)
5.3. Proteasomes
| Drug | Cell Structure Affected | Effect | Reference |
|---|---|---|---|
| Miltefosine | Plasma membrane; nucleus; hydrogenosomes; flagella | Cell clusters; membrane blebbing; wrinkled cells; pseudocysts formation; myelin-like figures; condensed chromatin; vacuolization; decreased size of hydrogenosomes | [57] |
| Methyl jasmonate | Hydrogenosome | cell death; loss of hydrogenosomal membrane potential | [67] |
| Δ(24(25))-sterol methyltransferase inhibitors | Golgi; Hydrogenosomes; plasma membrane | cell clusters; wrinkled cells; membrane blebbing; cell disruption. abnormal Golgi; damaged hydrogenosomes; autophagic vacuoles | [47] |
| 3-(biphenyl-4-yl)-3-hydoxyquinuclidine (BPQ-OH) | Plasma membrane; vacuoles | inhibits in vitro proliferation; rounded and wrinkled cells, membrane blebbing; intense vacuolization, o cell death; cell death | [62] |
| Lactacystin | ER | increase in the number of endoplasmic reticulum membranes | [64] |
| Zinc-clotrimazole complexes | ER; Golgi; plasma membrane | atypical ER; a high number of filopodia; irregular distribution of Golgi lamellas | [61] |
| Amiodarone | Nucleus; hydrogenosome | Cytokinesis blockage; duplicated cell structures; large multinucleated cells; Hydrogenosome alteration; glycogen disturbance | [63] |
| Amioder and Dronedarone | Hydrogenosome; plasma membrane | Apoptosis; deformed andaggregated cells; hydrogenosomes alterations | [63] |
| Thiosemicarbazones 49, 51, and 63 | Structural cell changes;Hydrogenosomes are not affected | Pseudocysts, cell surface alterations | [68] |
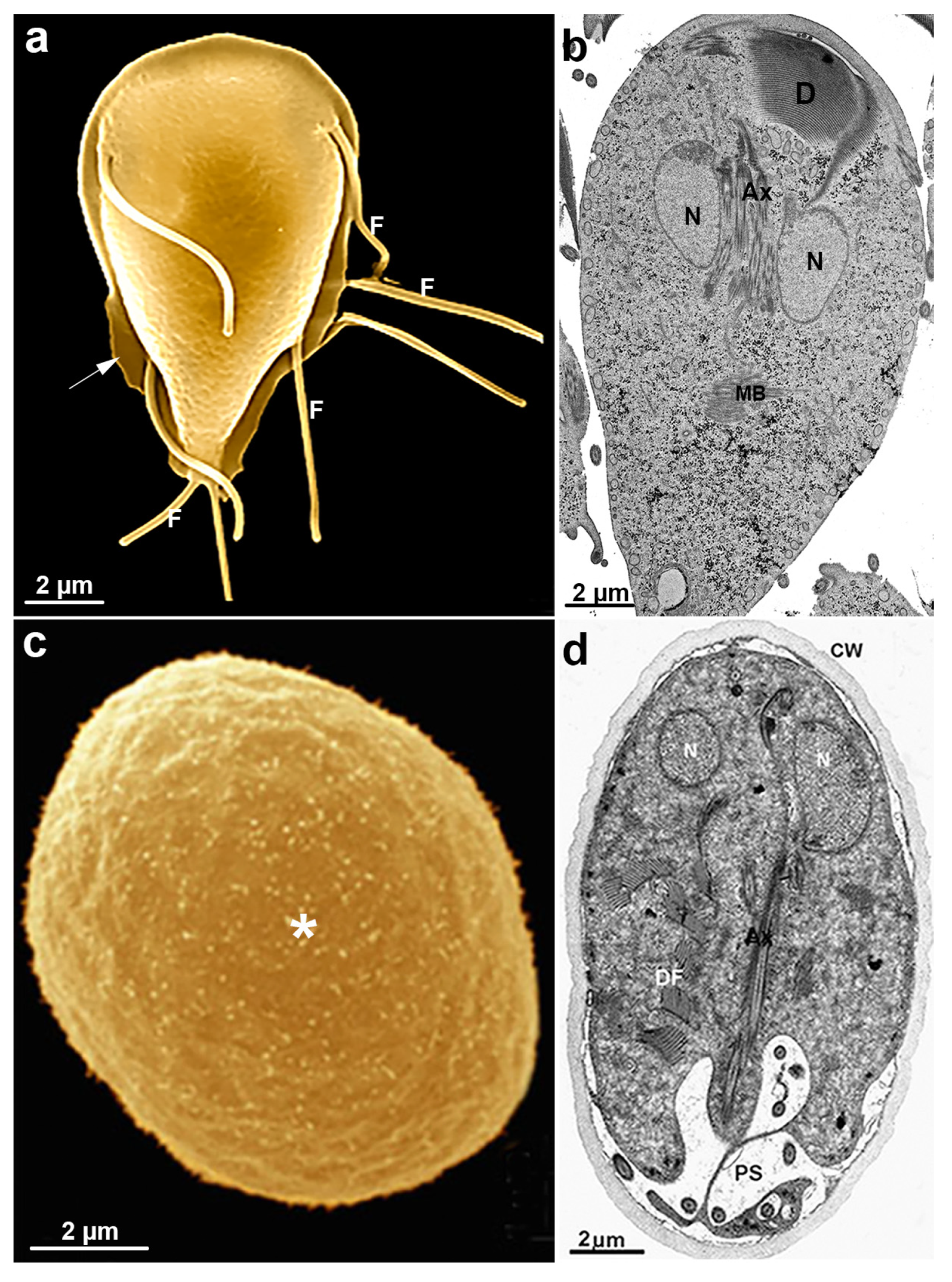
6. Giardia intestinalis
6.1. Giardia intestinalis Features
6.2. Giardia Cyst
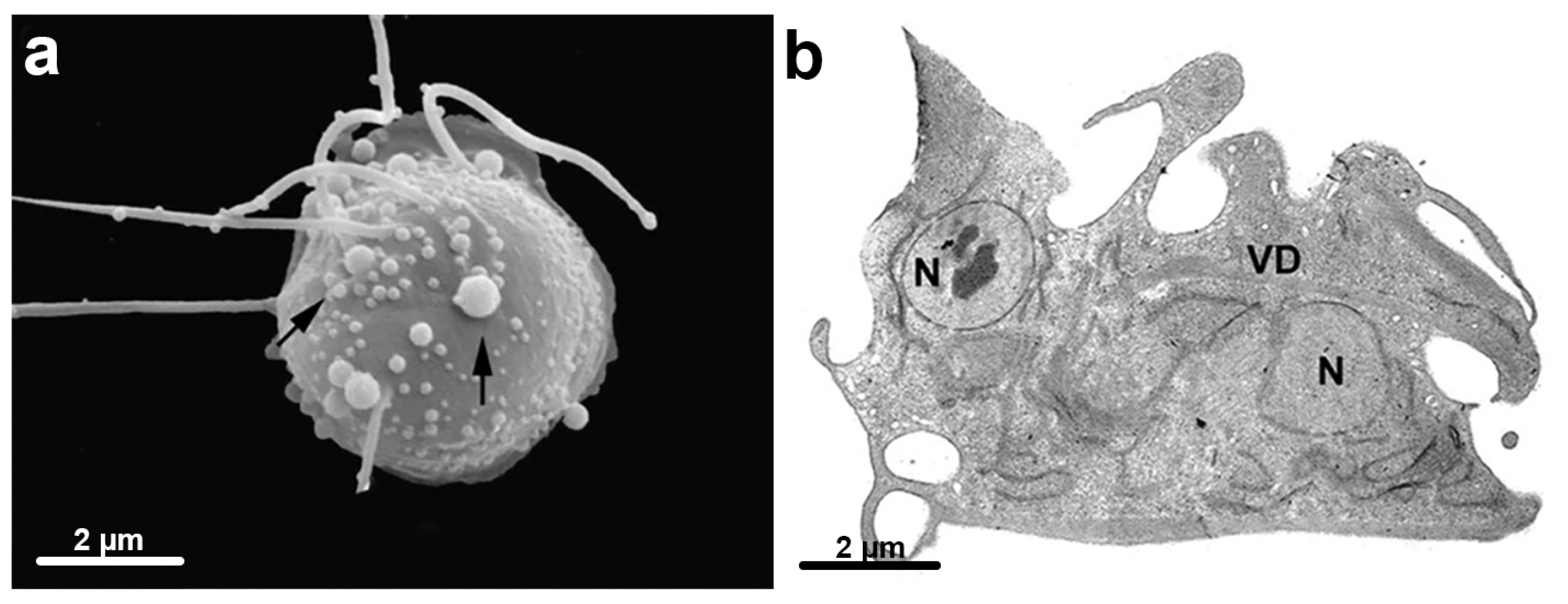
6.3. Giardia and Unusual Cell Structures
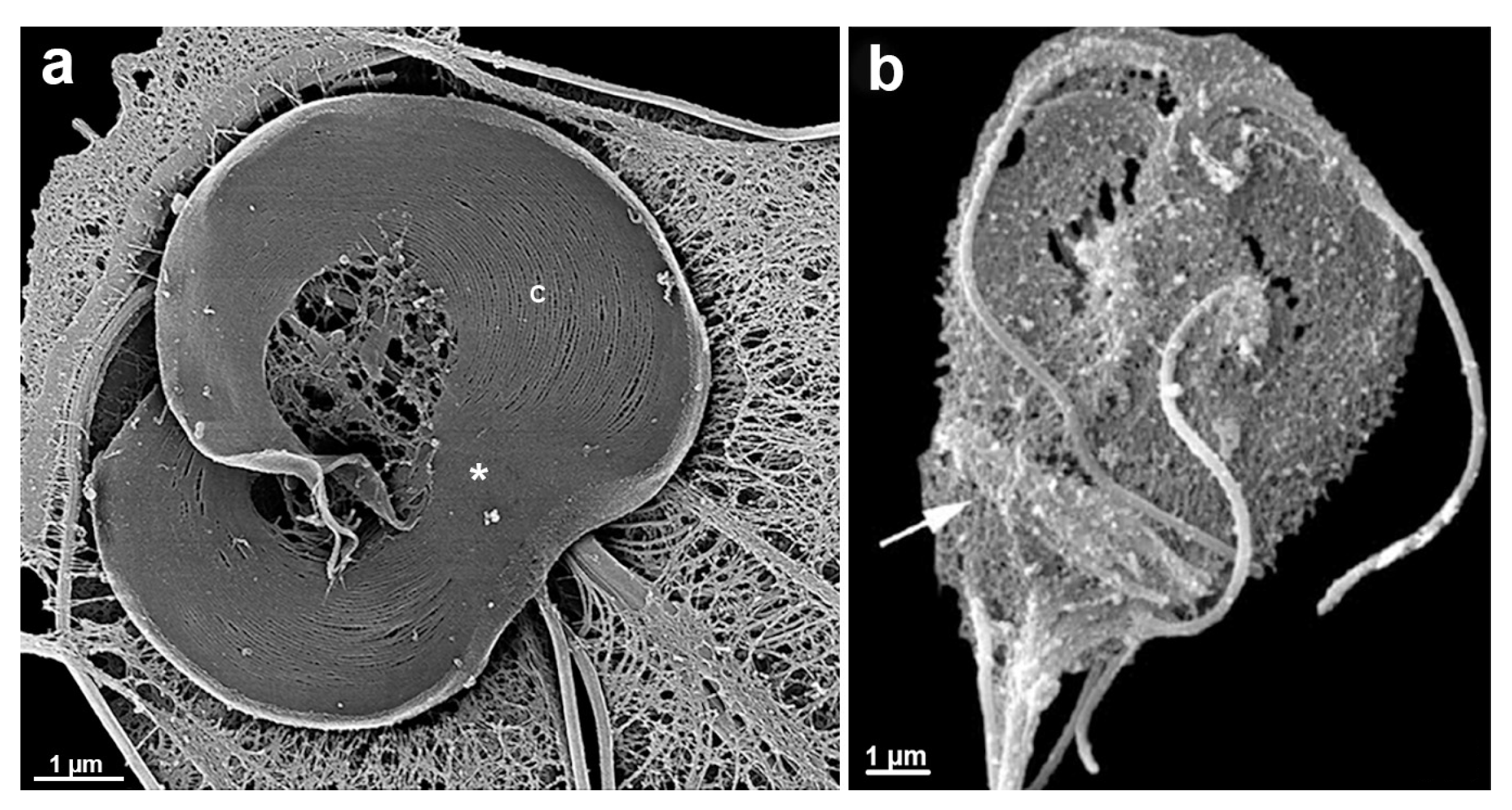
6.3.1. Ventral Disc
6.3.2. Median Body
6.3.3. Flagella
6.3.4. Mitosomes
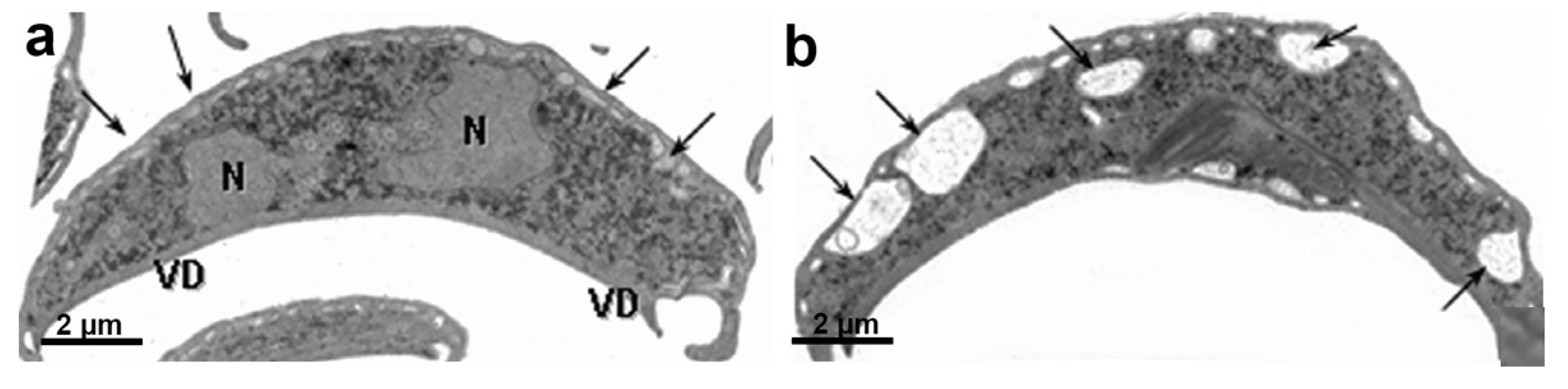
7. Endocytic and Exocytic System
8. Peroxisomes in Giardia
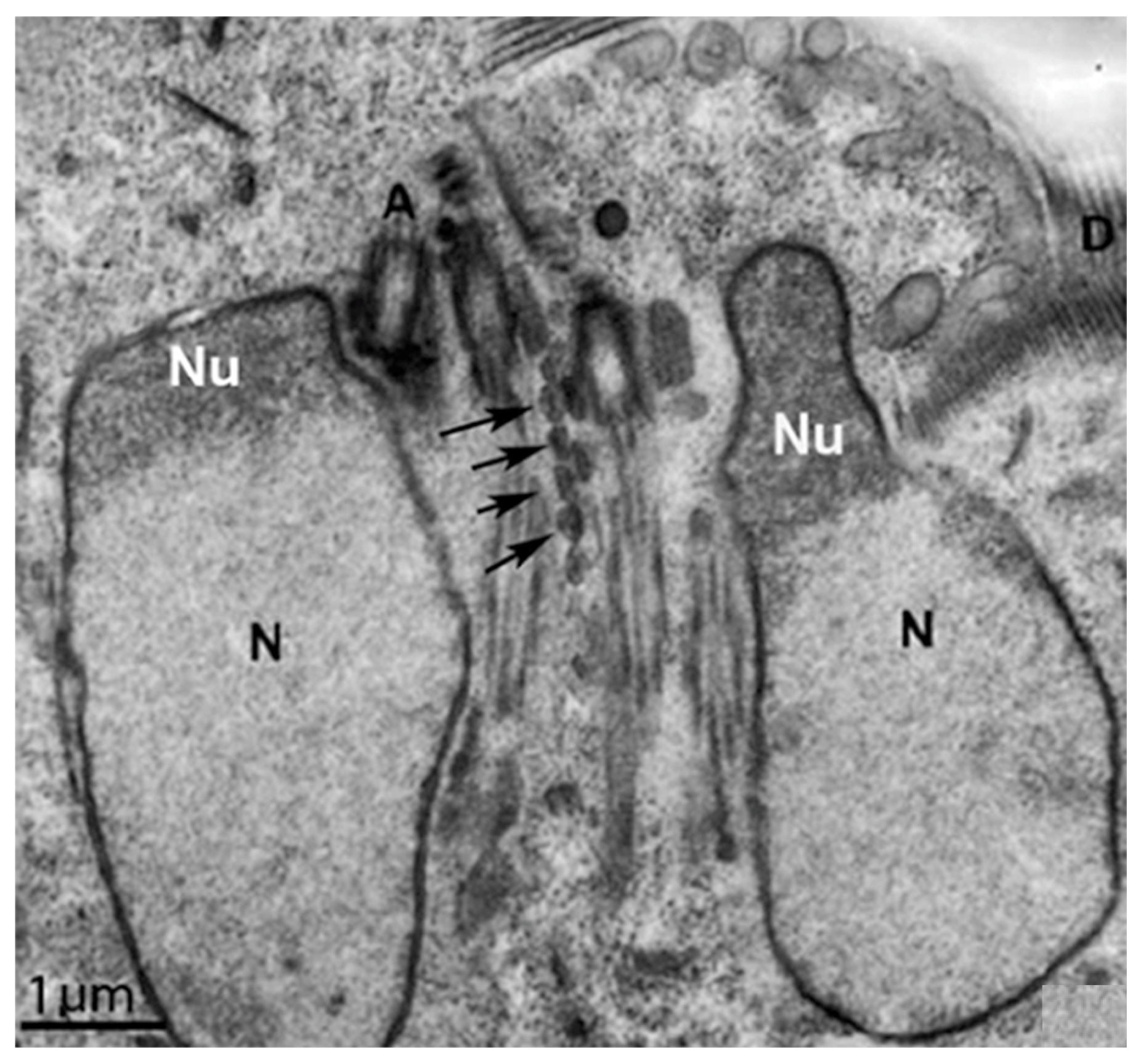
9. Nuclei
Mitosis
10. Drugs Affecting Giardia
10.1. Metronidazole, Albendazole and Nitazoxanide
10.2. Effects on Giardia Structures by Other Drugs (Table 2)
| Drug | Cell Structure Affected | Effect | Reference |
|---|---|---|---|
| Metronidazole | Nuclei Vacuoles | DNA damage; accumulation of cells in the G2 phase; myelin-like figures | [101,107] |
| Albendazole | Ventral disc Mitosis | ventral disc disruption; accumulation of cells in the G2 phase; | [109,110] |
| Mebendazole | Ventral disc | ventral disc disrupted | [110] |
| Nitazoxanide and CMC-20 | Plasma membrane Ventral disc | plasma membrane disruption; ventral disc fragmentation | [112] |
| Beta-lapachone | Nuclei Vacuoles Plasm membrane | chromatin condensation; vacuolization; membrane blebbing | [113] |
| [(10H-phenothiazin-10-yl)methyl]-N-hydroxybenzamide | Vacuoles Cytokinesis | Myelinic figures; multinucleated parasites | [116] |
| Furazolidone | Cytoplasm contents Vacuoles | depleted cytoplasm; lamellar structures | [107] |
| Bisphosphonates | Vacuoles | Myelinic figures | [118] |
| 24,25-(R,S)-epiminolanosterol | Vacuoles Peripheral Vesicles | Myelinic figures; peripheral vesicle increased | [117] |
| Nocodazole | Flagella Ventral disc Median Body | flagellar length increased; ventral disc disrupted; median body reduced | [119,120] |
| Colchicine | Ventral disc Mitosis | ventral disc disrupted; cytokinesis blocked | [120] |
| Taxol | Flagella Median Body | flagellar length decreased; median body increased | [120] |
| Oryzalin | Flagella | flagella bending | [121] |
| Polo-like kinases specific inhibitor | Flagella | Flagella length increased | [122] |
| Cytochalasin D | Flagella Cytokinesis Peripheral vesicles (endocytosis) | flagella internalization multiflagellated unshaped cells; ceramide absorption reduced | [125,131] |
| Pyrantel pamoate | Flagella Peripheral vesicles | flagella beating decreased peripheral vesicle increased | [124] |
| Small gatekeeper kinases and aurora kinases Kinase inhibitors | Cytokinesis | multinucleated parasites | [126,127] |
| 3-arylideneindolin-2-one-type sirtuin inhibitor | Nuclei Vacuoles | DNA fragmentation Myelin figures | [9] |
11. Ribosomes
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas-López, L.; Krakovka, S.; Einarsson, E.; Ribacke, U.; Xu, F.; Jerlström-Hultqvist, J.; Svärd, S.G. A detailed gene expression map of Giardia encystation. Genes 2021, 12, 1932. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M.; de Souza, W. Observation of Giardia sp. in the termite gut of Heterotermes tenuis. Parasitol. Res. 2021, 120, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Angulo, E.E.; Estrella-Hernandez, P.; Salgado-Lugo, H.; Ochoa-Leyva, A.; Gomez-Puyou, A.; Campos, S.S.; Montero-Moran, G.; Ortega-López, J.; Saab-Rincón, G.; Arroyo, R.; et al. Cellular and biochemical characterization of two closely related triosephosphate isomerases from Trichomonas vaginalis. Parasitology 2012, 139, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Cardoza, C.G.; Brieba, L.G.; Arroyo, R.; Rojo-Dominguez, A.M.; Vique-Sánchez, J.L. Triosephosphate isomerase as a therapeutic target Against trichomoniasis. Mol. Biochem. Parasitol. 2021, 246, 111413. [Google Scholar] [CrossRef]
- Ali, V.; Nozaki, T. Current therapeutics, their problems and sulfur-containing-amino-acid metabolism as a novel target against infections by amitochondriate protozoan parasites. Clin. Microbiol. Rev. 2007, 20, 164–187. [Google Scholar] [CrossRef] [Green Version]
- Santos, H.L.C.; Rebello, K.M. An overview of mucosa-associated protozoa: Challenges in chemotherapy and future perspectives. Front. Cell Infect. Microbiol. 2022. [CrossRef]
- Walters, H.Á.; Temesvari, L.A. Target acquired: Transcriptional regulators as drug targets for protozoan parasites. Int. J. Parasitol. 2021, 51, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Fleck, K.; Nitz, M.; Jeffers, V. Reading a new chapter in protozoan parasite transcriptional regulation. PLoS Path. 2021, 17, e1010056. [Google Scholar] [CrossRef]
- Gadelha, A.P.R.; Bravim, B.; Vidal, J.; Reignault, L.C.; Cosme, B.; Huber, K.; Bracher, F.; de Souza, W. Alterations on growth and cell organization of Giardia intestinalis trophozoites after treatment with KH-TFMDI, a novel class III histone deacetylase inhibitor. Int. J. Med. Microbiol. 2019, 309, 130–142. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Bazán-Tejeda, M.L.; Villa-Rosa, E.G.; Bermudez-Cruz, R.M. Nicotinamide induces G2 cell cycle arrest in Giardia duodenalis trophozoites and promotes changes in sirtuins transcriptional expression. Exp. Parasitol. 2019, 209, 107822. [Google Scholar] [CrossRef]
- World Health Organization. Report on Global Sexually-Transmitted Infection Surveillance; WHO: Geneva, Switzerland, 2018.
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutcliffe, S. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 939–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Pol, B.; Kwok, C.; Pierre-Louis, B.; Rinaldi, A.; Salata, R.A.; Chen, P.L.; van de Wijgert, J.; Mmiro, F.; Mugerwa, R.; Chipato, T.; et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J. Infect. Dis. 2008, 197, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Noel, J.C.; Fayt, I.; Romero Munoz, M.R.; Simon, P.; Engohan-Aloghe, C. High prevalence of high-risk human papillomavirus infection among women with Trichomonas vaginalis infection on monolayer cytology. Arch. Gynecol. Obstet. 2010, 282, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.S. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 1957, 43, 488–490. [Google Scholar] [CrossRef]
- Benchimol, M. The mastigont system. In Structures and Organelles in Pathogenic Protists; de Souza, W., Ed.; Springer: London, UK, 2010; Volume 1, pp. 1–26. [Google Scholar]
- Pereira-Neves, A.; Ribeiro, K.C.; Benchimol, M. Pseudocysts in trichomonads—New insights. Protist 2003, 154, 313–329. [Google Scholar] [CrossRef]
- Lehker, M.W.; Alderete, J.F. Resolution of six chromosomes of Trichomonas vaginalis and conservation of size and number among isolates. J. Parasitol. 1999, 85, 976–979. [Google Scholar] [CrossRef]
- Ribeiro, K.C.; Monteiro-Leal, L.H.; Benchimol, M. Contributions of the axostyle and flagella to closed mitosis in the protists Tritichomons foetus and Trichomonas vaginalis. J. Euk. Microbiol. 2000, 47, 481–492. [Google Scholar] [CrossRef]
- Benchimol, M.; Kachar, B.; De Souza, W. Surface domains in the pathogenic protozoan Tritrichomonas foetus. J. Protozool. 1992, 39, 480–484. [Google Scholar] [CrossRef]
- Benchimol, M.; Elias, C.A.; De Souza, W. Specializations in the flagellar membrane of Tritrichomonas foetus. J. Parasitol. 1981, 67, 174–178. [Google Scholar] [CrossRef]
- Benchimol, M.; Kachar, B.; de Souza, W. The structural organization of the pathogenic protozoan Tritrichomonas foetus as seen in replicas of quick frozen, freeze-fractured and deep etched cells. Biol. Cell 1993, 77, 289–295. [Google Scholar] [CrossRef]
- Viscogliosi, E.; Brugerolle, G. Striated fibers in Trichomonads: Costa proteins represent a new class of proteins forming striated roots. Cell Motil. Cytoskeleton 1994, 29, 82–93. [Google Scholar] [CrossRef]
- Benchimol, M. The hydrogenosome as a drug target. Curr. Pharm. Des. 2008, 14, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, M.B.; Brugerolle, G. Structure in Trichomonads Parasitic in Human; Honigberg, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 5–35. [Google Scholar]
- Honigberg, B.M.; Mattern, C.F.; Daniel, W.A. Fine structure of the mastigont system in Tritrichomonas foetus (Riedmüller). J. Protozool. 1971, 18, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M. Trichomonads under microscopy. Microsc Microanal. 2004, 10, 528–550. [Google Scholar] [CrossRef]
- de Andrade Rosa, I.; Caruso, M.B.; de Oliveira Santos, E.; Gonzaga, L.; Zingali, R.B.; de Vasconcelos, A.T.R.; de Souza, W.; Benchimol, M. The costa of trichomonads: A complex macromolecular cytoskeleton structure made of uncommon proteins. Biol. Cell. 2017, 109, 238–253. [Google Scholar] [CrossRef]
- De Andrade Rosa, I.; de Souza, W.; Benchimol, M. High-resolution scanning electron microscopy of the cytoskeleton of Tritrichomonas foetus. J. Struct. Biol. 2013, 183, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, P.T.; de Souza, W. Costain 1 (ARM19800.1)—The first identified protein of the costa of the pathogenic protozoan Tritrichomonas foetus. Exp. Parasitol. 2022, 232, 108177. [Google Scholar] [CrossRef]
- Chapman, A.; Hann, A.O.C.; Lindstead, D.; Lloyd, D. Energy dispersive X-ray microanalysis of membrane-associated inclusion in hydrogenosomes isolated from Trichomonas vaginalis. J. Gen. Microbiol. 1985, 131, 2933–2939. [Google Scholar] [CrossRef] [Green Version]
- De Souza, W.; Benchimol, M. Electron spectroscopic imaging of calcium in the hydrogenosomes of Tritrichomonas foetus. J. Submicrosc. Cytol. Pathol. 1988, 20, 619–621. [Google Scholar]
- Ribeiro, K.C.; Benchimol, M.; Farina, M. Contribution of cryofixation and freeze-substitution to analytical microscopy: A study of the Tritrichomonas foetus hydrogenosome. Microsc. Res. Tech. 2001, 53, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. The hydrogenosome. J. Gen. Microbiol. 1993, 139, 2879–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchimol, M.; Johnson, P.J.; De Souza, W. Morphogenesis of the hydrogenosome: An ultrastructural study. Biol. Cell 1996, 87, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Lahti, C.J.; Bradley, P.J. Biogenesis of the hydrogenosome: An unusual organelle in the anaerobic protist Trichomonas vaginalis. J. Parasitol. 1993, 79, 664–670. [Google Scholar] [CrossRef]
- Lindmark, D.G.; Müller, M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus and its role in pyruvate metabolism. J. Biol. Chem. 1973, 248, 7724–7728. [Google Scholar] [CrossRef]
- Clemens, D.L.; Johnson, P.J. Failure to detect DNA in hydrogenosomes of Trichomonas vaginalis by nick translation and immunomicroscopy. Mol. Biochem. Parasitol. 2000, 106, 307–313. [Google Scholar] [CrossRef]
- Hrdy, I.; Tachezy, J.; Muller, M. Metabolism of trichomonad hydrogenosomes. In Hydrogenosomes and Mitosomes: Mitochondria of Anaerobic Eukaryotes; Tachezy, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 114–145. [Google Scholar]
- de Andrade Rosa, I.; Einicker-Lamas, M.; Roney Bernardo, R.; Previatto, L.M.; Mohana-Borges, R.; Morgado-Díaz, J.A.; Benchimol, M. Cardiolipin in hydrogenosomes: Evidence of symbiotic origin. Eukaryot Cell. 2006, 5, 784–787. [Google Scholar] [CrossRef] [Green Version]
- Rada, P.; Doležal, P.; Jedelský, P.L.; Bursac, D.; Perry, A.J.; Šedinová, M.; Smíšková, K.; Novotný, M.; Beltrán, N.C.; Hrdý, I.; et al. The core components of organelle biogenesis and membrane transport in the hydrogenosomes of Trichomonas vaginalis. PLoS ONE 2011, 6, e24428. [Google Scholar] [CrossRef] [Green Version]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.M.; Besteiro, S.; et al. Draft Genome Sequence of the Sexually Transmitted Pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Tachezy, J.; Sanchez, L.B.; Muller, M. Mitochondrial type iron-sulfur cluster assembly in the amitochondriate eukaryotes Trichomonas vaginalis and Giardia intestinalis, as indicated by the phylogeny of IscS. Mol. Biol. Evol. 2001, 18, 1919–1928. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.E.; Brown, M.T.; Shiflett, A.M.; Dyall, S.D.; Hayes, R.D.; Xie, Y.; Loo, J.A.; Johnson, P.J. The Trichomonas vaginalis hydrogenosome proteome is highly reduced relative to mitochondria, yet complex compared with mitosomes. Int. J. Parasitol. 2011, 41, 1421–1434. [Google Scholar] [CrossRef]
- Tachezy, J.; Makki, A.; Hrdy, I. The hydrogenosome of Trichomonas vaginalis. J. Euk. Microbiol. 2022; early view. [Google Scholar] [CrossRef] [PubMed]
- Rosa Ide, A.; Rocha, D.A.; de Souza, W.; Urbina, J.A.; Benchimol, M. Ultrastructural alterations induced by Δ(24)-sterol methyltransferase inhibitors on Trichomonas vaginalis. FEMS Microbiol. Lett. 2011, 315, 72–78. [Google Scholar] [CrossRef]
- Lossick, J.G. Treatment of sexually transmitted vaginosis/vaginitis. Clin. Infect. Dis. 1990, 6, S665–S681. [Google Scholar] [CrossRef] [PubMed]
- Land, K.M.; Clemens, D.L.; Johnson, P.J. Loss of multiple hydrogenosomal proteins associated with organelle metabolism and high-level drug resistance in trichomonads. Exp. Parasitol. 2001, 97, 102–110. [Google Scholar] [CrossRef]
- Hrdý, I.; Cammack, R.; Stopka, P.; Kulda, J.; Tachezy, J. Alternative pathway of metronidazole activation in Trichomonas vaginalis hydrogenosomes. Antimicrob. Agents Chemother. 2005, 49, 5033–5036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Lindmark, D.G. Uptake of metronidazole and its effect on viability in trichomonads and Entamoeba invadens under anaerobic and aerobic conditions. Antimicrob. Agents Chemother. 1976, 9, 696–700. [Google Scholar] [CrossRef] [Green Version]
- Paunkov, A.; Sóki, J.; Leitsch, D. Modulation of iron import and Metronidazole resistance in Bacterioides fragilis. Front. Microbiol. 2022, 13, 898453. [Google Scholar] [CrossRef]
- Krakovka, S.; Ribacke, L.; Miyamoto, Y.; Eckmann, L.; Svard, S. Characterization of Metronidazole-resistant Giardia intestinalis lines by comparative transcriptomics and proteomics. Front. Microbiol. 2022, 13, 834008. [Google Scholar] [CrossRef]
- Huang, P.J.; Huang, C.Y.; Li, Y.X.; Liu, Y.C.; Chu, L.J.; Yeh, Y.M.; Cheng, W.H.; Chen, R.M.; Lee, C.C.; Chen, L.C.; et al. Dissecting the Transcriptomes of Multiple Metronidazole-Resistant and Sensitive Trichomonas vaginalis Strains Identified Distinct Genes and Pathways Associated with Drug Resistance and Cell Death. Biomedicines 2021, 9, 1817. [Google Scholar] [CrossRef]
- Narcisi, E.M.; Secor, W.E. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob. Agents Chemother. 1996, 40, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M. Hydrogenosome morphological variation induced by fibronectin and other drugs in Trichomonas vaginalis and Tritrichomonas foetus. Parasitol. Res. 2001, 87, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.A.; de Andrade Rosa, I.; de Souza, W.; Benchimol, M. Evaluation of the effect of miltefosine on Trichomonas vaginalis. Parasitol. Res. 2014, 113, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Coombs, G.H. Leishmaniasis-Current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003, 19, 502–508. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A.; Rai, M.; Prajapati, S.; Singh, A.K.; Ostyn, B.; Boelaert, M.; Dujardin, J.; Chakravarty, J. Efficacy of Miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infect. Dis. 2012, 55, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Magoulas, G.E.; Afroudakis, P.; Georgikopoulou, K.; Roussaki, M.; Borsari, C.; Fotopoulou, T.; Santarem, N.; Barrias, E.; Nevado, P.T.; Hachenberg, J.; et al. Design, synthesis, and anti-parasitic evaluation of click phospholipids. Molecules 2021, 26, 4204. [Google Scholar] [CrossRef]
- Midlej, V.; Rubim, F.; Villarreal, W.; Martins-Duarte, É.S.; Navarro, M.; de Souza, W.; Benchimol, M. Zinc-clotrimazole complexes are effective against Trichomonas vaginalis. Parasitology 2019, 146, 1206–1216. [Google Scholar] [CrossRef]
- Rocha, D.A.; de Andrade Rosa, I.; Urbina, J.A.; de Souza, W.; Benchimol, M. The effect of 3-(biphenyl-4-yl)-3-hydoxyquinuclidine (BPQ-OH) and Metronidazole on Trichomonas vaginalis: A comparative study. Parasitol. Res. 2014, 113, 2185–2197. [Google Scholar] [CrossRef]
- De Souza, T.G.; Benaim, G.; de Souza, W.; Benchimol, M. Effects of amiodarone, amioder, and dronedarone on Trichomonas vaginalis. Parasitol. Res. 2022, 121, 1761–1773. [Google Scholar] [CrossRef]
- Pereira-Neves, A.; Gonzaga, L.; Menna-Barreto, R.F.; Benchimol, M. Characterisation of 20S Proteasome in Tritrichomonas foetus and its role during the cell cycle and transformation into endoflagellar form. PLoS ONE 2015, 10, e0129165. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, A.J.; Bibo-Verdugo, B.; Miyamoto, Y.; Wang, S.C.; Yang, J.Z.; Zuill, D.E.; Matsuka, S.; Jiang, Z.; Almaliti, J.; Caffrey, C.R.; et al. 20S proteasome as drug target in Trichomonas vaginalis. Antimicrob. Agents Chemother. 2019, 63, e00448-19. [Google Scholar] [CrossRef]
- Pereira-Neves, A.; Menna-Barreto, R.F.; Benchimol, M. The fungal metabolite gliotoxin inhibits proteasome proteolytic activity and induces an irreversible pseudocystic transformation and cell death in Tritrichomonas foetus. Parasitol. Res. 2016, 115, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Vilela, R.; Menna-Barreto, R.F.; Benchimol, M. Methyl jasmonate induces cell death and loss of hydrogenosomal membrane potential in Trichomonas vaginalis. Parasitol. Int. 2010, 59, 387–393. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Fonseca-Berzal, C.; Martínez-Montiel, M.; Álvarez-Márquez, M.; Gómez-Núñez, M.; LacuevaArnedo, M.; Espinosa-Buitrago, T.; Martín-Pérez, T.; Escario, J.A.; Merino-Montiel, P.; et al. Thio-and selenosemicarbazones as antiprotozoal agents against Trypanosoma cruzi and Trichomonas vaginalis. J. Enzyme Inhibit Med. Chem. 2022, 37, 781–791. [Google Scholar] [CrossRef]
- Dixon, B.R. Giardia duodenalis in humans and animals—Transmission and disease. Res. Vet. Sci. 2021, 135, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Roger, A.J.; Svärd, S.G.; Tovar, J.; Clark, C.G.; Smith, M.W.; Gillin, F.D.; Sogin, M.L. A mitochondrial-like chaperonin 60 gene in Giardia lamblia: Evidence that diplomonads once harbored an endosymbiont related to the progenitor of mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovar, J.; León-Avila, G.; Sánchez, L.B.; Sutak, R.; Tachezy, J.; van der Giezen, M.; Hernández, M.; Müller, M.; Lucocq, J.M. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 2003, 426, 172–176. [Google Scholar] [CrossRef]
- He, D.; Dong, J.; Wen, J.; Xin, D.; Lu, S. Phylogenetic positions of several amitochondriate protozoa--evidence from phylogenetic analysis of DNA topoisomerase II. Sci. China C Life Sci. 2005, 48, 565–573. [Google Scholar] [CrossRef]
- Benchimol, M. The nuclei of Giardia lamblia -new ultrastructural observations. Arch. Microbiol. 2005, 183, 62–72. [Google Scholar] [CrossRef]
- Gadelha, A.P.R.; Benchimol, M.; de Souza, W. The structural organization of Giardia intestinalis cytoskeleton. Adv. Parasitol. 2020, 107, 1–23. [Google Scholar]
- Piva, B.; Benchimol, M. The median body of Giardia lamblia: An ultrastructural study. Biol. Cell 2004, 96, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Lanfredi-Rangel, A.; Attias, M.; Carvalho, T.M.U.; Kattenbach, W.M.; De Souza, W. The peripheral vesicles of trophozoites of the primitive protozoan Giardia lamblia may correspond to early and late endosomes and to lysosomes. J. Struct. Biol. 1998, 123, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Lanfredi-Rangel, A.; Kattenbach, W.M.; Diniz JAJr de Souza, W. Trophozoites of Giardia lamblia may have a Golgi-like structure. FEMS Microbiol. Lett. 1999, 181, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchimol, M.; Gadelha, A.P.R.; de Souza, W. Cell biology of the life cycle of Giardia intestinalis. In Lifecycles of Pathogenic Protists in Humans; de Souza, W., Ed.; Microbiology Monographs; Springer: Cham, Switzerland, 2022; Volume 35. [Google Scholar]
- Gadelha, A.P.R.; Benchimol, M.; de Souza, W. Nanoarchitecture of the ventral disc of Giardia intestinalis as revealed by high-resolution scanning electron microscopy and helium ion microscopy. Histochem. Cell Biol. 2022, 157, 251–265. [Google Scholar] [CrossRef]
- Gadelha, A.P.; Benchimol, M.; de Souza, W. Helium ion microscopy and ultra-high-resolution scanning electron microscopy analysis of membrane-extracted cells reveals novel characteristics of the cytoskeleton of Giardia intestinalis. J. Struct. Biol. 2015, 190, 271–278. [Google Scholar] [CrossRef]
- House, S.A.; Richter, D.J.; Pham, J.K.; Dawson, S.C. Giardia flagellar motility is not directly required to maintain attachment to surfaces. PLoS Pathog. 2011, 7, e1002167. [Google Scholar] [CrossRef] [Green Version]
- Benchimol, M. Participation of the adhesive disc during karyokinesis in Giardia lamblia. Biol. Cell 2004, 96, 291–301. [Google Scholar] [CrossRef]
- Brown, J.R.; Schwartz, C.L.; Heumann, J.M.; Dawson, S.C.; Hoenger, A. A detailed look at the cytoskeletal architecture of the Giardia lamblia ventral disc. J. Struct. Biol. 2016, 194, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Hagen, K.D.; Hirakawa, M.P.; House, S.A.; Schwartz, C.L.; Pham, J.K.; Cipriano, M.J.; De La Torre, M.J.; Sek, A.C.; Du, G.; Forsythe, B.M.; et al. Novel structural components of the ventral disc and lateral crest in Giardia intestinalis. PLoS Negl. Trop. Dis. 2011, 5, e1442. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, D.; Andrade, I.S.; Terra, L.L.; Guimarães, P.R.; Zingali, R.B.; de Souza, W. Proteomic analysis of the ventral disc of Giardia lamblia. BMC Res. Notes 2012, 19, 5–41. [Google Scholar] [CrossRef] [Green Version]
- Nosala, C.; Hagen, K.D.; Hilton, N.; Chase, T.M.; Jones, K.; Loudermilk, R.; Nguyen, K.; Dawson, S.C. Disc-associated proteins mediate the unusual hyperstability of the ventral disc in Giardia lamblia. J. Cell Sci. 2020, 133, jcs227355. [Google Scholar] [CrossRef]
- Campanati, L.; Holloshi, A.; Troester, H.; Spring, H.; Souza, W.; Monteiro-Leal, L.H. Video-Microscopy observations of fast dynamic process in the protozoon Giardia lamblia. Cell Motil. Cytoskeleton 2002, 51, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Lenaghan, S.C.; Davis, C.A.; Henson, W.R.; Zhang, Z.; Zhang, M. High-speed microscopic imaging of flagella motility and swimming in Giardia lamblia trophozoites. Proc. Natl. Acad. Sci. USA 2011, 108, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia-Brigagão, C.; Gadelha, A.P.; de Souza, W. New associated structures of the anterior flagella of Giardia duodenalis. Microsc. Microanal. 2013, 19, 1374–1376. [Google Scholar] [CrossRef]
- Benchimol, M.; Piva, B.; Campanati, L.; de Souza, W. Visualization of the funis of Giardia lamblia by high-resolution field emission scanning electron microscopy—New insights. J. Struct. Biol. 2004, 147, 102–115. [Google Scholar] [CrossRef]
- Tůmová, P.; Voleman, L.; Klingl, A.; Nohýnková, E.; Wanner, G.; Doležal, P. Inheritance of the reduced mitochondria of Giardia intestinalis is coupled to the flagellar maturation cycle. BMC Biol. 2021, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Jedelský, P.L.; Doležal, P.; Rada, P.; Pyrih, J.; Smíd, O.; Hrdý, I.; Sedinová, M.; Marcinčiková, M.; Voleman, L.; Perry, A.J.; et al. The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis. PLoS ONE 2011, 6, e17285. [Google Scholar] [CrossRef] [Green Version]
- Rout, S.; Zumthor, J.P.; Schraner, E.M.; Faso, C.; Hehl, A.B. An interactome-centered protein discovery approach reveals novel components involved in mitosome function and homeostasis in Giardia lamblia. PLoS Pathog. 2016, 12, e1006036. [Google Scholar] [CrossRef]
- Abodeely, M.; DuBois, K.N.; Hehl, A.; Stefanic, S.; Sajid, M.; De Souza, W.; Attias, M.; Engel, J.C.; Hsieh, I.; Fetter, R.D.; et al. A contiguous compartment functions as endoplasmic reticulum and endosome/lysosome in Giardia lamblia. Eukaryot. Cell 2009, 8, 1665–1676. [Google Scholar]
- Ward, W.; Alvarado, L.; Rawlings, N.D.; Engel, J.C.; Franklin, C.; McKerrow, J.H. A primitive enzyme for a primitive cell: The protease required for excystation of Giardia. Cell 1997, 89, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Midlej, V.; de Souza, W.; Benchimol, M. The peripheral vesicles gather multivesicular bodies with different behavior during the Giardia intestinalis life cycle. J. Struct. Biol. 2019, 207, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Virgen, K.; Chávez-Munguía, B.; Talamás-Lara, D.; Lagunes-Guillén, A.; Martínez-Higuera, A.; Lazcano, A.; Martínez-Palomo, A.; Espinosa-Cantellano, M. Giardia lamblia: Identification of peroxisomal-like proteins. Exp. Parasitol. 2018, 191, 36–43. [Google Scholar] [CrossRef]
- Bernander, R.; Palm, J.E.; Svärd, S.G. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell Microbiol. 2001, 3, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M. Behavior of the nuclear envelope in Giardia lamblia. Parasitol. Res. 2004, 94, 254–264. [Google Scholar] [CrossRef]
- Jiménez-García, L.F.; Zavala, G.; Chávez-Munguía, B.; Ramos-Godínez, M.P.; López-Velásques, G.; Segura-Valdez, M.L.; Montañez, C.; Hehl, A.B.; Argüello-García, R.; Ortega-Pierres, G. Identification of nucleoli in the early branching protist Giardia duodenalis. Int. J. Parasitol. 2008, 38, 1297–1304. [Google Scholar] [CrossRef]
- Uzlikova, M.; Nohynkova, E. The effect of Metronidazole on the cell cycle and DNA in metronidazole-susceptible and -resistant Giardia cell lines. Mol. Biochem. Parasitol. 2014, 198, 75–81. [Google Scholar] [CrossRef]
- Auriostigue-Bautista, J.C.; Hernández-Vázquez, E.; González-Calderón, D.; Figueroa-Romero, J.L.; Castillo-Villanueva, A.; Torres-Arroyo, A.; Ponce-Macotela, M.; Rufino-González, Y.; Martínez-Gordillo, M.; Miranda, L.D.; et al. Discovery of Benzopyrrolizidines as Promising Antigiardiasic Agents. Front. Cell. Infect. Microbiol. 2022, 11, 828100. [Google Scholar] [CrossRef]
- Sagolla, M.S.; Dawson, S.C.; Mancuso, J.J.; Cande, W.Z. Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. J. Cell Sci. 2006, 119, 4889–4900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchimol, M. Mitosis in Giardia lamblia: Multiple modes of cytokinesis. Protist 2004, 155, 33–44. [Google Scholar] [CrossRef]
- Saghaug, C.S.; Klotz, C.; Kallio, J.P.; Brattbakk, H.R.; Stokowy, T.; Aebischer, T.; Kursula, I.; Langeland, N.; Hanevik, K. Genetic variation in metronidazole metabolism and oxidative stress pathways in clinical Giardia lamblia assemblage A and B isolates. Infect. Drug Resist. 2019, 12, 1221–1235. [Google Scholar] [CrossRef] [Green Version]
- Riches, A.; Hart, C.J.S.; Trenholme, K.R.; Skinner-Adams, T.S. Anti-Giardia Drug Discovery: Current Status and Gut Feelings. J. Med. Chem. 2020, 63, 13330–13354. [Google Scholar] [CrossRef]
- Campanati, L.; Monteiro-Leal, L.H. The effects of the antiprotozoal drugs metronidazole and furazolidone on trophozoites of Giardia lamblia (P1 strain). Parasitol. Res. 2002, 88, 80–85. [Google Scholar] [CrossRef]
- MacDonald, L.M.; Armson, A.; Thompson, A.R.; Reynoldson, J.A. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol. Biochem. Parasitol. 2004, 138, 89–96. [Google Scholar] [CrossRef]
- Oxberry, M.E.; Thompson, R.C.; Reynoldson, J.A. Evaluation of the effects of Albendazole and Metronidazole on the ultrastructure of Giardia duodenalis, Trichomonas vaginalis and Spironucleus muris using transmission electron microscopy. Int. J. Parasitol. 1994, 24, 695–703. [Google Scholar] [CrossRef]
- Chavez, B.; Cedillo-Rivera, R.; Martinez-Palomo, A. Giardia lamblia: Ultrastructural study of the in vitro effect of benzimidazoles. J. Protozool. 1992, 39, 510–515. [Google Scholar] [CrossRef]
- Müller, J.; Wastling, J.; Sanderson, S.; Müller, N.; Hemphill, A. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 2007, 51, 1979–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matadamas-Martínez, F.; Castillo, R.; Hernández-Campos, A.; Méndez-Cuesta, C.; de Souza, W.; Gadelha, A.P.; Nogueda-Torres, B.; Hernández, J.M.; Yépez-Mulia, L. Proteomic and ultrastructural analysis of the effect of a new nitazoxanide-N-methyl-1H-benzimidazole hybrid against Giardia intestinalis. Res. Vet. Sci. 2016, 105, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pelissier, M.A.; Vasquez, N.; Balamurugan, R.; Pereira, E.; Dossou-Yovo, F.; Suau, A.; Pochart, P.; Magne, F. Metronidazole effects on microbiota and mucus layer thickness in the rat gut. FEMS Microbiol. Ecol. 2010, 73, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, G.; Vilela, R.; Menna-Barreto, R.F.; Midlej, V.; Benchimol, M. Cell death induction in Giardia lamblia: Effect of beta-lapachone and starvation. Parasitol. Int. 2009, 58, 424–437. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, F.; Palomo-Ligas, L.; Hernández-Hernández, J.M.; Pérez-Rangel, A.; Aguayo-Ortiz, R.; Hernández-Campos, A.; Castillo, R.; González-Pozos, S.; Cortés-Zárate, R.; Ramírez-Herrera, M.A.; et al. Curcumin alters the cytoskeleton and microtubule organization on trophozoites of Giardia lamblia. Acta Trop. 2017, 172, 113–121. [Google Scholar] [CrossRef]
- Oliveira, R.V.F.; de Souza, W.; Vögerl, K.; Bracher, F.; Benchimol, M.; Gadelha, A.P.R. In vitro effects of the 4-[(10H-phenothiazin-10-yl)methyl]-N-hydroxybenzamide on Giardia intestinalis trophozoites. Acta Trop. 2022, 232, 106484. [Google Scholar] [CrossRef]
- Maia, C.; Attias, M.; Urbina, J.; Gilbert, I.; Magaraci, F.; de Souza, W. Azasterols impair Giardia lamblia proliferation and induces encystation. Biochem. Biophys. Res. Commun. 2007, 363, 310–316. [Google Scholar] [CrossRef]
- Gadelha, A.P.R.; Brigagao, C.M.; da Silva, M.B.; Rodrigues, A.B.M.; Guimarães, A.C.R.; Paiva, F.; de Souza, W.; Henriques, C. Insights about the structure of farnesyl diphosphate synthase (FPPS) and the activity of bisphosphonates on the proliferation and ultrastructure of Leishmania and Giardia. Parasit. Vectors 2020, 13, 168. [Google Scholar] [CrossRef]
- Mariante, R.M.; Vancini, R.G.; Melo, A.L.; Benchimol, M. Giardia lamblia: Evaluation of the in vitro effects of nocodazole and colchicine on trophozoites. Exp. Parasitol. 2005, 11, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.C.; Sagolla, M.S.; Mancuso, J.J.; Woessner, D.J.; House, S.A.; Fritz-Laylin, L.; Cande, W.Z. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot. Cell. 2007, 6, 2354–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terra, L.L.; Campanati, L.; De Souza, W. Heterogeneity in the sensitivity of microtubules of Giardia lamblia to the herbicide oryzalin. Parasitol. Res. 2010, 107, 47–54. [Google Scholar] [CrossRef]
- Park, E.A.; Kim, J.; Shin, M.Y.; Park, S.J. A polo-like kinase modulates cytokinesis and flagella biogenesis in Giardia lamblia. Parasit. Vectors 2021, 14, 182. [Google Scholar] [CrossRef]
- Castillo-Romero, A.; Leon-Avila, G.; Perez Rangel, A.; Cortes Zarate, R.; Garcia Tovar, C.; Hernandez, J.M. Participation of actin on Giardia lamblia growth and encystation. PLoS ONE 2009, 4, e7156. [Google Scholar] [CrossRef] [Green Version]
- Campanati, L.; Gadelha, A.P.; Monteiro-Leal, L.H. Electron and video-light microscopy analysis of the in vitro effects of pyrantel pamoate on Giardia lamblia. Exp. Parasitol. 1999, 97, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, G.; Benchimol, M. Giardia lamblia behavior under cytochalasins treatment. Parasitol. Res. 2006, 98, 250–256. [Google Scholar] [CrossRef]
- Davids, B.J.; Williams, S.; Lauwaet, T.; Palanca, T.; Gillin, F.D. Giardia lamblia aurora kinase: A regulator of mitosis in a binucleate parasite. Int. J. Parasitol. 2008, 38, 353–369. [Google Scholar] [CrossRef]
- Hennessey, K.M.; Smith, T.R.; Xu, J.W.; Alas, G.C.; Ojo, K.K.; Merritt, E.A.; Paredez, A.R. Identification and Validation of Small-Gatekeeper Kinases as Drug Targets in Giardia lamblia. PLoS Negl. Trop. Dis. 2016, 10, e0005107. [Google Scholar] [CrossRef] [Green Version]
- Hardin, W.R.; Li, R.; Xu, J.; Shelton, A.M.; Alas, G.C.M.; Minin, V.N.; Paredez, A.R. Myosin-independent cytokinesis in Giardia utilizes flagella to coordinate force generation and direct membrane trafficking. Proc. Natl. Acad. Sci. USA 2017, 114, 5854–5863. [Google Scholar] [CrossRef]
- Paredez, A.R.; Assaf, Z.J.; Sept, D.; Timofejeva, L.; Dawson, S.C.; Wang, C.J.; Cande, W.Z. An actin cytoskeleton with evolutionarily conserved functions in the absence of canonical actin-binding proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 6151–6156. [Google Scholar] [CrossRef] [Green Version]
- Amador, E.; López-Pacheco, K.; Morales, N.; Coria, R.; López-Villaseñor, I. Characterization of cyclin-dependent kinases and Cdc2/Cdc28 kinase subunits in Trichomonas vaginalis. Parasitology 2017, 144, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Castillo, C.; Roychowdhury, S.; Hehl, A.; Aley, S.B.; Das, S. Clathrin-dependent pathways and the cytoskeleton network are involved in ceramide endocytosis by a parasitic protozoan, Giardia lamblia. Int. J. Parasitol. 2007, 37, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Kunz, S.; Balmer, V.; Sterk, G.J.; Pollastri, M.P.; Leurs, R.; Müller, N.; Hemphill, A.; Spycher, C. The single cyclic nucleotide-specific phosphodiesterase of the intestinal parasite Giardia lamblia represents a potential drug target. PLoS Negl. Trop. Dis. 2017, 11, e0005891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalle, M.; Camerini, S.; Cecchetti, S.; Finelli, R.; Sferra, G.; Müller, J.; Ricci, G.; Pozio, E. The FAD-dependent glycerol-3-phosphate dehydrogenase of Giardia duodenalis: An unconventional enzyme that interacts with the g14-3-3 and it is a target of the antitumoral compound NBDHEX. Front. Microbiol. 2015, 6, 544. [Google Scholar] [CrossRef] [Green Version]
- Hiregange, D.G.; Rivalta, A.; Bose, T.; Breiner-Goldstein, E.; Samiya, S.; Cimicata, G.; Kulakova, L.; Zimmerman, E.; Bashan, A.; Herzberg, O.; et al. Cryo-EM structure of the ancient eukaryotic ribosome from the human parasite Giardia lamblia. Nucleic Acids Res. 2022, 50, 1770–1782. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Yépez-Mulia, L.; González-Sánchez, I.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; Sainz-Espuñes, T.R.; Cerbón, M.A.; Rodríguez-Villar, K.; Rodríguez-Vicente, A.K.; Cortés-Gines, M.; et al. Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents. Molecules 2017, 22, 1864. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benchimol, M.; Gadelha, A.P.; de Souza, W. Unusual Cell Structures and Organelles in Giardia intestinalis and Trichomonas vaginalis Are Potential Drug Targets. Microorganisms 2022, 10, 2176. https://doi.org/10.3390/microorganisms10112176
Benchimol M, Gadelha AP, de Souza W. Unusual Cell Structures and Organelles in Giardia intestinalis and Trichomonas vaginalis Are Potential Drug Targets. Microorganisms. 2022; 10(11):2176. https://doi.org/10.3390/microorganisms10112176
Chicago/Turabian StyleBenchimol, Marlene, Ana Paula Gadelha, and Wanderley de Souza. 2022. "Unusual Cell Structures and Organelles in Giardia intestinalis and Trichomonas vaginalis Are Potential Drug Targets" Microorganisms 10, no. 11: 2176. https://doi.org/10.3390/microorganisms10112176
APA StyleBenchimol, M., Gadelha, A. P., & de Souza, W. (2022). Unusual Cell Structures and Organelles in Giardia intestinalis and Trichomonas vaginalis Are Potential Drug Targets. Microorganisms, 10(11), 2176. https://doi.org/10.3390/microorganisms10112176










