Candida albicans Promotes the Antimicrobial Tolerance of Escherichia coli in a Cross-Kingdom Dual-Species Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Escherichia coli Isolates
2.2. Biofilm Formation (E. coli Only)
2.3. Drug Susceptibility Testing of Planktonic Cells and Biofilms (E. coli Only)
2.4. Drug Susceptibility Testing of Biofilms (C. albicans Only)
2.5. Drug Susceptibility Testing of Dual-Species Biofilms (E. coli and C. albicans)
2.6. Effect of C. albicans Culture Supernatant on E. coli Biofilm Formation in the Presence of MEPM, CMZ, CTRX or LVFX
2.7. Treatment of Candida Culture Supernatant
2.8. Fractionation of Candida Supernatant
2.9. Statistical Analysis
3. Results
3.1. Biofilm Formation by E. coli Isolates
3.2. Drug Susceptibilities of E. coli Planktonic Cells and Biofilms
3.3. Drug Susceptibilities of Dual-Species Biofilms with E. coli and C. albicans
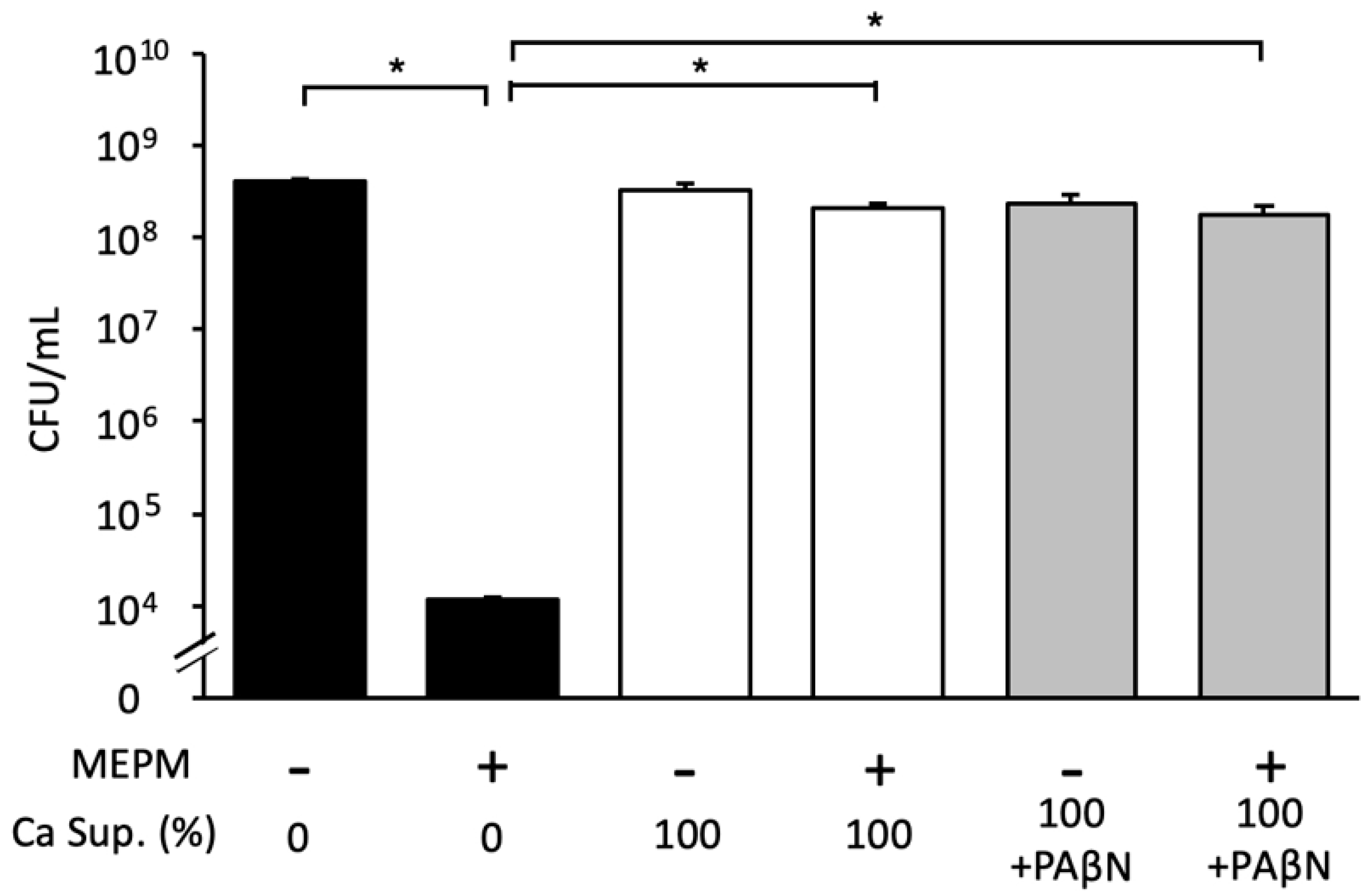
3.4. Effect of C. albicans Culture Supernatant on E. coli Biofilm Formation in the Presence of Antimicrobial Agents
3.5. Effects of Various Treatments on the Candida Culture Supernatant
3.6. Effect of Candida Culture Supernatant Fractions on E. coli Biofilm Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Gupta, V.; Gombar, S.; Chander, J.; Sahoo, T. Incidence, risk factors, microbiology of venous catheter associated bloodstream infections: A prospective study from a tertiary care hospital. Indian J. Med. Microbiol. 2015, 33, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug. Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Buonomo, A.; Pagano, M.C.; Alfonsi, L.; Foggia, M.; Mottola, M.; Marinosci, G.Z.; Contaldo, F.; Pasanisi, F. Central venous catheter-related bloodstream infections in adult patients on home parenteral nutrition: Prevalence, predictive factors, therapeutic outcome. Clin. Nutr. 2016, 35, 1394–1398. [Google Scholar] [CrossRef]
- Downes, K.J.; Metlay, J.P.; Bell, L.M.; McGowan, K.L.; Elliott, M.R.; Shah, S. Polymicrobial bloodstream infections among children and adolescents with central venous catheters evaluated in ambulatory care. Clin. Infect. Dis. 2008, 46, 387–394. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; de Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal biofilms and polymicrobial diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Pereira, R.; dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation, and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Mody, R.K.; Walker, T.; Chiller, T. The Global burden of fungal diseases. Infect. Dis. Clin. N. Am. 2016, 30, 1–11. [Google Scholar] [CrossRef]
- Bartlett, J.G. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar]
- Klotz, S.A.; Chasin, B.S.; Powell, B.; Gaur, N.K.; Lipke, P.N. Polymicrobial bloodstream infections involving Candida species: Analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 2007, 59, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Vihta, K.D.; Stoesser, N.; Llewelyn, M.J.; Quan, T.P.; Davies, T.; Fawcett, N.J.; Dunn, L.; Jeffery, K.; Butler, C.C.; Hayward, G.; et al. Trends over time in Escherichia coli bloodstream infections, urinary tract infections, and antibiotic susceptibilities in Oxfordshire, UK, 1998–2016: A study of electronic health records. Lancet Infect. Dis. 2018, 18, 1138–1149. [Google Scholar] [CrossRef]
- de Lastours, V.; Laouénan, C.; Royer, G.; Carbonnelle, E.; Lepeule, R.; Esposito-Farèse, M.; Clermont, O.; Duval, X.; Fantin, B.; Mentré, F.; et al. Mortality in Escherichia coli bloodstream infections: Antibiotic resistance still does not make it. J. Antimicrob. Chemother. 2020, 75, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Lefort, A.; Panhard, X.; Clermont, O.; Woerther, P.L.; Branger, C.; Mentré, F.; Fantin, B.; Wolff, M.; Denamur, E. Host factors and portal of entry outside bacterial determinants to predict the severity of Escherichia coli bacteremia. J. Clin. Microbiol. 2011, 49, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Schlackow, I.; Stoesser, N.; Walker, S.A.; Crook, D.W.; Peto, T.E.A.; Wyllie, D.H. The increasing incidence of Escherichia coli bacteremia is driven by an increase in antibiotic-resistant isolates: An electronic database study in Oxfordshire 1999–2011. J. Antimicrob. Chemother. 2012, 67, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, P.; Natarajan, V.; Sevanan, M. In Vitro biofilm formation by uropathogenic Escherichia coli and their antimicrobial susceptibility patterns. Asian Pac. J. Trop. Med. 2012, 5, 210–213. [Google Scholar] [CrossRef]
- Martínez, J.A.; Soto, S.; Fabrega, A.; Almela, M.; Mensa, J.; Soriano, A.; Marco, F.; Jimenez de Anta, M.T.; Vila, J. Relationship of phylogenetic background, biofilm production, and time to detection of growth in blood culture vials with clinical variables and prognosis associated with Escherichia coli bacteremia. J. Clin. Microbiol. 2006, 44, 1468–1474. [Google Scholar] [CrossRef]
- Abdel-Halim, M.S.; Askoura, M.; Mansour, B.; Yahya, G.; El-Ganiny, A.M. In Vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates. J. Antibiot. 2022, 22, 566. [Google Scholar] [CrossRef]
- Yahya, G.; Ebada, A.; Khalaf, E.M.; Mansour, B.; Nouh, N.A.; Mosbah, R.A.; Saber, S.; Moustafa, M.; Negm, S.; El-Sokkary, M.; et al. Soil-associated Bacillus Species: A reservoir of bioactive compounds with potential therapeutic activity against human pathogens. Microorganisms 2021, 9, 1131. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 2016, 7, e01365-16. [Google Scholar] [CrossRef]
- Lopez-Medina, E.; Fan, D.; Coughlin, L.A.; Ho, E.X.; Lamont, I.L.; Reimmann, C.; Hooper, L.V.; Koh, A.Y. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog. 2015, 11, e1005129. [Google Scholar] [CrossRef] [PubMed]
- Kostoulias, X.; Murray, G.L.; Cerqueira, G.M.; Kong, J.B.; Bantun, F.; Mylonakis, E.; Khoo, C.A.; Peleg, A.Y. Impact of a cross-kingdom signaling molecule of Candida albicans on Acinetobacter baumannii physiology. Antimicrob. Agents Chemother. 2015, 60, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- de Brucker, K.; Tan, Y.; Vints, K.; de Cremer, K.; Braem, A.; Verstraeten, N.; Michiels, J.; Vleugels, J.; Cammue, B.P.; Thevissen, K. Fungal β-1,3-Glucan increases ofloxacin tolerance of Escherichia coli in a polymicrobial E. coli/Candida albicans biofilm. Antimicrob. Agents Chemother. 2015, 59, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Farrokhi, Y.; Al-shibli, B.; Al-hameedawi, D.F.J.; Neshati, Z.; Makhdoumi, A. Escherichia coli enhances the virulence factors of Candida albicans, the cause of vulvovaginal candidiasis, in a dual bacterial/fungal biofilm. Res. Microbiol. 2021, 172, 103849. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Catlow, D.; di Maio, A.; Blair, J.M.A.; Hall, R.A. Candida albicans enhances meropenem tolerance of Pseudomonas aeruginosa in a dual-species biofilm. J. Antimicrob. Chemother. 2020, 75, 925–935. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S. Modulation of Staphylococcus aureus Candida albicans quorum sensing. Antimicrob. Agents Chemother. 2017, 61, 1–14. [Google Scholar] [CrossRef]
- Lobo, C.I.V.; Rinaldi, T.B.; Christiano, C.M.S.; De Sales Leite, L.; Barbugli, P.A.; Klein, M.I. Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J. Oral Microbiol. 2019, 20, 1581520. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Zarnowski, R.; Westler, W.M.; Lacmbouh, G.A.; Marita, J.M.; Bothe, J.R.; Bernhardt, J.; Sahraoui, A.L.H.; Fontainei, J.; Sanchez, H.; Hatfeld, R.D.; et al. Novel entries in the fungal biofilm matrix encyclopedia. mBio 2014, 5, e01333-14. [Google Scholar] [CrossRef]
- Wu, Y.; Outten, F.W. IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J. Bacteriol. 2009, 191, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation by Staphylococcus aureus. mBio 2012, 3, e00198-12. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sorribas, A.; Poilvache, H.; van Bambeke, F. Pharmacodynamics of moxifloxacin, meropenem, caspofungin, and their combinations against in vitro polymicrobial inter-kingdom biofilms. Antimicrob. Agents Chemother. 2022, 66, e0214921. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshima, S.; Kurakado, S.; Matsumoto, Y.; Kudo, T.; Sugita, T. Candida albicans Promotes the Antimicrobial Tolerance of Escherichia coli in a Cross-Kingdom Dual-Species Biofilm. Microorganisms 2022, 10, 2179. https://doi.org/10.3390/microorganisms10112179
Eshima S, Kurakado S, Matsumoto Y, Kudo T, Sugita T. Candida albicans Promotes the Antimicrobial Tolerance of Escherichia coli in a Cross-Kingdom Dual-Species Biofilm. Microorganisms. 2022; 10(11):2179. https://doi.org/10.3390/microorganisms10112179
Chicago/Turabian StyleEshima, Shintaro, Sanae Kurakado, Yasuhiko Matsumoto, Takayuki Kudo, and Takashi Sugita. 2022. "Candida albicans Promotes the Antimicrobial Tolerance of Escherichia coli in a Cross-Kingdom Dual-Species Biofilm" Microorganisms 10, no. 11: 2179. https://doi.org/10.3390/microorganisms10112179
APA StyleEshima, S., Kurakado, S., Matsumoto, Y., Kudo, T., & Sugita, T. (2022). Candida albicans Promotes the Antimicrobial Tolerance of Escherichia coli in a Cross-Kingdom Dual-Species Biofilm. Microorganisms, 10(11), 2179. https://doi.org/10.3390/microorganisms10112179





