Animal Models Used in Monkeypox Research
Abstract
1. Introduction
2. Reservoirs of MPXV
3. Animal Models Used to Study Infection Biology
3.1. Prairie Dog Model
3.2. Squirrel Model
3.3. Gambian Pouched Rat Model
3.4. Non-Human Primates
4. Virulence Factors of MPXVs
5. Animal Models Used in Vaccine Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sklenovská, N.; van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A Tale of Two Clades: Monkeypox Viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Damon, I.K. Outbreaks of Human Monkeypox after Cessation of Smallpox Vaccination. Trends Microbiol. 2012, 20, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human Monkeypox—After 40 Years, an Unintended Consequence of Smallpox Eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef]
- Nakoune, E.; Lampaert, E.; Ndjapou, S.G.; Janssens, C.; Zuniga, I.; Van Herp, M.; Fongbia, J.P.; Koyazegbe, T.D.; Selekon, B.; Komoyo, G.F.; et al. A Nosocomial Outbreak of Human Monkeypox in the Central African Republic. Open Forum Infect. Dis. 2017, 4, ofx168. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043. [Google Scholar] [CrossRef]
- Hutson, C.L.; Carroll, D.S.; Gallardo-Romero, N.; Drew, C.; Zaki, S.R.; Nagy, T.; Hughes, C.; Olson, V.A.; Sanders, J.; Patel, N.; et al. Comparison of Monkeypox Virus Clade Kinetics and Pathology within the Prairie Dog Animal Model Using a Serial Sacrifice Study Design. BioMed Res. Int. 2015, 2015, 965710. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Monkeypox; World Health Organization (WHO): Geneva, Switzerland, 2022.
- Hobson, G.; Adamson, J.; Adler, H.; Firth, R.; Gould, S.; Houlihan, C.; Johnson, C.; Porter, D.; Rampling, T.; Ratcliffe, L.; et al. Family Cluster of Three Cases of Monkeypox Imported from Nigeria to the United Kingdom, May 2021. Eurosurveillance 2021, 26, 2100745. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’Connor, C.; Dunning, J.; Ghebrehewet, S.; et al. Two Cases of Monkeypox Imported to the United Kingdom, September 2018. Eurosurveillance 2018, 23, 1800509. [Google Scholar] [CrossRef]
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I.; et al. Community Transmission of Monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 2022, 27, 2200422. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Mileto, D.; Riva, A.; Cutrera, M.; Moschese, D.; Mancon, A.; Meroni, L.; Giacomelli, A.; Bestetti, G.; Rizzardini, G.; Gismondo, M.R.; et al. New Challenges in Human Monkeypox Outside Africa: A Review and Case Report from Italy. Travel Med. Infect. Dis. 2022, 49, 102386. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Damon, I.K. Monkeypox Virus Infections in Small Animal Models for Evaluation of Anti-Poxvirus Agents. Viruses 2010, 2, 2763–2776. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-Like Disease in Cynomolgus Monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Arita, I.; Jezek, Z.; Ruti, K.; Khodakevich, L. Human Monkeypox: A Newly Emerged Orthopoxvirus Zoonosis in the Tropical Rain Forests of Africa. Am. J. Trop. Med. Hyg. 1985, 34, 781–789. [Google Scholar] [CrossRef]
- Gispen, R.; Brand-Saathof, B.B.; Hekker, A.C. Monkeypox-Specific Antibodies in Human and Simian Sera from the Ivory Coast and Nigeria. Bull. World Health Organ. 1976, 53, 355–360. [Google Scholar]
- Reynolds, M.G.; Doty, J.B.; McCollum, A.M.; Olson, V.A.; Nakazawa, Y. Monkeypox Re-Emergence in Africa: A Call to Expand the Concept and Practice of One Health. Expert Rev. Anti-infect. Ther. 2019, 17, 129–139. [Google Scholar] [CrossRef]
- Breman, J.G.; Bernadou, J.; Nakano, J.H. Poxvirus in West African Nonhuman Primates: Serological Survey Results. Bull. World Health Organ. 1977, 55, 605–612. [Google Scholar]
- Khodakevich, L.; Jezek, Z.; Kinzanzka, K. Isolation of Monkeypox Virus from Wild Squirrel Infected in Nature. Lancet 1986, 327, 98–99. [Google Scholar] [CrossRef]
- Khodakevich, L.; Szczeniowski, M.; Manbu-ma-Disu; Jezek, Z.; Marennikova, S.; Nakano, J.; Messinger, D. The Role of Squirrels in Sustaining Monkeypox Virus Transmission. Trop. Geogr. Med. 1987, 39, 115–122. [Google Scholar] [PubMed]
- Khodakevich, L.; Jezek, Z.; Messinger, D. Monkeypox Virus: Ecology and Public Health Significance. Bull. World Health Organ. 1988, 66, 747–752. [Google Scholar] [PubMed]
- Suu-Ire, R.; Karem, K.; Root, J.J.; Galley, J.; Carroll, D.S.; Abel, J.; Kwasi, M.O.; Damon, I.K.; Likos, A.; Olson, V.A.; et al. A Silent Enzootic of an Orthopoxvirus in Ghana, West Africa: Evidence for Multi-Species Involvement in the Absence of Widespread Human Disease. Am. J. Trop. Med. Hyg. 2010, 82, 746–754. [Google Scholar] [CrossRef]
- Hutson, C.L.; Lee, K.N.; Abel, J.; Carroll, D.S.; Montgomery, J.M.; Olson, V.A.; Li, Y.; Davidson, W.; Hughes, C.; Dillon, M.; et al. Monkeypox Zoonotic Associations: Insights from Laboratory Evaluation of Animals Associated with the Multi-State US Outbreak. Am. J. Trop. Med. Hyg. 2007, 76, 757–768. [Google Scholar] [CrossRef]
- Gispen, R.; Verlinde, J.D.; Zwart, P. Histopathological and Virological Studies on Monkeypox. Arch. Virusforsch. 1967, 21, 205–216. [Google Scholar] [CrossRef]
- Radonić, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A.; Mätz-Rensing, K.; Boesch, C.; Leendertz, F.H.; Nitsche, A. Fatal Monkeypox in Wild-Living Sooty Mangabey, Côte d’Ivoire, 2012. Emerg. Infect. Dis. 2014, 20, 1009–1011. [Google Scholar] [CrossRef]
- Hutin, Y.J.F.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef]
- Doty, J.; Malekani, J.; Kalemba, L.; Stanley, W.; Monroe, B.; Nakazawa, Y.; Mauldin, M.; Bakambana, T.; Liyandja, T.L.D.; Braden, Z.; et al. Assessing Monkeypox Virus Prevalence in Small Mammals at the Human–Animal Interface in the Democratic Republic of the Congo. Viruses 2017, 9, 283. [Google Scholar] [CrossRef]
- Guarner, J.; Johnson, B.J.; Paddock, C.D.; Shieh, W.-J.; Goldsmith, C.S.; Reynolds, M.G.; Damon, I.K.; Regnery, R.L.; Zaki, S.R.; the Veterinary Monkeypox Virus Working Group. Monkeypox Transmission and Pathogenesis in Prairie Dogs. Emerg. Infect. Dis. 2004, 10, 426–431. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC) Update: Multistate Outbreak of Monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb. Mortal Wkly. Rep. 2003, 52, 616–618.
- Khodakevich, L.; Szczeniowski, M.; Nambu-ma-Disu; Jezek, Z.; Marennikova, S.; Nakano, J.; Meier, F. Monkeypox Virus in Relation to the Ecological Features Surrounding Human Settlements in Bumba Zone, Zaire. Trop. Geogr. Med. 1987, 39, 56–63. [Google Scholar] [PubMed]
- Monroe, B.P.; Doty, J.B.; Moses, C.; Ibata, S.; Reynolds, M.; Carroll, D. Collection and Utilization of Animal Carcasses Associated with Zoonotic Disease in Tshuapa District, the Democratic Republic of the Congo, 2012. J. Wildl. Dis. 2015, 51, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Rimoin, A.W.; Alfonso, V.H.; Hoff, N.A.; Doshi, R.H.; Mulembakani, P.; Kisalu, N.K.; Muyembe, J.-J.; Okitolonda, E.W.; Wright, L.L. Human Exposure to Wild Animals in the Sankuru Province of the Democratic Republic of the Congo. EcoHealth 2017, 14, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Marennikova, S.S.; Seluhina, E.M. Susceptibility of Some Rodent Species to Monkeypox Virus, and Course of the Infection. Bull. World Health Organ. 1976, 53, 13–20. [Google Scholar]
- Hutson, C.L.; Gallardo-Romero, N.; Carroll, D.S.; Clemmons, C.; Salzer, J.S.; Nagy, T.; Hughes, C.M.; Olson, V.A.; Karem, K.L.; Damon, I.K. Transmissibility of the Monkeypox Virus Clades via Respiratory Transmission: Investigation Using the Prairie Dog-Monkeypox Virus Challenge System. PLoS ONE 2013, 8, e55488. [Google Scholar] [CrossRef]
- Falendysz, E.A.; Londoño-Navas, A.M.; Meteyer, C.U.; Pussini, N.; Lopera, J.G.; Osorio, J.E.; Rocke, T.E. Evaluation of Monkeypox Virus Infection of Black-Tailed Prairie Dogs (Cynomys ludovicianus) Using In Vivo Bioluminescent Imaging. J. Wildl. Dis. 2014, 50, 524–536. [Google Scholar] [CrossRef]
- Weiner, Z.P.; Salzer, J.S.; LeMasters, E.; Ellison, J.A.; Kondas, A.V.; Morgan, C.N.; Doty, J.B.; Martin, B.E.; Satheshkumar, P.S.; Olson, V.A.; et al. Characterization of Monkeypox Virus Dissemination in the Black-Tailed Prairie Dog (Cynomys Ludovicianus) through in Vivo Bioluminescent Imaging. PLoS ONE 2019, 14, e0222612. [Google Scholar] [CrossRef]
- Falendysz, E.A.; Lopera, J.G.; Doty, J.B.; Nakazawa, Y.; Crill, C.; Lorenzsonn, F.; Kalemba, L.N.; Ronderos, M.D.; Mejia, A.; Malekani, J.M.; et al. Characterization of Monkeypox Virus Infection in African Rope Squirrels (Funisciurus Sp.). PLoS Negl. Trop. Dis. 2017, 11, e0005809. [Google Scholar] [CrossRef]
- Tesh, R.B.; Watts, D.M.; Sbrana, E.; Siirin, M.; Popov, V.L.; Xiao, S.-Y. Experimental Infection of Ground Squirrels (Spermophilus Tridecemlineatus) with Monkeypox Virus. Emerg. Infect. Dis. 2004, 10, 1563–1567. [Google Scholar] [CrossRef]
- Hutson, C.L.; Nakazawa, Y.J.; Self, J.; Olson, V.A.; Regnery, R.L.; Braden, Z.; Weiss, S.; Malekani, J.; Jackson, E.; Tate, M.; et al. Laboratory Investigations of African Pouched Rats (Cricetomys gambianus) as a Potential Reservoir Host Species for Monkeypox Virus. PLoS Negl. Trop. Dis. 2015, 9, e0004013. [Google Scholar] [CrossRef] [PubMed]
- Falendysz, E.A.; Lopera, J.G.; Lorenzsonn, F.; Salzer, J.S.; Hutson, C.L.; Doty, J.; Gallardo-Romero, N.; Carroll, D.S.; Osorio, J.E.; Rocke, T.E. Further Assessment of Monkeypox Virus Infection in Gambian Pouched Rats (Cricetomys gambianus) Using In Vivo Bioluminescent Imaging. PLoS Negl. Trop. Dis. 2015, 9, e0004130. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.A.; Sagartz, J.E.; Huso, D.L.; Buller, R.M.L. Experimental Infection of an African Dormouse (Graphiurus kelleni) with Monkeypox Virus. Virology 2009, 383, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Abel, J.A.; Carroll, D.S.; Olson, V.A.; Braden, Z.H.; Hughes, C.M.; Dillon, M.; Hopkins, C.; Karem, K.L.; Damon, I.K.; et al. Comparison of West African and Congo Basin Monkeypox Viruses in BALB/c and C57BL/6 Mice. PLoS ONE 2010, 5, e8912. [Google Scholar] [CrossRef]
- Osorio, J.E.; Iams, K.P.; Meteyer, C.U.; Rocke, T.E. Comparison of Monkeypox Viruses Pathogenesis in Mice by In Vivo Imaging. PLoS ONE 2009, 4, e6592. [Google Scholar] [CrossRef]
- Parker, S.; Buller, R.M. A Review of Experimental and Natural Infections of Animals with Monkeypox Virus between 1958 and 2012. Future Virol. 2013, 8, 129–157. [Google Scholar] [CrossRef]
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Iizuka, I.; Shiota, T.; Sakai, K.; Ogata, M.; et al. Virulence and Pathophysiology of the Congo Basin and West African Strains of Monkeypox Virus in Non-Human Primates. J. Gen. Virol. 2009, 90, 2266–2271. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence Differences between Monkeypox Virus Isolates from West Africa and the Congo Basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Totmenin, A.V.; Safronov, P.F.; Mikheev, M.V.; Gutorov, V.V.; Ryazankina, O.I.; Petrov, N.A.; Babkin, I.V.; Uvarova, E.A.; Sandakhchiev, L.S.; et al. Analysis of the Monkeypox Virus Genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef]
- Gubser, C.; Hué, S.; Kellam, P.; Smith, G.L. Poxvirus Genomes: A Phylogenetic Analysis. J. Gen. Virol. 2004, 85, 105–117. [Google Scholar] [CrossRef]
- Estep, R.D.; Messaoudi, I.; O’Connor, M.A.; Li, H.; Sprague, J.; Barron, A.; Engelmann, F.; Yen, B.; Powers, M.F.; Jones, J.M.; et al. Deletion of the Monkeypox Virus Inhibitor of Complement Enzymes Locus Impacts the Adaptive Immune Response to Monkeypox Virus in a Nonhuman Primate Model of Infection. J. Virol. 2011, 85, 9527–9542. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.N.; Self, J.; Weiss, S.; Braden, Z.; Xiao, Y.; Girgis, N.M.; Emerson, G.; Hughes, C.; Sammons, S.A.; Isaacs, S.N.; et al. Elucidating the Role of the Complement Control Protein in Monkeypox Pathogenicity. PLoS ONE 2012, 7, e35086. [Google Scholar] [CrossRef] [PubMed]
- Kindrachuk, J.; Arsenault, R.; Kusalik, A.; Kindrachuk, K.N.; Trost, B.; Napper, S.; Jahrling, P.B.; Blaney, J.E. Systems Kinomics Demonstrates Congo Basin Monkeypox Virus Infection Selectively Modulates Host Cell Signaling Responses as Compared to West African Monkeypox Virus. Mol. Cell. Proteom. 2012, 11, M111.015701. [Google Scholar] [CrossRef]
- Weaver, J.R.; Isaacs, S.N. Monkeypox Virus and Insights into Its Immunomodulatory Proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. Orthopoxvirus Genes That Mediate Disease Virulence and Host Tropism. Adv. Virol. 2012, 2012, 524743. [Google Scholar] [CrossRef]
- Eaglesham, J.B.; Pan, Y.; Kupper, T.S.; Kranzusch, P.J. Viral and Metazoan Poxins Are CGAMP-Specific Nucleases That Restrict CGAS–STING Signalling. Nature 2019, 566, 259–263. [Google Scholar] [CrossRef]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox Virus Evades Antiviral CD4+ and CD8+ T Cell Responses by Suppressing Cognate T Cell Activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Rahman, M.M.; Lanchbury, J.S.; Shattuck, D.; Neff, C.; Dufford, M.; van Buuren, N.; Fagan, K.; Barry, M.; Smith, S.; et al. Proteomic Screening of Variola Virus Reveals a Unique NF-ΚB Inhibitor That Is Highly Conserved among Pathogenic Orthopoxviruses. Proc. Natl. Acad. Sci. USA 2009, 106, 9045–9050. [Google Scholar] [CrossRef]
- Song, H.; Josleyn, N.; Janosko, K.; Skinner, J.; Reeves, R.K.; Cohen, M.; Jett, C.; Johnson, R.; Blaney, J.E.; Bollinger, L.; et al. Monkeypox Virus Infection of Rhesus Macaques Induces Massive Expansion of Natural Killer Cells but Suppresses Natural Killer Cell Functions. PLoS ONE 2013, 8, e77804. [Google Scholar] [CrossRef]
- Zaucha, G.M.; Jahrling, P.B.; Geisbert, T.W.; Swearengen, J.R.; Hensley, L. The Pathology of Experimental Aerosolized Monkeypox Virus Infection in Cynomolgus Monkeys (Macaca fascicularis). Lab. Investig. 2001, 81, 1581–1600. [Google Scholar] [CrossRef]
- Alkhalil, A.; Hammamieh, R.; Hardick, J.; Ichou, M.A.; Jett, M.; Ibrahim, S. Gene Expression Profiling of Monkeypox Virus-Infected Cells Reveals Novel Interfaces for Host-Virus Interactions. Virol. J. 2010, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.K.; Leung, M.K.; Hauhart, R.; Buller, R.M.L.; Bertram, P.; Wang, X.; Rosengard, A.M.; Kotwal, G.J.; Atkinson, J.P. Structure and Regulatory Profile of the Monkeypox Inhibitor of Complement: Comparison to Homologs in Vaccinia and Variola and Evidence for Dimer Formation. J. Immunol. 2006, 176, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Cann, J.A.; Jahrling, P.B.; Hensley, L.E.; Wahl-Jensen, V. Comparative Pathology of Smallpox and Monkeypox in Man and Macaques. J. Comp. Pathol. 2013, 148, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Mucker, E.M.; Golden, J.W.; Hammerbeck, C.D.; Kishimori, J.M.; Royals, M.; Joselyn, M.D.; Ballantyne, J.; Nalca, A.; Hooper, J.W. A Nucleic Acid-Based Orthopoxvirus Vaccine Targeting the Vaccinia Virus L1, A27, B5, and A33 Proteins Protects Rabbits against Lethal Rabbitpox Virus Aerosol Challenge. J. Virol. 2022, 96, e01504-21. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.C.; Kaufman, D.M. Anticipating Smallpox and Monkeypox Outbreaks: Complications of the Smallpox Vaccine. Neurologist 2004, 10, 265–274. [Google Scholar] [CrossRef]
- Rezza, G. Emergence of Human Monkeypox in West Africa. Lancet Infect. Dis. 2019, 19, 797–799. [Google Scholar] [CrossRef]
- Parrino, J.; Graham, B. Smallpox Vaccines: Past, Present, and Future. J. Allergy Clin. Immunol. 2006, 118, 1320–1326. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A.; et al. Immunogenicity of a Highly Attenuated MVA Smallpox Vaccine and Protection against Monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; di Pilato, M.; Esteban, M. The Evolution of Poxvirus Vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef]
- Nalca, A.; Zumbrun, E.E. ACAM2000™: The New Smallpox Vaccine for United States Strategic National Stockpile. DDDT 2010, 2010, 71–79. [Google Scholar] [CrossRef]
- Meyer, H.; Ehmann, R.; Smith, G.L. Smallpox in the Post-Eradication Era. Viruses 2020, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Sakiyama, T.; Hasegawa, H.; Saijo, M.; Maeda, A.; Kurane, I.; Maeno, G.; Kimura, J.; Hirama, C.; Yoshida, T.; et al. An Attenuated LC16m8 Smallpox Vaccine: Analysis of Full-Genome Sequence and Induction of Immune Protection. J. Virol. 2005, 79, 11873–11891. [Google Scholar] [CrossRef]
- Eto, A.; Saito, T.; Yokote, H.; Kurane, I.; Kanatani, Y. Recent Advances in the Study of Live Attenuated Cell-Cultured Smallpox Vaccine LC16m8. Vaccine 2015, 33, 6106–6111. [Google Scholar] [CrossRef]
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Ogata, M.; Fukushi, S.; Mizutani, T.; Sata, T.; et al. LC16m8, a Highly Attenuated Vaccinia Virus Vaccine Lacking Expression of the Membrane Protein B5R, Protects Monkeys from Monkeypox. J. Virol. 2006, 80, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.N.; Cecchinato, V.; Andresen, V.; Heraud, J.-M.; Hryniewicz, A.; Parks, R.W.; Venzon, D.; Chung, H.; Karpova, T.; McNally, J.; et al. Smallpox Vaccine Safety Is Dependent on T Cells and Not B Cells. J. Infect. Dis. 2011, 203, 1043–1053. [Google Scholar] [CrossRef]
- Iizuka, I.; Ami, Y.; Suzaki, Y.; Nagata, N.; Fukushi, S.; Ogata, M.; Morikawa, S.; Hasegawa, H.; Mizuguchi, M.; Kurane, I.; et al. A Single Vaccination of Nonhuman Primates with Highly Attenuated Smallpox Vaccine, LC16m8, Provides Long-Term Protection against Monkeypox. Jpn. J. Infect. Dis. 2017, 70, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.W.; Kabamba, J.; McCollum, A.M.; Lushima, R.S.; Wemakoy, E.O.; Tamfum, J.-J.M.; Nguete, B.; Hughes, C.M.; Monroe, B.P.; Reynolds, M.G. Vaccinating against Monkeypox in the Democratic Republic of the Congo. Antivir. Res. 2019, 162, 171–177. [Google Scholar] [CrossRef]
- Stittelaar, K.J.; Kuiken, T.; de Swart, R.L.; van Amerongen, G.; Vos, H.W.; Niesters, H.G.M.; van Schalkwijk, P.; van der Kwast, T.; Wyatt, L.S.; Moss, B.; et al. Safety of Modified Vaccinia Virus Ankara (MVA) in Immune-Suppressed Macaques. Vaccine 2001, 19, 3700–3709. [Google Scholar] [CrossRef]
- Stittelaar, K.J.; van Amerongen, G.; Kondova, I.; Kuiken, T.; van Lavieren, R.F.; Pistoor, F.H.M.; Niesters, H.G.M.; van Doornum, G.; van der Zeijst, B.A.M.; Mateo, L.; et al. Modified Vaccinia Virus Ankara Protects Macaques against Respiratory Challenge with Monkeypox Virus. J. Virol. 2005, 79, 7845–7851. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox Vaccine–Induced Antibodies Are Necessary and Sufficient for Protection against Monkeypox Virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Bray, M.; Whitehouse, C.A.; Miller, D.; Mucker, E.; Manischewitz, J.; King, L.R.; Robert-Guroff, M.; Hryniewicz, A.; Venzon, D.; et al. Smallpox Vaccine Does Not Protect Macaques with AIDS from a Lethal Monkeypox Virus Challenge. J. Infect. Dis. 2005, 191, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxviridae. In Fields of Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Hooper, J.W.; Thompson, E.; Wilhelmsen, C.; Zimmerman, M.; Ichou, M.A.; Steffen, S.E.; Schmaljohn, C.S.; Schmaljohn, A.L.; Jahrling, P.B. Smallpox DNA Vaccine Protects Nonhuman Primates against Lethal Monkeypox. J. Virol. 2004, 78, 4433–4443. [Google Scholar] [CrossRef]
- Golden, J.W.; Josleyn, M.; Mucker, E.M.; Hung, C.-F.; Loudon, P.T.; Wu, T.C.; Hooper, J.W. Side-by-Side Comparison of Gene-Based Smallpox Vaccine with MVA in Nonhuman Primates. PLoS ONE 2012, 7, e42353. [Google Scholar] [CrossRef]
- Hirao, L.A.; Draghia-Akli, R.; Prigge, J.T.; Yang, M.; Satishchandran, A.; Wu, L.; Hammarlund, E.; Khan, A.S.; Babas, T.; Rhodes, L.; et al. Multivalent Smallpox DNA Vaccine Delivered by Intradermal Electroporation Drives Protective Immunity in Nonhuman Primates Against Lethal Monkeypox Challenge. J. Infect. Dis. 2011, 203, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, R.J.; Smedley, J.V.; Perera, P.-Y.; Silvera, P.M.; Waldmann, T.A.; Capala, J.; Perera, L.P. Smallpox Vaccine with Integrated IL-15 Demonstrates Enhanced in Vivo Viral Clearance in Immunodeficient Mice and Confers Long Term Protection against a Lethal Monkeypox Challenge in Cynomolgus Monkeys. Vaccine 2010, 28, 7081–7091. [Google Scholar] [CrossRef] [PubMed]
- Heraud, J.-M.; Edghill-Smith, Y.; Ayala, V.; Kalisz, I.; Parrino, J.; Kalyanaraman, V.S.; Manischewitz, J.; King, L.R.; Hryniewicz, A.; Trindade, C.J.; et al. Subunit Recombinant Vaccine Protects against Monkeypox. J. Immunol. 2006, 177, 2552–2564. [Google Scholar] [CrossRef]
- Buchman, G.W.; Cohen, M.E.; Xiao, Y.; Richardson-Harman, N.; Silvera, P.; DeTolla, L.J.; Davis, H.L.; Eisenberg, R.J.; Cohen, G.H.; Isaacs, S.N. A Protein-Based Smallpox Vaccine Protects Non-Human Primates from a Lethal Monkeypox Virus Challenge. Vaccine 2010, 28, 6627–6636. [Google Scholar] [CrossRef]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; le Pluart, D.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of Human-to-Dog Transmission of Monkeypox Virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
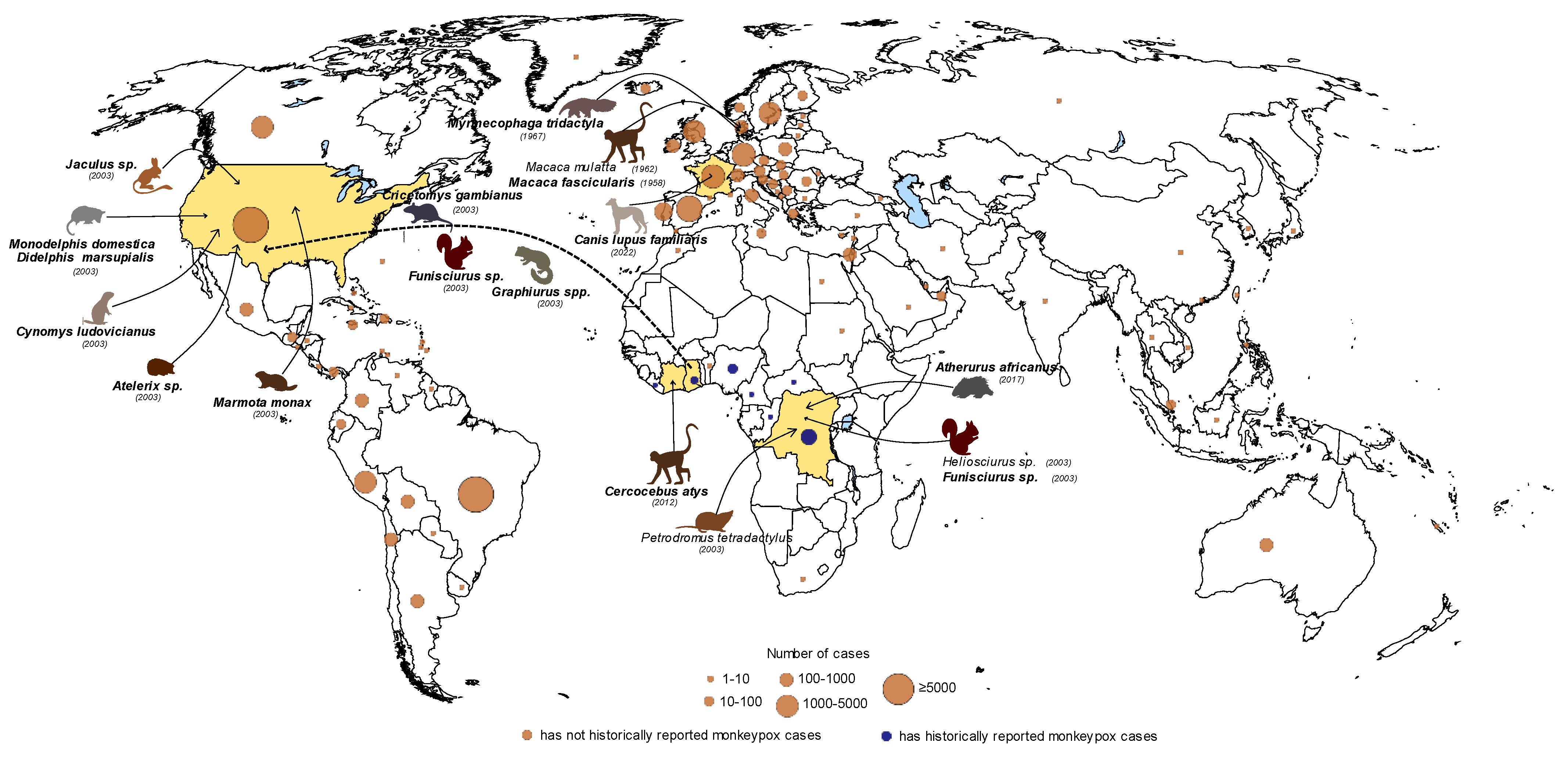
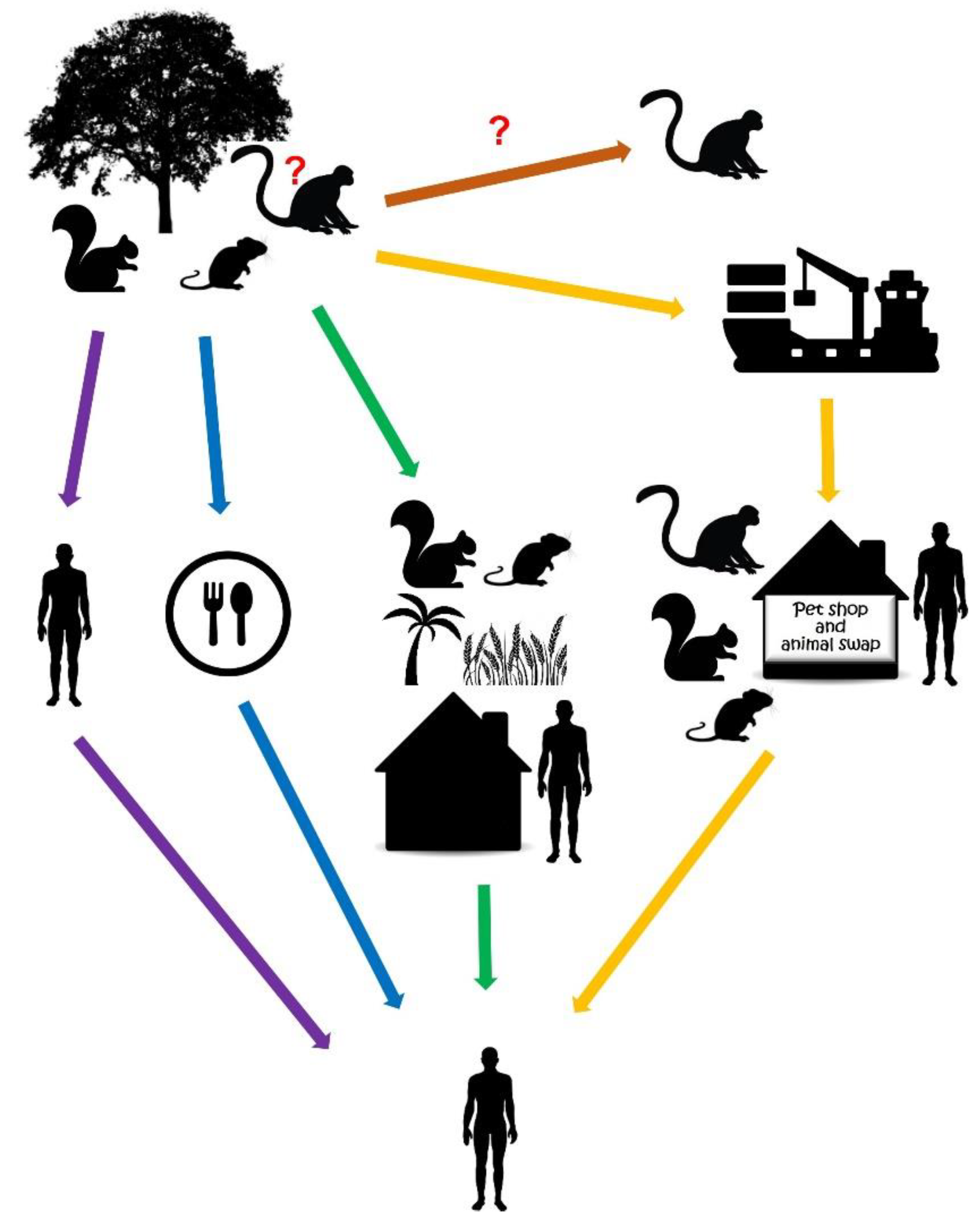
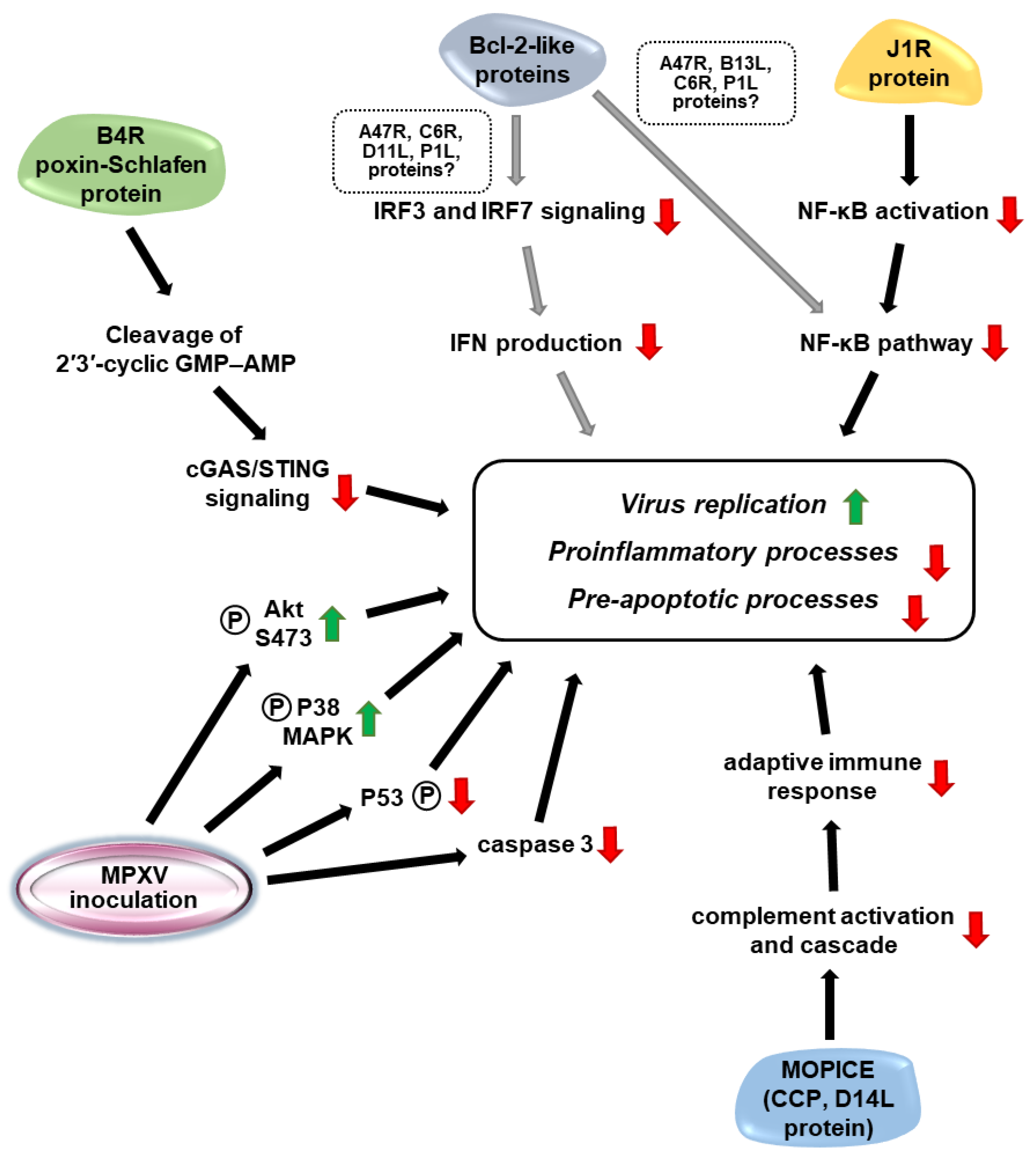
| Animal Model | Inoculation Route | Infection Dose | Clinical Signs | Gross Pathology | Viral Shedding (Viral Titer/mL) | References |
|---|---|---|---|---|---|---|
| prairie dog | intranasal | 1 × 108 pfu CB or WA strain | disseminated cutaneous lesions, inappetence, nasal discharge | lymphadenopathy, inflamed oviducts, hemorrhagic foci of adipose tissue and lungs | 7.8 × 107 pfu (WA MPXV) 2.3 × 108 pfu (CB MPXV) | Hutson et al. 2015 [7] |
| intranasal | 6 × 103 pfu WA | lesions, crusty noses, dehydration and inappetence | not examined | 2 × 105–1 × 106 pfu | Hutson et al. 2013 [37] | |
| intranasal | high dose 5 × 103 pfu CB | inappetence, dehydration, nasal congestion, labored breathing, facial edema, swollen paws | not examined extreme morbidity | 2 × 107–6 × 107 pfu | ||
| intranasal | low dose 7 × 102 pfu CB strain | skin lesions, inappetence, labored breathing | not examined | 1.2 × 104–7.8 × 104 pfu | ||
| intranasal | 104 pfu WA strain | maculopapular skin lesions distended abdomen, diarrhea, ocular discharge, weight loss | subacute, necrotizing dermatitis, severe acute necrosis of lymphoid tissue and fibrinoid necrosis of blood vessels in the thymus and tonsil, multifocal lymphoplasmacytic interstitial pneumonia | 5 × 105–4 × 107 pfu | Falendysz et al. 2014 [38] | |
| intranasal | 4.3–5.9 × 104 pfu WA strain | skin lesions, inappetence, mild nasal discharge | not examined | 1.2 × 106–1.7 × 109 pfu | Weiner et al. 2019 [39] | |
| rope squirrel | intranasal or intradermal | 1 × 106 pfu CB strain | ID and IN group: skin and oral lesions, nasal discharge, lethargy only in IN group: severe respiratory disease | not examined | up to 1.34 × 107 pfu | Falendysz et al. 2017 [40] |
| ground squirrel | intraperitoneal or intranasal | 105 or 106 pfu WA strain | lethargy | IP group: centrilobular hepatocytic degeneration or necrosis in the liver, moderate-to- marked necrosis of the spleen IN group: multifocal steatosis of the liver, diffuse hepatocytic necrosis, moderate-to- severe necrosis of the spleen | not examined | Tesh et al. 2004 [41] |
| Gambian pouched rat | scarification | 4 × 104 pfu WA or CB strain | skin and tongue lesions, lesions near eyes, lethargy, weight loss, hypopigmentation | not examined | inoculation site: 108 pfu oral and nasal shedding: 105 pfu (WA) and 107 pfu (CB) | Hutson et al. 2015 [42] |
| intradermal or intranasal | 106 pfu CB strain | ID group: weight loss, skin lesions, vesicles on the tongue, necrosis of the gingiva, lethargy IN group: no clinical signs | not examined | up to 1.85 × 106 pfu | Falendysz et al. 2015 [43] | |
| dormouse | intranasal | 2 × 104 CB strain | dehydration, conjunctivitis | upper gastrointestinal hemorrhage, hepatomegaly, lymphadenopathy, lymphoid necrosis in the submandibular lymph nodes, spleen and thymus, hepatocellular necrosis in the liver | ~ 105 pfu | Schultz et al. 2009 [44] |
| mouse (BALB/c and C57BL/6) | subcutaneous or intranasal | 105 pfu WA or CB strain | SC group (CB strain): edema at the site of inoculation, weight loss (only in BALB/c) IN group (CB strain): weight loss SC group (WA strain): slight edema at the site of inoculation IN group (WA strain): no clinical signs | not examined | not examined | Hutson et al. 2010 [45] |
| mouse (BALB/c) | intraperitoneal | 105 pfu WA or CB strain | rough coat, inappetence, decreased activity, multifocal lesions on the skin of the feet | severely necrotic ovary | not examined | Osorio et al. 2009 [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domán, M.; Fehér, E.; Varga-Kugler, R.; Jakab, F.; Bányai, K. Animal Models Used in Monkeypox Research. Microorganisms 2022, 10, 2192. https://doi.org/10.3390/microorganisms10112192
Domán M, Fehér E, Varga-Kugler R, Jakab F, Bányai K. Animal Models Used in Monkeypox Research. Microorganisms. 2022; 10(11):2192. https://doi.org/10.3390/microorganisms10112192
Chicago/Turabian StyleDomán, Marianna, Enikő Fehér, Renáta Varga-Kugler, Ferenc Jakab, and Krisztián Bányai. 2022. "Animal Models Used in Monkeypox Research" Microorganisms 10, no. 11: 2192. https://doi.org/10.3390/microorganisms10112192
APA StyleDomán, M., Fehér, E., Varga-Kugler, R., Jakab, F., & Bányai, K. (2022). Animal Models Used in Monkeypox Research. Microorganisms, 10(11), 2192. https://doi.org/10.3390/microorganisms10112192









