Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Non-Inseminated Mares
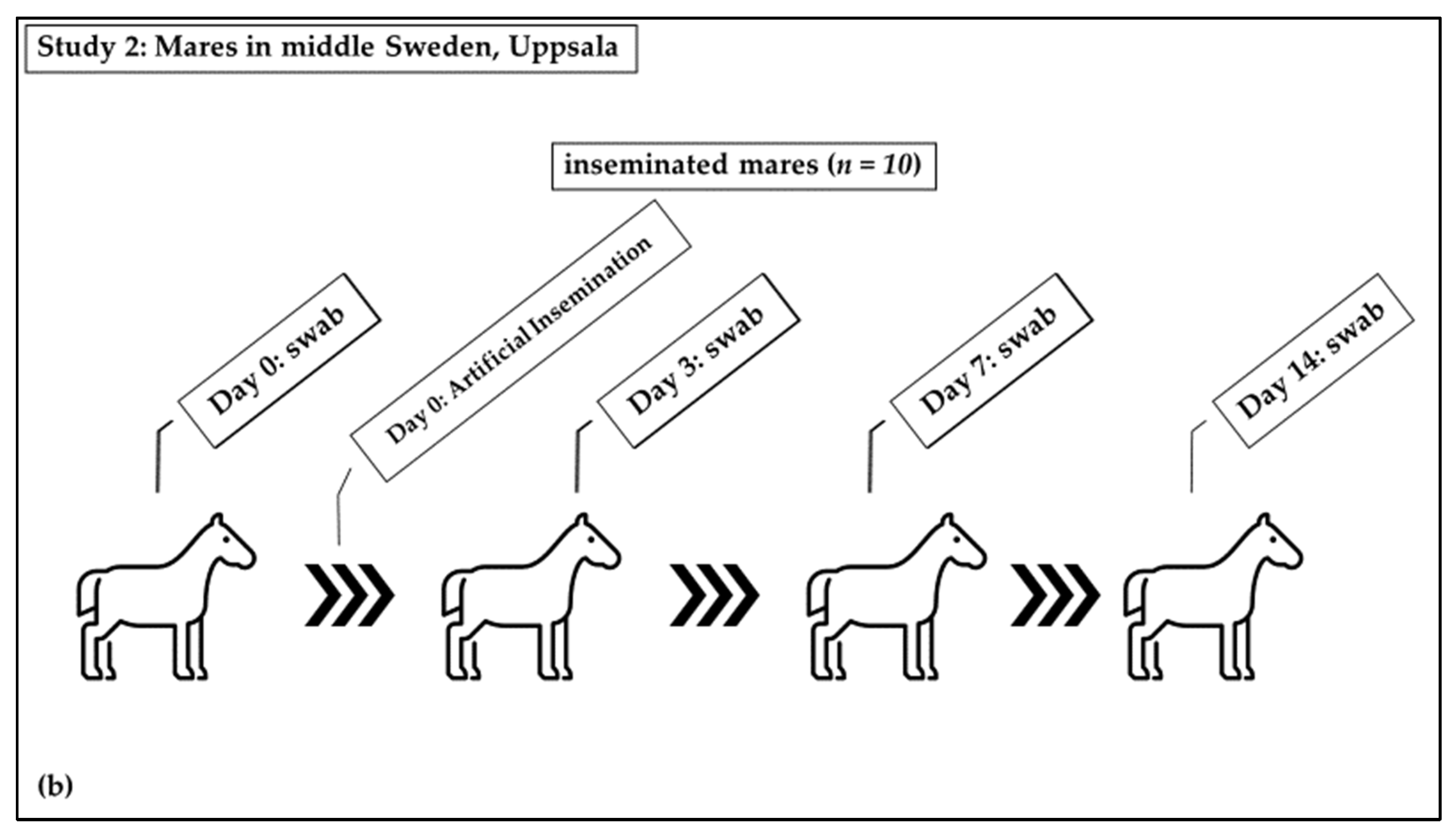
2.2. Inseminated Mares
2.3. Sampling Technique
2.4. Bacteriological Analysis
2.5. Antimicrobial Susceptibility Testing
2.6. Whole-Genome Sequencing
2.7. Statistical Analysis
3. Results
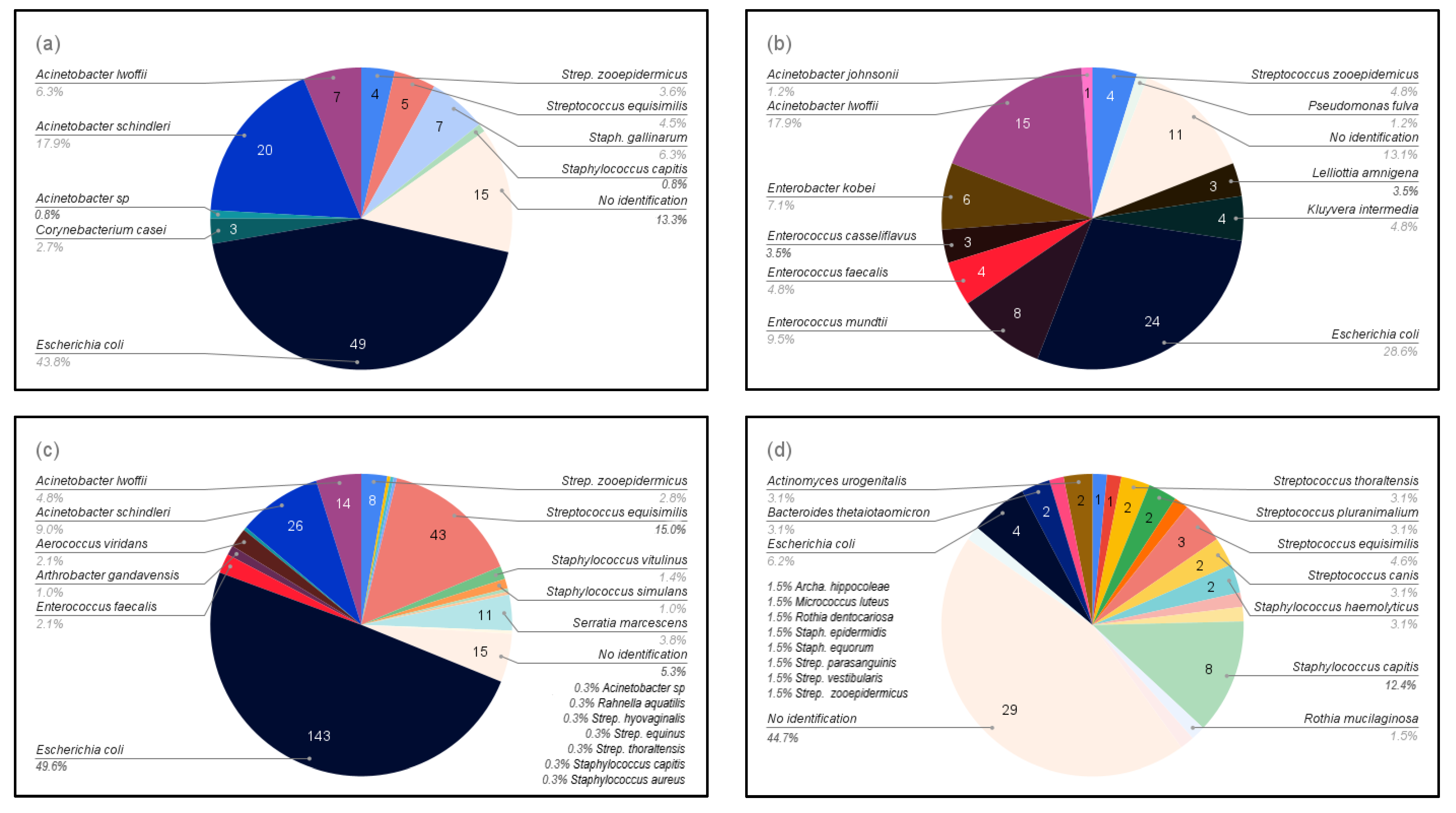
3.1. Bacterial Isolation
3.2. Antimicrobial Susceptibility
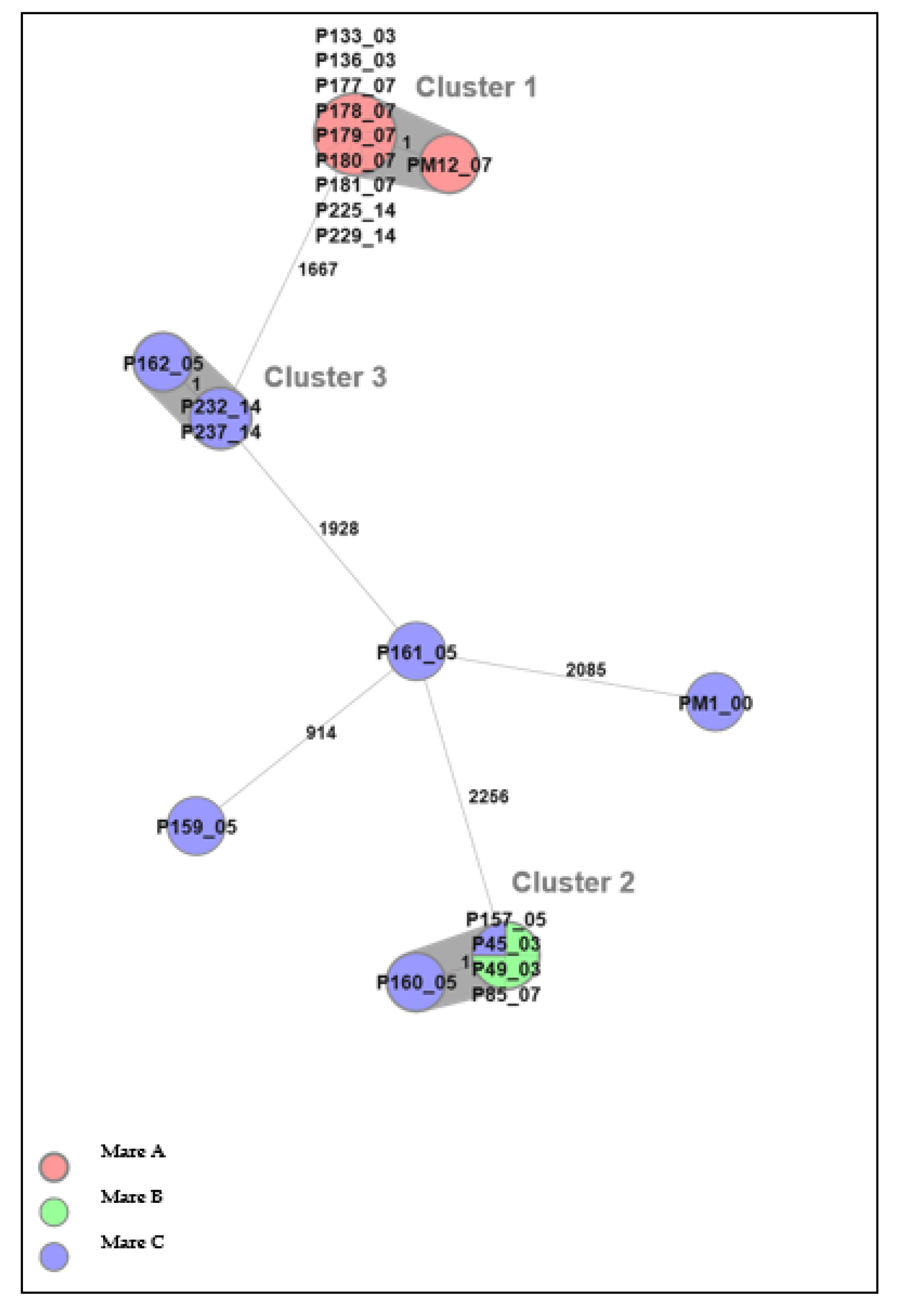
3.3. Whole-Genome Sequencing
3.4. AMR Genes
4. Discussion
4.1. Bacteria Isolation
4.2. Antimicrobial Susceptibility
4.3. Whole-Genome Sequencing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eggleston, K.; Zhang, R.; Zeckhauser, R.J. The Global Challenge of Antimicrobial Resistance: Insights from Economic Analysis. Int. J. Environ. Res. Public Health 2010, 7, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, R.; Grigoryan, L.; Lundborg, C.S.; Hofstede, G.; Cohen, J.; van der Kelen, G.; Deliens, L.; Haaijer-Ruskamp, F.M. Are Cultural Dimensions Relevant for Explaining Cross-National Differences in Antibiotic Use in Europe? BMC Health Serv. Res. 2008, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- European Union. Council Directive 92/65/EEC of 13 July 1992 Laying down Animal Health Requirements Governing Trade in and Imports into the Community of Animals, Semen, Ova and Embryos Not Subject to Animal Health Requirements Laid down in Specific Community Rules Referre. Off. J. Eur. Union 1992, L268, 54–72. [Google Scholar]
- Malaluang, P.; Wilén, E.; Lindahl, J.; Hansson, I.; Morrell, J.M. Antimicrobial Resistance in Equine Reproduction. Animals 2021, 11, 3035. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Swedres-Svarm Sales of Antibiotics and Occurrence of Resistance in Sweden; Public Health Agency of Sweden and National Veterinary Institute: Uppsala, Sweden, 2021.
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.H.; McDermott, P.F.; et al. Validating the AMRFINder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A Novel Web Tool for WGS-Based Detection of Antimicrobial Resistance Associated with Chromosomal Point Mutations in Bacterial Pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Lee, S.G.; Yang, J.H.; Cho, G.J. Distribution and Antimicrobial Susceptibility Patterns of Bacteria Isolated from Genital Tract in Thoroughbred Mares. J. Vet. Clin. 2007, 24, 19–25. [Google Scholar]
- Singh, B.R. Occurrence of Multiple Drug Resistant (MDR) Aerobic Bacteria in Vaginal Swabs of Mares and Their Association with Infertility. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 2009, 30, 105–112. [Google Scholar]
- Goncagul, G.; Gocmen, H.; Yendim, S.K.; Yilmaz, K.; Intas, K.S. Bacteriologic and Cytologic Examination Results of Mares with Pneumovagina in Bursa Region. Int. J. Vet. Sci. 2016, 5, 295–298. [Google Scholar]
- Nocera, F.P.; Papulino, C.; del Prete, C.; Palumbo, V.; Pasolini, M.P.; de Martino, L. Endometritis Associated with Enterococcus Casseliflavus in a Mare: A Case Report. Asian Pac. J. Trop. Biomed. 2017, 7, 760–762. [Google Scholar] [CrossRef]
- Goncagül, G.; Seyrek-İntas, K. Antimicrobial Susceptibility of Bacteria Isolated from Uteri of Thoroughbred Mares with Fertility Problems. Kafkas. Univ. Vet. Fak. Derg. 2013, 19, A105–A109. [Google Scholar] [CrossRef]
- Ferrer, M.S.; Palomares, R. Aerobic Uterine Isolates and Antimicrobial Susceptibility in Mares with Post-Partum Metritis. Equine Vet. J. 2018, 50, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Pisello, L.; Rampacci, E.; Stefanetti, V.; Beccati, F.; Hyatt, D.R.; Coletti, M.; Passamonti, F. Temporal Efficacy of Antimicrobials against Aerobic Bacteria Isolated from Equine Endometritis: An Italian Retrospective Analysis (2010–2017). Vet. Rec. 2019, 185, 598. [Google Scholar] [CrossRef] [PubMed]
- Frontoso, R.; de Carlo, E.; Pasolini, M.P.; van der Meulen, K.; Pagnini, U.; Iovane, G.; de Martino, L. Retrospective Study of Bacterial Isolates and Their Antimicrobial Susceptibilities in Equine Uteri during Fertility Problems. Res. Vet. Sci. 2008, 84, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Benko, T.; Boldizar, M.; Novotny, F.; Hura, V.; Valocky, I.; Dudrikova, K.; Karamanova, M.; Petrovic, V. Incidence of Bacterial Pathogens in Equine Uterine Swabs, Their Antibiotic Resistance Patterns, and Selected Reproductive Indices in English Thoroughbred Mares during the Foal Heat Cycle. Vet. Med. 2015, 60, 613–620. [Google Scholar] [CrossRef]
- Albihn, A.; Båverud, V.; Magnusson, U. Uterine Microbiology and Antimicrobial Susceptibility in Isolated Bacteria from Mares with Fertility Problems. Acta Vet. Scand. 2003, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotic Resistance in the Environment: A Link to the Clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Greko, C.; Engström, B.E.; Karlsson, M. Antimicrobial Susceptibility of Swedish, Norwegian and Danish Isolates of Clostridium Perfringens from Poultry, and Distribution of Tetracycline Resistance Genes. Vet. Microbiol. 2004, 99, 251–257. [Google Scholar] [CrossRef]

| Mares in Northern Sweden, Boden | |
|---|---|
| Gram-positive (n = 14) | Corynebacterium casei, Enterococcus casseliflavus, Enterococcus faecalis, Enterococcus mundtii, Staphylococcus aureus, Staphylococcus capitis, Staphylococcus gallinarum, Staphylococcus simulans, Staphylococcus vitulinus, Streptococcus equinus, Streptococcus equisimilis, Streptococcus hyovaginalis, Streptococcus thoraltensis, and Streptococcus zooepidemicus |
| Gram-negative (n = 13) | Acinetobacter johnsonii, Acinetobacter lwoffii, Acinetobacter schindleri, Acinetobacter sp., Aerococcus viridans, Arthrobacter gandavensis, Enterobacter kobei, Escherichia coli, Kluyvera intermedia, Lelliottia amnigena, Pseudomonas fulva, Rahnella aquatilis, and Serratia marcescens |
| Mares in middle Sweden, Uppsala | |
| Gram-positive (n = 16) | Actinomyces urogenitalis, Archanobacterium hippocoleae, Micrococcus luteus, Rothia dentocariosa, Rothia mucilaginosa, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus equorum, Staphylococcus haemolyticus, Streptococcus canis, Streptococcus equisimilis, Streptococcus parasanguinis, Streptococcus pluranimalium, Streptococcus thoraltensis, Streptococcus vestibularis, and Streptococcus zooepidemicus |
| Gram-negative (n = 2) | Bacteroides thetaiotaomicron and Escherichia coli |
| Res | <0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | >1024 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | I (n = 34) | 0 | 8.8 | 76.5 | 14.7 | ||||||||||||||
| NI (n = 45) | 0 | 15.6 | 71.1 | 13.3 | |||||||||||||||
| Azithromycin | I (n = 34) | 0 | 2.9 | 44.1 | 53.0 | ||||||||||||||
| NI (n = 45) | 0 | 4.4 | 13.3 | 64.5 | 17.8 | ||||||||||||||
| Cefotaxime | I (n = 34) | 0 | 100 | ||||||||||||||||
| NI (n = 45) | 0 | 100 | |||||||||||||||||
| Ceftazidime | I (n = 34) | 0 | 100 | ||||||||||||||||
| NI (n = 45) | 0 | 100 | |||||||||||||||||
| Ciprofloxacin | I (n = 34) | 0 | 85.3 | 14.7 | |||||||||||||||
| NI (n = 45) | 0 | 93.3 | 6.7 | ||||||||||||||||
| Chloramphenicol * | I (n = 34) | 11.8 | 88.2 | 11.8 | |||||||||||||||
| NI (n = 45) | 0 | 100 | |||||||||||||||||
| Colistin | I (n = 34) | 2.9 | 97.1 | 2.9 | |||||||||||||||
| NI (n = 45) | 0 | 100 | |||||||||||||||||
| Gentamicin | I (n = 34) | 0 | 58.8 | 41.2 | |||||||||||||||
| NI (n = 45) | 0 | 91.1 | 8.9 | ||||||||||||||||
| Meropenem | I (n = 34) | 0 | 100 | ||||||||||||||||
| NI (n = 45) | 0 | 100 | |||||||||||||||||
| Nalidixic acid | I (n = 34) | 0 | 100 | ||||||||||||||||
| NI (n = 45) | 0 | 97.8 | 2.2 | ||||||||||||||||
| Sulfamethoxazole * | I (n = 34) | 32.4 | 2.9 | 14.7 | 47.1 | 2.9 | 32.4 | ||||||||||||
| NI (n = 45) | 60.0 | 2.2 | 4.4 | 17.8 | 15.6 | 60.0 | |||||||||||||
| Tetracycline | I (n = 34) | 0 | 70.6 | 11.8 | 17.6 | ||||||||||||||
| NI (n = 45) | 0 | 84.4 | 15.6 | ||||||||||||||||
| Tigecycline | I (n = 34) | 0 | 100 | ||||||||||||||||
| NI (n = 45) | 0 | 100 | |||||||||||||||||
| Trimethoprim * | I (n = 34) | 32.4 | 2.9 | 55.9 | 8.8 | 2.9 | 29.5 | ||||||||||||
| NI (n = 45) | 0 | 48.9 | 51.1 |
| Res | <0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >64 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefalotin | I (n = 39) | 0 | 100.0 | |||||||||||
| NI (n = 61) | 0 | 100.0 | ||||||||||||
| Cefoxitin | I (n = 39) | - | 5.1 | 7.6 | 71.8 | 10.3 | 2.6 | 2.6 | ||||||
| NI (n = 61) | - | 1.7 | 18.0 | 80.3 | ||||||||||
| Clindamycin | I (n = 39) | 12.8 | 87.2 | 5.1 | 7.7 | |||||||||
| NI (n = 61) | 3.2 | 96.8 | 1.6 | 1.6 | ||||||||||
| Enrofloxacin | I (n = 39) | - | 23.1 | 76.9 | ||||||||||
| NI (n = 61) | - | 19.7 | 80.3 | |||||||||||
| Erythromycin * | I (n = 39) | 12.8 | 87.2 | 12.8 | ||||||||||
| NI (n = 61) | 1.6 | 98.4 | 1.6 | |||||||||||
| Fusidic acid | I (n = 39) | - | 53.8 | 46.2 | ||||||||||
| NI (n = 61) | - | 3.3 | 96.7 | |||||||||||
| Gentamicin | I (n = 39) | - | 10.3 | 89.7 | ||||||||||
| NI (n = 61) | - | 19.7 | 8.2 | 72.1 | ||||||||||
| Nitrofurantoin | I (n = 39) | 0 | 76.9 | 23.1 | ||||||||||
| NI (n = 61) | 0 | 85.2 | 14.8 | |||||||||||
| Oxacillin | I (n = 39) | 0 | 100.0 | |||||||||||
| NI (n = 61) | 0 | 100.0 | ||||||||||||
| Penicillin | I (n = 39) | 5.2 | 87.2 | 7.6 | 5.2 | |||||||||
| NI (n = 61) | 0 | 100.0 | ||||||||||||
| Tetracycline | I (n = 39) | 56.3 | 10.3 | 2.6 | 7.7 | 23.1 | 56.3 | |||||||
| NI (n = 61) | 77.0 | 23.0 | 77.0 | |||||||||||
| Trimethoprim/ | I (n = 39) | 7.7 | 84.6 | 7.7 | 7.7 | |||||||||
| Sulfamethoxazole | NI (n = 61) | 1.6 | 88.6 | 8.2 | 1.6 | 1.6 |
| Res | <0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >64 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefalotin | I (n = 20) | - | 95.0 | 5.0 | ||||||||||
| Cefoxitin | I (n = 20) | - | 5.0 | 5.0 | 50.0 | 30.0 | 10.0 | |||||||
| Clindamycin | I (n = 20) | - | 95.0 | 5.0 | ||||||||||
| Enrofloxacin | I (n = 20) | - | 95.0 | 5.0 | ||||||||||
| Erythromycin | I (n = 20) | 10 | 85.0 | 5.0 | 10.0 | |||||||||
| Fusidic acid | I (n = 20) | 10 | 90.0 | 10.0 | ||||||||||
| Gentamicin | I (n = 20) | 10 | 90.0 | 10.0 | ||||||||||
| Nitrofurantoin | I (n = 20) | - | 100.0 | |||||||||||
| Oxacillin | I (n = 20) | 10 | 90.0 | 10.0 | ||||||||||
| Penicillin | I (n = 20) | 35 | 55.0 | 5.0 | 5.0 | 5.0 | 15.0 | 15.0 | ||||||
| Tetracycline | I (n = 20) | 0 | 45.0 | 35.0 | 10.0 | 10.0 | ||||||||
| T/S | I (n = 20) | 0 | 70.0 | 25.0 | 5.0 |
| Res | <0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | NI (n = 4) | 0 | 100.0 | |||||||||||||||
| Chloramphenicol | NI (n = 4) | - | 100.0 | |||||||||||||||
| Ciprofloxacin | NI (n = 4) | 0 | 25.0 | 75.0 | ||||||||||||||
| Daptomycin | NI (n = 4) | - | 75.0 | 25.0 | ||||||||||||||
| Erythromycin | NI (n = 4) | - | 100.0 | |||||||||||||||
| Gentamicin | NI (n = 4) | 0 | 100.0 | |||||||||||||||
| Linezolid | NI (n = 4) | 0 | 50.0 | 50.0 | ||||||||||||||
| Q/D | NI (n = 4) | - | 100.0 | |||||||||||||||
| Teicoplanin | NI (n = 4) | 0 | 100.0 | |||||||||||||||
| Tetracycline | NI (n = 4) | - | 75.0 | 25.0 | ||||||||||||||
| Tigecycline | NI (n = 4) | 0 | 25.0 | 50.0 | 25.0 | |||||||||||||
| Vancomycin | NI (n = 4) | - | 100.0 |
| Antimicrobial | E. coli | Streptococcus spp. | Streptococcus zooepidemicus | Streptococcus equisimilis | Staphylococcus spp. | E. faecalis | |||
|---|---|---|---|---|---|---|---|---|---|
| I (n = 34) | NI (n = 45) | I (n = 10) | I (n = 21) | NI (n = 8) | I (n = 8) | NI (n = 48) | I (n = 20) | NI (n = 4) | |
| Trimethoprim | 32 | 0 | NA | NA | NA | NA | NA | NA | NA |
| Tetracycline | NA | NA | 40 | 76 | 25 | 63 | 88 | NA | NA |
| Chloramphenicol | 12 | 0 | NA | NA | NA | NA | NA | NA | NA |
| Colistin | 3 | 0 | NA | NA | NA | NA | NA | NA | NA |
| Gentamicin | 0 | 0 | NA | NA | NA | NA | NA | 10 | 0 |
| Sulfamethoxazole | 32 | 60 | NA | NA | NA | NA | NA | NA | NA |
| Penicillin | NA | NA | 10 | 0 | 0 | 0 | 0 | 35 | NA |
| Oxacillin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | NA |
| Fusidic acid | NA | NA | NA | NA | NA | NA | NA | 10 | NA |
| Erythromycin | NA | NA | 40 | 10 | 100 | 0 | 2 | 5 | NA |
| Clindamycin | NA | NA | 30 | 14 | 25 | 0 | 0 | NA | NA |
| T/S | NA | NA | 30 | 0 | 13 | 0 | 0 | 0 | NA |
| Bacteria | Day 0 | Day 3 | Day 7 | Day 14 | ||||
|---|---|---|---|---|---|---|---|---|
| Isolate ID | Resistance | Isolate ID | Resistance | Isolate ID | Resistance | Isolate ID | Resistance | |
| E. coli | P136 | Tri and Chl | P177, P178, P179, P180, P181, P192, and PM12 | Tri | P229 | Tri and Chl | ||
| P159 and P160 | Chl | P85 | Col | P225 | Tri | |||
| P133 | Tri | |||||||
| S. equisimilis | P5 | Tet | P57 | Tet | ||||
| S. zooepidemicus | P158 | Tet | P148 | Ery, Cli, and Tet | ||||
| P126, P151, and PM5 | Tet | |||||||
| Streptococcus spp. | P11 | Ery and Tet | P109 | Ery, Cli, Tet, and T/S | P217 and PM6 | Ery, Cli, Tet, and T/S | ||
| Staphylococcus spp. | P21 | Pen, Oxa, Fus, and Ery | P198 | Fus and Gen | P207 | Pen | P245 | Pen |
| P2, P143, and P146 | Pen | P156 | Gen | |||||
| P10 | Ery | P76 | Pen and Oxa | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaluang, P.; Wilén, E.; Frosth, S.; Lindahl, J.; Hansson, I.; Morrell, J.M. Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance. Microorganisms 2022, 10, 2204. https://doi.org/10.3390/microorganisms10112204
Malaluang P, Wilén E, Frosth S, Lindahl J, Hansson I, Morrell JM. Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance. Microorganisms. 2022; 10(11):2204. https://doi.org/10.3390/microorganisms10112204
Chicago/Turabian StyleMalaluang, Pongpreecha, Elin Wilén, Sara Frosth, Johanna Lindahl, Ingrid Hansson, and Jane M. Morrell. 2022. "Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance" Microorganisms 10, no. 11: 2204. https://doi.org/10.3390/microorganisms10112204
APA StyleMalaluang, P., Wilén, E., Frosth, S., Lindahl, J., Hansson, I., & Morrell, J. M. (2022). Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance. Microorganisms, 10(11), 2204. https://doi.org/10.3390/microorganisms10112204







