Seasonal Patterns of Zoonotic Cutaneous Leishmaniasis Caused by L. major and Transmitted by Phlebotomus papatasi in the North Africa Region, a Systematic Review and a Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review, Data Set Collection, and Localization of Sites
2.1.1. Systematic Review
2.1.2. Data Set Collection and Sites Localization
2.2. Normalization of ZCL Case Number and/Vector Density (Nj)
2.3. Statistical Analysis
3. Results and Discussion
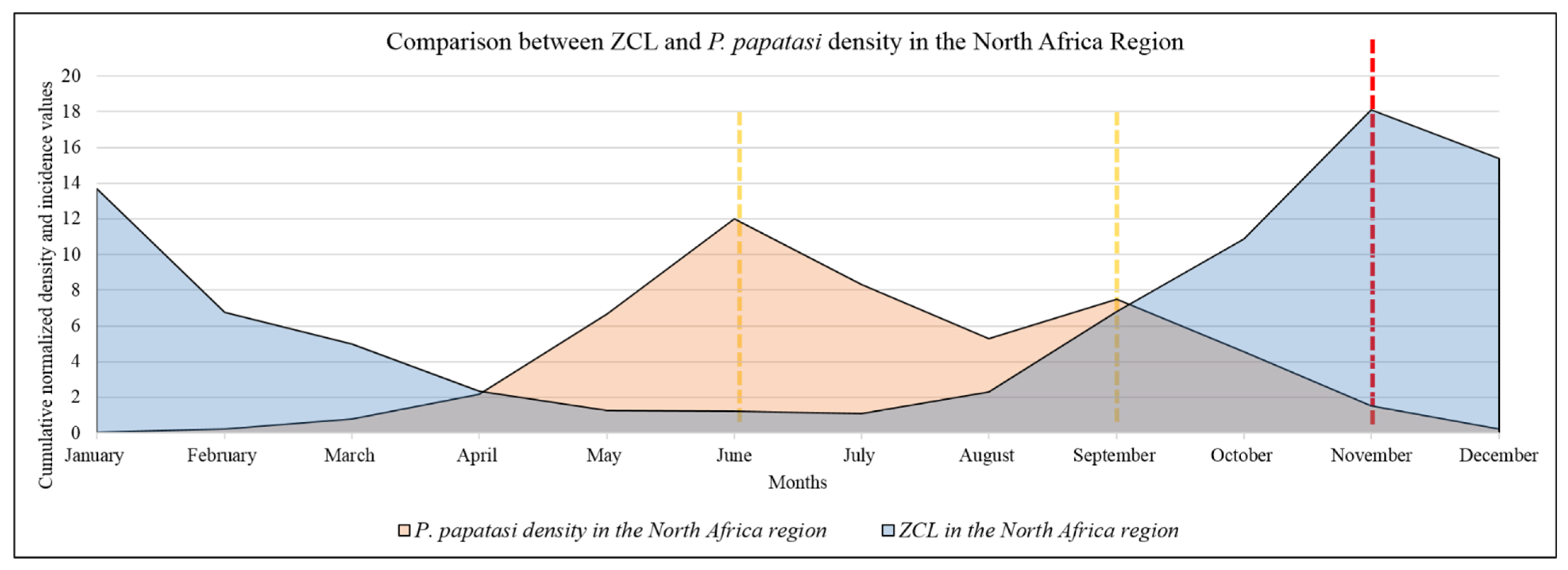
3.1. Annual Distribution of ZCL Cases in the North African Region
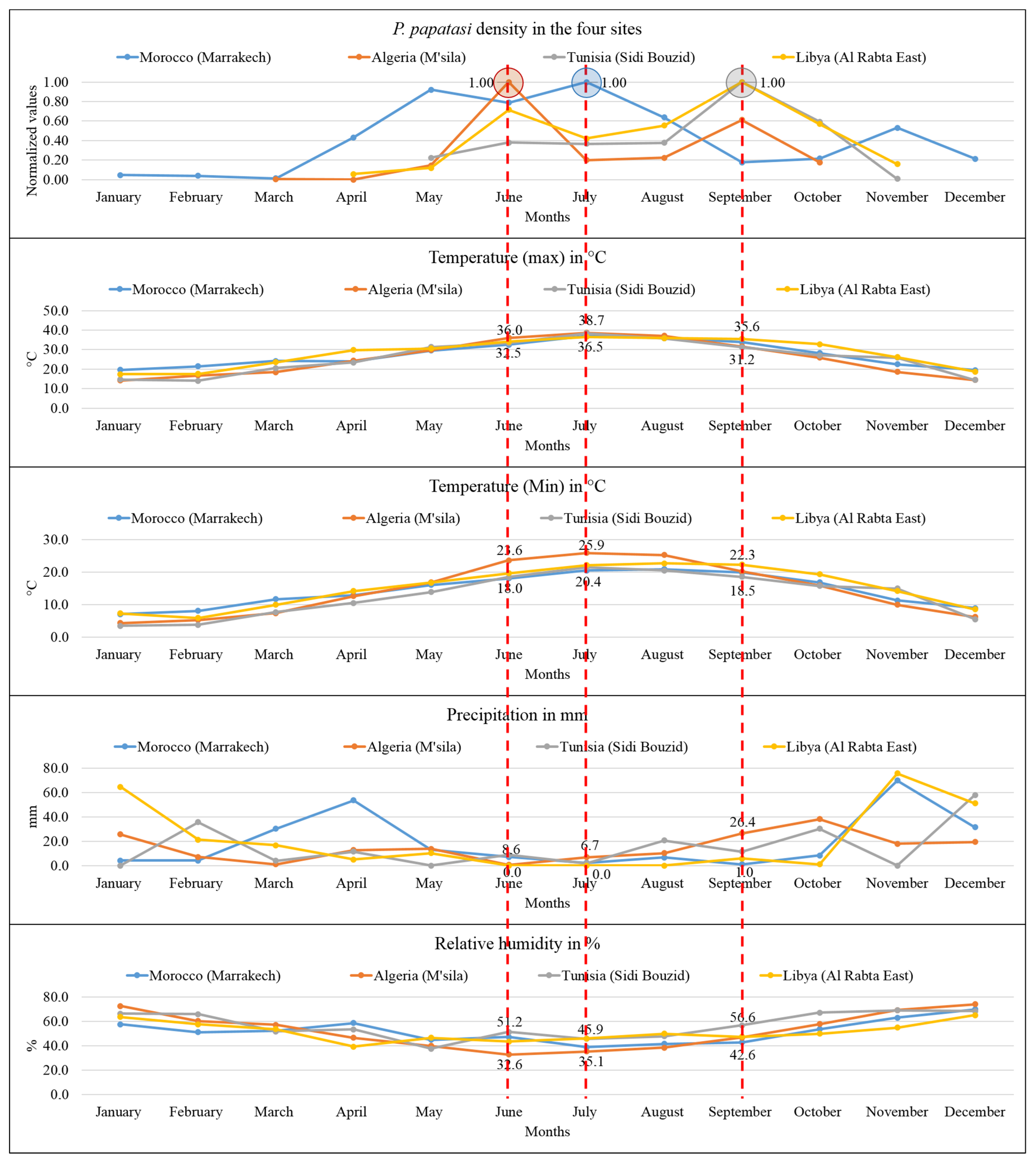
3.2. Monthly Distribution of P. papatasi in the North African Region
3.3. Monthly Records of ZCL Cases in the North African Region
3.4. Association of ZCL Incidence and the Climatic Parameters in North Africa
3.5. Association of the P. papatasi Activity and the Climatic Parameters in the North African Region
3.6. Strengths and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 8 January 2022).
- Aoun, K.; Bouratbine, A. Cutaneous leishmaniasis in North Africa: A review. Parasite 2014, 21, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulde, M.K.; Heyl, G.; Amirih, M.L. Zoonotic cutaneous leishmaniasis, Afghanistan. Emerg. Infect. Dis. 2006, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Jalali, H.; Enayati, A.A.; Fakhar, M.; Motevalli-Haghi, F.; Charati, J.Y.; Dehghan, O.; Hosseini-Vasoukolaei, N. Reemergence of zoonotic cutaneous leishmaniasis in an endemic focus, northeastern Iran. Parasite Epidemiol. Control 2021, 13, e00206. [Google Scholar] [CrossRef] [PubMed]
- Abuzaid, A.A.; Abdoon, A.M.; Aldahan, M.A.; Alzahrani, A.G.; Alhakeem, R.F.; Asiri, A.M.; Alzahrani, M.H.; Memish, Z.A. Cutaneous leishmaniasis in Saudi Arabia: A comprehensive overview. Vector-Borne Zoonotic Dis. 2017, 17, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Kholoud, K.; Bounoua, L.; Sereno, D.; El Hidan, M.; Messouli, M. Emerging and Re-emerging leishmaniases in the mediterranean area: What can Be learned from a retrospective review analysis of the situation in Morocco during 1990 to 2010? Microorganisms 2020, 8, 1511. [Google Scholar] [CrossRef]
- Barhoumi, W.; Chelbi, I.; Fares, W.; Zhioua, S.; Abbas, M.; Derbali, M.; Ramalho-Ortigao, M.; Zhioua, E. Risk Assessment of the Role of the Ecotones in the Transmission of Zoonotic Cutaneous Leishmaniasis in Central Tunisia. Int. J. Environ. Res. Public Health 2021, 18, 9274. [Google Scholar] [CrossRef]
- Mollalo, A.; Alimohammadi, A.; Shirzadi, M.R.; Malek, M.R. Geographic information system-based analysis of the spatial and spatio-temporal distribution of zoonotic cutaneous leishmaniasis in Golestan Province, north-east of Iran. Zoonoses Public Health 2015, 62, 18–28. [Google Scholar] [CrossRef]
- Bellali, H.; Hchaichi, A.; Harizi, C.; Mrabet, A.; Chahed, M.K. Comparison between active surveillance and passive detection of zoonotic cutaneous leishmaniasis in endemic rural areas in Central Tunisia, 2009 to 2014. Asian Pac. J. Trop. Dis. 2015, 5, 515–519. [Google Scholar] [CrossRef]
- Bounoua, L.; Kahime, K.; Houti, L.; Blakey, T.; Ebi, K.L.; Zhang, P.; Imhoff, M.L.; Thome, K.J.; Dudek, C.; Sahabi, S.A.; et al. Linking climate to incidence of zoonotic cutaneous leishmaniasis (L. major) in pre-Saharan North Africa. Int. J. Environ. Res. Public Health 2013, 10, 3172–3191. [Google Scholar] [CrossRef]
- Karmaoui, A.; Sereno, D.; Maia, C.; Campino, L.; El Jaafari, S.; Taybi, A.; Hajj, L. A conceptual model for understanding the zoonotic cutaneous leishmaniasis transmission risk in the Moroccan pre-Saharan area. Parasite Epidemiol. Control 2022, 17, e00243. [Google Scholar] [CrossRef]
- Karmaoui, A.; El Qorchi, F.; Hajji, L.; Zerouali, S. Eco-epidemiological aspects of Zoonotic cutaneous leishmaniasis in Ouarzazate Province, Morocco. J. Parasit. Dis. 2021, 45, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R. Climate change and waterborne and vectorborne disease. J. Appl. Microbiol. Symp. Suppl. 2003, 94, 37S–46S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaie, E.; Alesheikh, A.A.; Tabasi, M. Spatial modeling of zoonotic cutaneous leishmaniasis with regard to potential environmental factors using ANFIS and PCA-ANFIS methods. Acta Trop. 2022, 228, 106296. [Google Scholar] [CrossRef] [PubMed]
- Mohammadbeigi, A.; Khazaei, S.; Heidari, H.; Asgarian, A.; Arsangjang, S.; Saghafipour, A.; Mohammadsalehi, N.; Ansari, H. An investigation of the effects of environmental and ecologic factors on cutaneous leishmaniasis in the old world: A systematic review study. Rev. Environ. Health 2021, 36, 117–128. [Google Scholar] [CrossRef]
- Toumi, A.; Chlif, S.; Bettaieb, J.; Alaya, N.B.; Boukthir, A.; Ahmadi, Z.E.; Salah, A.B. Temporal dynamics and impact of climate factors on the incidence of zoonotic cutaneous leishmaniasis in central Tunisia. PLoS Negl. Trop. Dis. 2012, 6, e1633. [Google Scholar] [CrossRef]
- WHO EMRO. Cutaneous Leishmaniasis Factsheet. Neglected Tropical Diseases. Available online: http://www.emro.who.int/neglected-tropical-diseases/information-resources-leishmaniasis/cl-factsheet.html (accessed on 14 July 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Nakagawa, S.; Noble, D.W.; Senior, A.M.; Lagisz, M. Meta-evaluation of meta-analysis: Ten appraisal questions for biologists. BMC Biol. 2017, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Archives of the Provincial Health; Delegation of Zagora: Draa Tafilalet Region, Morocco, 2005.
- Ben tayeb, R. Etude Epidémiologique de la Leishmaniose Cutanée dans la Province d’Errachidia entre 2012 et 2017. Ph.D. Thesis, University Sid Mohamed Ben Abdellah, Fez city, Morocco, 2019. [Google Scholar]
- Bennis, I.; De Brouwere, V.; Ameur, B.; El Idrissi Laamrani, A.; Chichaoui, S.; Hamid, S.; Boelaert, M. Control of cutaneous leishmaniasis caused by Leishmania major in south-eastern Morocco. Trop. Med. Int. Health 2015, 20, 1297–1305. [Google Scholar] [CrossRef]
- Archives of the Provincial Health; Delegation of Errachidia: Draa Tafilalet region, Morocco, 2020.
- Cherif, K. Étude Éco-Épidémiologique de la Leishmaniose Cutanée dans le Bassin du Hodna (M’SILA). Ph.D. Thesis, Université Ferhat Abbas-Setif, Sétif, Algeria, 2014; 197p. [Google Scholar]
- Talmoudi, K.; Bellali, H.; Ben-Alaya, N.; Saez, M.; Malouche, D.; Chahed, M.K. Modeling zoonotic cutaneous leishmaniasis incidence in central Tunisia from 2009-2015: Forecasting models using climate variables as predictors. PLoS Negl. Trop. Dis. 2017, 11, e0005844. [Google Scholar] [CrossRef] [Green Version]
- Fichet-Calvet, E.; Jomaa, I.; Ben Ismail, R.; Ashford, R.W. Leishmania major infection in the fat sand rat Psammomys obesus in Tunisia: Interaction of host and parasite populations. Ann. Trop. Med. Parasitol. 2003, 97, 593–603. [Google Scholar] [CrossRef]
- Chelbi, I.; Derbali, M.; Al-Ahmadi, Z.; Zaafouri, B.; El Fahem, A.; Zhioua, E. Phenology of Phlebotomus papatasi (Diptera: Psychodidae) relative to the seasonal prevalence of zoonotic cutaneous leishmaniasis in central Tunisia. J. Med. Entomol. 2007, 44, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Aoun, K.; Abda, I.B.; Bousslimi, N.; Bettaieb, J.; Siala, E.; Abdallah, R.B.; Benmously, R.; Bouratbine, A. Caractérisation comparative des trois formes de leishmaniose cutanée endémiques en Tunisie. Ann. De Dermatol. Et De Vénéréologie 2012, 139, 452–458. [Google Scholar] [CrossRef]
- Amro, A.; Gashout, A.; Al-Dwibe, H.; Zahangir Alam, M.; Annajar, B.; Hamarsheh, O.; Shubar, H.; Schönian, G. First molecular epidemiological study of cutaneous leishmaniasis in Libya. PLoS Negl. Trop. Dis. 2012, 6, e1700. [Google Scholar] [CrossRef] [Green Version]
- Amro, A.; Al-Dwibe, H.; Gashout, A.; Moskalenko, O.; Galafin, M.; Hamarsheh, O.; Frohme, M.; Jaeschke, A.; Schönian, G.; Kuhls, K. Spatiotemporal and molecular epidemiology of cutaneous leishmaniasis in Libya. PLoS Negl. Trop. Dis. 2017, 11, e0005873. [Google Scholar] [CrossRef] [Green Version]
- Al-Koleeby, Z.; El Aboudi, A.; Aboulfadl, S.; Faraj, C. Diversity and Bionomics of Sandflies (Diptera: Psychodidae) of an Endemic Focus of Cutaneous Leishmaniasis in Zagora Province, Southeast of Morocco. J. Parasitol. Res. 2021, 2021, 8812691. [Google Scholar] [CrossRef]
- El Omari, H.; Chahlaoui, A.; Ouarrak, K.; Faraj, C.; Lalami, A.E.O. Surveillance of leishmaniasis: Inventory and seasonal fluctuation of phlebotomine sandflies (Diptera: Psychodidae), at the prefecture of Meknes (center of Morocco). Bull. Soc. Pathol. Exot 2018, 111, 309–315. [Google Scholar] [CrossRef]
- Talbi, F.Z.; El Ouali Lalami, A.; Fadil, M.; Najy, M.; Ech-Chafay, H.; Lachhab, M.; Lotfi, S.; Nouayti, N.; Lahouiti, K.; Faraj, C.; et al. Entomological Investigations, Seasonal Fluctuations and Impact of Bioclimate Factors of Phlebotomines Sand Flies (Diptera: Psychodidae) of an Emerging Focus of Cutaneous Leishmaniasis in Aichoun, Central Morocco. J. Parasitol. Res. 2020. [Google Scholar] [CrossRef]
- Boussaa, S.; Guernaoui, S.; Pesson, B.; Boumezzough, A. Seasonal fuctuations of phlebotomine sand fy populations (Diptera: Psychodidae) in the urban area of Marrakech, Morocco. Acta Trop. 2005, 95, 86–91. [Google Scholar] [CrossRef]
- Talbi, F.Z.; El OualiLalami, A.; Janati Idrissi, A.; Sebti, F.; Faraj, C. Leishmaniasis in central Morocco: Seasonal fuctuations of phlebotomine sand fy in Aichoun locality, from Sefrou province. Pathol. Res. Int. 2015, 438749. [Google Scholar] [CrossRef]
- Lahouiti, K.; Maniar, S.; Bekhti, K. Seasonal fuctuations of phlebotomines sand fy populations (Diptera: Psychodidae) in the Moulay Yacoub province, centre Morocco: Efect of ecological factors. Afr. J. Env. Sci. Technol. 2013, 7, 1028–1031. [Google Scholar]
- Zouirech, M.; Belghyti, D.; El Kohli, M.; Faraj, C. Entomological investigation of an emerging leishmaniasis focus in Azilal province, Morocco. Pak. Entomol. 2013, 35, 11–15. [Google Scholar]
- Boudrissa, A. Étude éco-Epidémiologique de la Leishmaniose Cutanée Dans la Wilaya de M’Sila. Master’s Thesis, Université de Sétif, Sétif, Algérie, 2005. [Google Scholar]
- Benikhlef, R.; Aoun, K.; Boudrissa, A.; Ben Abid, M.; Cherif, K.; Aissi, W.; Benrekta, S.; Boubidi, S.C.; Späth, G.F.; Bouratbine, A.; et al. Cutaneous Leishmaniasis in Algeria; Highlight on the Focus of M’Sila. Microorganisms 2021, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Dokhan, M.R.; Kenawy, M.A.; Doha, S.A.; El-Hosary, S.S.; Shaibi, T.; Annajar, B.B. Entomological studies of phlebotomine sand fies (Diptera: Psychodidae) in relation to cutaneous leishmaniasis transmission in Al Rabta, North West of Libya. Acta Trop. 2016, 154, 95–101. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Ali, R.M.; Loutfy, N.F.; Awad, O.M.; Suliman, N.K. Bionomics of phlebotomine sandfy species in west Alexandria, Egypt. J. Entomol. Zool. Stud. 2016, 4, 349–353. [Google Scholar]
- Yaghoobi-Ershadi, M.R.; Akhavan, A.A.; Abai, M.R.; Ebrahimi, B.; Zahraei-Ramazani, A.R.; Vafaei-Nezhad, R.; Jafari, R. Epidemiological study in a new focus of cutaneous leishmaniasis in the Islamic Republic of Iran. East. Mediterr. Health J. 2004, 10, 688. Available online: https://www.who.int/iris/handle/10665/119335 (accessed on 19 November 2022). [CrossRef] [PubMed]
- Shirzadi, M.R.; Mollalo, A.; Yaghoobi-Ershadi, M.R. Dynamic relations between incidence of zoonotic cutaneous leishmaniasis and climatic factors in Golestan Province, Iran. J. Arthropod-Borne Dis. 2015, 9, 148. [Google Scholar]
- Faulde, M.; Schrader, J.; Heyl, G.; Amirih, M.; Hoerauf, A. Zoonotic cutaneous leishmaniasis outbreak in Mazar-e Sharif, northern Afghanistan: An epidemiological evaluation. Int. J. Med. Microbiol. 2008, 298, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kasap, O.E.; Alten, B. Comparative demography of the sand fly Phlebotomus papatasi (Diptera: Psychodidae) at constant temperatures. J. Vector Ecol. 2006, 31, 378–385. [Google Scholar] [CrossRef]
- Benkova, I.; Volf, P. Effect of temperature on metabolism of Phlebotomus papatasi (Diptera: Psychodidae). J. Med. Entomol. 2007, 44, 150–154. [Google Scholar] [CrossRef]
- Pekağırbaş, M.; Karakuş, M.; Kasap, O.E.; Demir, S.; Nalçacı, M.; Töz, S.; Eren, H.; Özbel, Y. Investigation of Phlebotominae (Diptera: Psychodidae) Fauna, Seasonal Dynamics, and Natural Leishmania spp. Infection in Muğla, Southwest of Turkey. Acta Trop. 2021, 216, 105827. [Google Scholar] [CrossRef]
- Karmaoui, A. Seasonal Distribution of Phlebotomus papatasi, Vector of Zoonotic Cutaneous Leishmaniasis. Acta Parasitol. 2020, 65, 585–598. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Allah-Kalteh, E.; Sofizadeh, A. The Effect of Geographical and Climatic Factors on the Distribution of Phlebotomus papatasi (Diptera: Psychodidae) in Golestan Province, an Endemic Focus of Zoonotic Cutaneous Leishmaniasis in Iran, 2014. J. Arthropod-Borne Dis. 2021, 15, 225. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Astaneh, F.; Akhavan, A.A.; Shirzadi, M.R.; Rassi, Y.; YaghoobiErshadi, M.R.; Hanaf-Bojd, A.A.; Rauf, H. Species diversity of sand fies and ecological niche model of Phlebotomus papatasi in central Iran. Acta Trop. 2015, 149, 246–253. [Google Scholar] [CrossRef]
- Boudrissa, A.; Cherif, K.; Kherrachi, I.; Benbetka, S.; Bouiba, L.; Boubidi, S.C.; Harrat, Z. Extension de Leishmania major au nord de l’Alg’erie Spread of Leishmania major to the north of Algeria. Bull. Soc. Pathol. Exot. 2012, 105, 30–35. [Google Scholar] [CrossRef]
- Ready, P.D. Leishmaniasis emergence and climate change. Rev. Sci. Tech. 2008, 27, 399–412. [Google Scholar] [CrossRef]
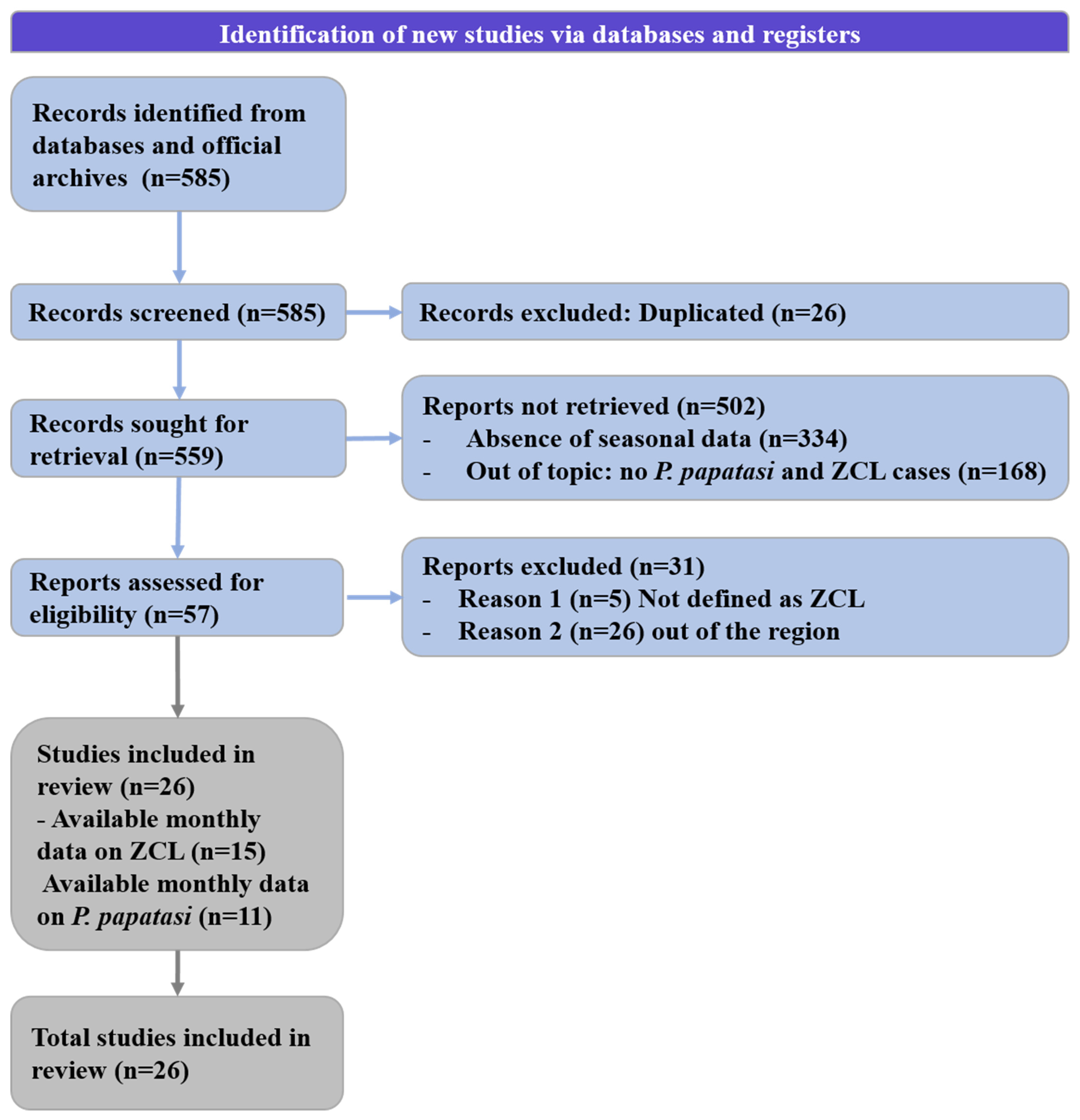
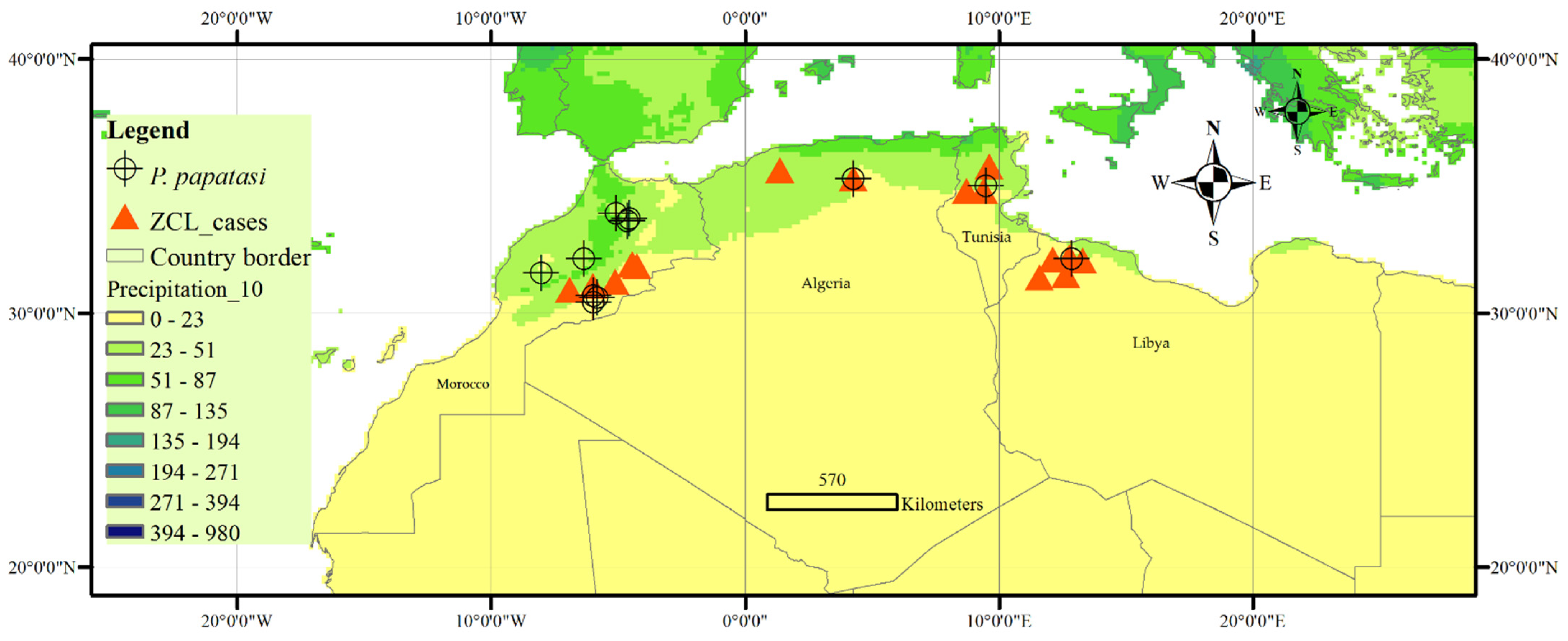

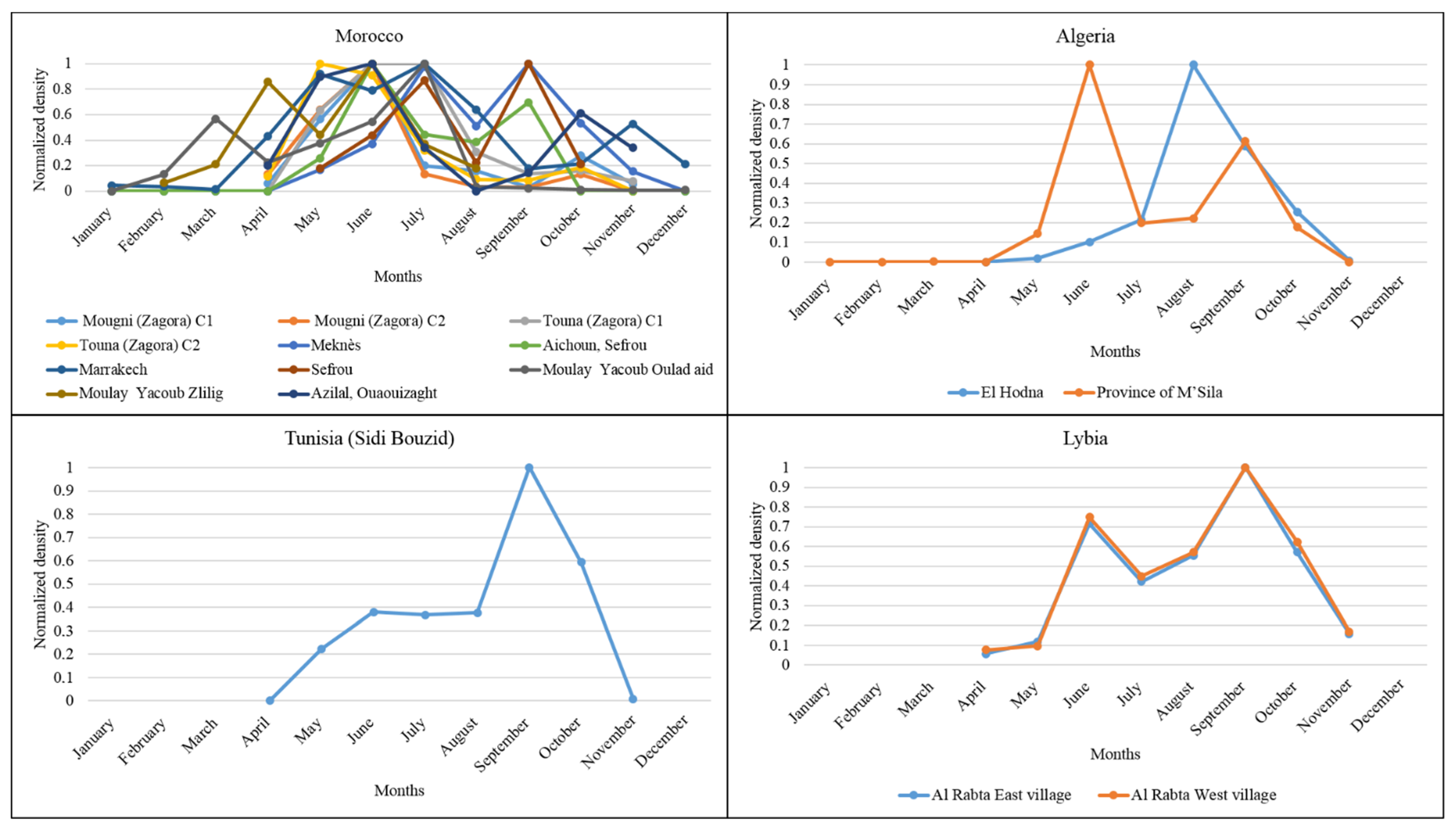

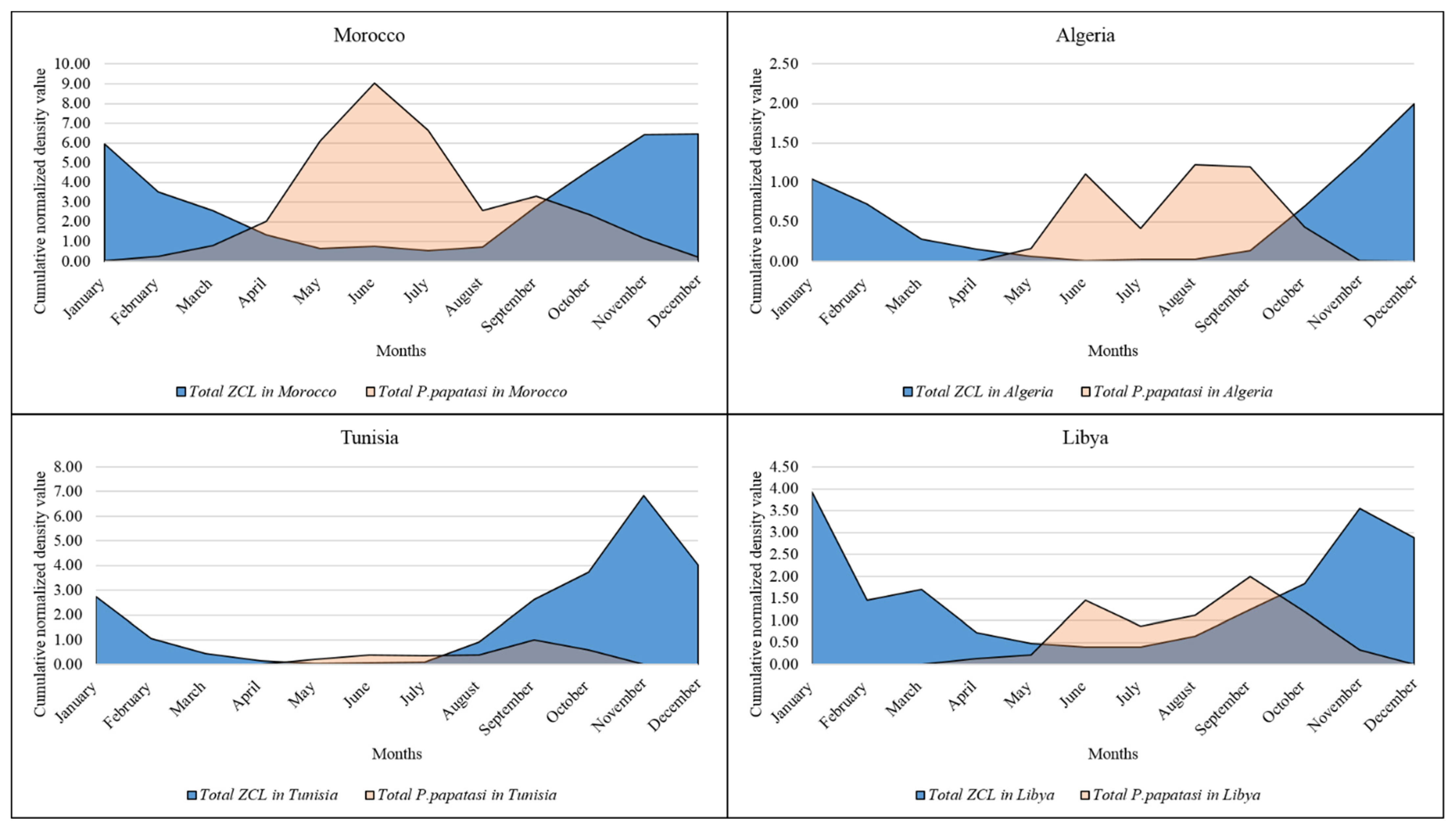

| Country | Zone | Latitude | Longitude | Altitude (m) | Annual Rainfall (mm) | Period | References |
|---|---|---|---|---|---|---|---|
| Morocco | Zagora province (2005) | ~30°18′27.60″ N | ~5°52′4.55″ W | 500 | 80 | 2005 | [20] |
| Zagora province (2017) | ~30°40′20.60″ N | ~5°7′4″ W | 500 | 80 | 2017 | [20] | |
| Errachidia Province (2012–2017) | ~31°55′5″ N | ~4°26′6″ W | ~1037 | 150 | 2012–2017 | [21] | |
| Errachidia Province (Urban) | ~31°55′46.27″ N | 4°26′55.83″ W | ~1037 | 134 | 2004–2013 | [22] | |
| Errachidia Province (Rural) | ~31°51′1″ N | ~4°16′4″ W | ~1011 | 134 | 2004–2013 | [22] | |
| Errachidia Province (1990–2009) | ~31°55′46″ N | ~4°26′55″ W | ~1037 | 134.28 | 1990–2009 | [10] | |
| Errachidia Province (2012–2020) | ~31°55′48″ N | ~4°26′58″ W | ~1037 | 134 | 2012–2020 | [23] | |
| Ouarzazate province (2002–2009) | ~30°55′59″ N | ~6°55′10″ W | ~1148 | 146.97 | 2002–2009 | [12] | |
| Algeria | El Hodna | 35°18′–35°32′N | 4°15′–5°06′ E | 400–1800 | 500–600 | 1995–2000 | [24] |
| Saida | ~34°50′29.47″ N | ~0°8′44.18″ E | ~802 | 353.47 | 1990–2009 | [10] | |
| Tunisia | Bir Badr, Hichria, and Zefzef (Sidi Bouzid) | ~34°49′45″ N | ~9°22′37″ E | 407 | 228 | 2009–2015 | [25] |
| R’milia, Sidi Bouzid (1995) | 35°46′ N | 9°36′ E | 280 | 260 | 1995 | [26] | |
| R’milia, Sidi Bouzid (1996) | 35°46′ N | 9°36′ E | 280 | 260 | 1996 | [26] | |
| R’milia, Sidi Bouzid (1997) | 35°46′ N | 9°36′ E | 280 | 260 | 1997 | [26] | |
| Sidi Bouzid (1991–2007) | ~35°02′00″ N | ~9°30′00″ E | ~373 | - | 1991–2007 | [16] | |
| Village of Felta, Sidi Bouzid (2005) | ~34°49′27″ N | 8°42′09″ E | ~768 | - | 2004–2005 | [27] | |
| Central Tunisia (2009–2014) | ~34°49′45″ N | ~9°22′37″ E | ~407 | ~228 | 2009–2014 | [9] | |
| Center and South | Large distribution in the center and south of the country | 2007–2011 | [28] | ||||
| Libya | Northwest (1995–2008) | Large distribution in the north-western districts of the country. | 1995–2008 | [29] | |||
| Northwest (2011–2012) | Large distribution in the north-western districts of the country, including Jafara, Tripoli, Misrata, and Nalut. | 2011–2012 | [30] | ||||
| Northwest (1995–2008) | Large distribution in the north-western districts of the country, including Jafara, Tripoli, Misrata, and Nalut. | 1995–2008 | [30] | ||||
| Northwest (1995–2012) | Large distribution in the north-western districts of the country, including Jafara, Tripoli, Misrata, and Nalut. | 1995–2012 | [30] | ||||
| Country | Zone | Latitude | Longitude | Altitude (m) | Annual Rainfall (mm) | Period | References |
|---|---|---|---|---|---|---|---|
| Morocco | Ksar Mougni, Tinzouline (Zagora) C1 | 30°27′3″ N | 5°58′26″ W | 775 | 37 | 2019 | [31] |
| Ksar Mougni, Tinzouline (Zagora) C2 | 30°27′3″ N | 5°58′26″ W | 775 | 37 | 2019 | [31] | |
| Touna, Tinzouline (Zagora) C1 | 30°37′28.2″ N | 5°49′56.1″ W | 910 | 26 | 2019 | [31] | |
| Touna, Tinzouline (Zagora) C2 | 30°37′28.2″ N | 5°49′56.1″ W | 910 | 26 | 2019 | [31] | |
| Meknes (prefecture) | ~33°45′02″ N | ~4°34′00″ O | 500 | 660 | 2016–2017 | [32] | |
| Aichoun, Tazouta, Sefrou | ~33°45′22″ N | ~5°32′26″ O | 750 | 400 | 2013–2014 | [33] | |
| Marrakech Urban | 31°36 N | 8°02 W | 471 | 2002–2003 | [34] | ||
| Sefrou | 33°39 N | 04°38 W | 809 | 2012 | [35] | ||
| Moulay Yacoub Oulad aid | ~34°05′N | ~4°45′ W | 345 | 2011–2012 | [36] | ||
| Moulay Yacoub Zlilig | 33°57 N | 5°05 W | 500 | 2011–2012 | [36] | ||
| Azilal province, Ouaouizaght district | 32°09′27.26″ N | 6° 20′57.58″ O | ~900–1200 | 2010 | [37] | ||
| Algeria | El Hodna | 35°18′−35°32′ N | 4°15′– 5°06′ | 500–800 | 2004 | [38] | |
| Province of M’Sila | 35°18′ and 35°32′ N | 4°15′ and 5°06′E | 2003/2004 | [39] | |||
| Tunisia | SidiBouzid | ~35°02′01″ N | ~9°28′54″ E | 350 | 2005 | [27] | |
| Lybia | Al Rabta East village | 32°9′46.59″ N | 12°50′50.65″ E | 300 | 2012–2013 | [40] | |
| Al Rabta West village | 32°9′46.59″ N | 12°50′50.65″ E | 300 | 2012–2013 | [40] |
| Correlation Method | Temperature (Maximum) | Temperature (Minimum) | Precipitation | Relative Humidity | |
|---|---|---|---|---|---|
| Tunisia (Sidi Bouzid) | R | 0.4326 | 0.3309 | 0.189 | 0.3225 |
| R2 | 0.1871 | 0.1095 | 0.0357 | 0.104 | |
| Algeria (Saida) | R | 0.3711 | 0.2273 | 0.4185 | 0.5764 |
| R2 | 0.1377 | 0.0517 | 0.1751 | 0.3322 | |
| Morocco (Ouarzazate) | R | 0.5197 | 0.2989 | 0.6254 | 0.6715 |
| R2 | 0.2701 | 0.0893 | 0.3911 | 0.4509 | |
| Libya (Tripoli) | R | 0.3438 | 0.2427 | 0.8641 | 0.3003 |
| R2 | 0.1182 | 0.0589 | 0.7467 | 0.0902 |
| Correlation Method | Temperature (Maximum) | Temperature (Minimum) | Precipitation | Relative Humidity | |
|---|---|---|---|---|---|
| Tunisia (Sidi Bouzid) | R | 0.5119 | 0.5685 | 0.498 | 0.4616 |
| R2 | 0.262 | 0.3232 | 0.248 | 0.2131 | |
| Algeria (M’SILA) | R | 0.494 | 0.5537 | 0.4893 | 0.3296 |
| R2 | 0.244 | 0.3066 | 0.2394 | 0.1086 | |
| Morocco (Marrakech) | R | 0.7509 | 0.5061 | 0.3147 | 0.5725 |
| R2 | 0.5639 | 0.2561 | 0.099 | 0.3278 | |
| Libya (Al Rabta East) | R | 0.5405 | 0.5309 | −0.2077 | 0.4894 |
| R2 | 0.2921 | 0.2819 | 0.0431 | 0.2395 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmaoui, A.; Sereno, D.; El Jaafari, S.; Hajji, L. Seasonal Patterns of Zoonotic Cutaneous Leishmaniasis Caused by L. major and Transmitted by Phlebotomus papatasi in the North Africa Region, a Systematic Review and a Meta-Analysis. Microorganisms 2022, 10, 2391. https://doi.org/10.3390/microorganisms10122391
Karmaoui A, Sereno D, El Jaafari S, Hajji L. Seasonal Patterns of Zoonotic Cutaneous Leishmaniasis Caused by L. major and Transmitted by Phlebotomus papatasi in the North Africa Region, a Systematic Review and a Meta-Analysis. Microorganisms. 2022; 10(12):2391. https://doi.org/10.3390/microorganisms10122391
Chicago/Turabian StyleKarmaoui, Ahmed, Denis Sereno, Samir El Jaafari, and Lhoussain Hajji. 2022. "Seasonal Patterns of Zoonotic Cutaneous Leishmaniasis Caused by L. major and Transmitted by Phlebotomus papatasi in the North Africa Region, a Systematic Review and a Meta-Analysis" Microorganisms 10, no. 12: 2391. https://doi.org/10.3390/microorganisms10122391
APA StyleKarmaoui, A., Sereno, D., El Jaafari, S., & Hajji, L. (2022). Seasonal Patterns of Zoonotic Cutaneous Leishmaniasis Caused by L. major and Transmitted by Phlebotomus papatasi in the North Africa Region, a Systematic Review and a Meta-Analysis. Microorganisms, 10(12), 2391. https://doi.org/10.3390/microorganisms10122391






