Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance
Abstract
:1. Introduction
2. Ecological Importance of Soil Nutrient Heterogeneity
3. Ecological Impacts of Soil Microorganisms
3.1. Molecular Transformation (Organic Matter and Inorganic Transformation)
3.2. Nutrient Cycling (Nitrogen Fixation and Carbon Cycling)
3.3. Soil Formation
4. Mechanisms of Plant Nutrient Acquisition in Heterogeneous Soil
4.1. Root Foraging Mechanism
4.2. Clonal Foraging Mechanism
4.3. Soil Microbe-Mediated Mechanisms of Plant Nutrient Acquisition in Heterogeneous Habitat
4.3.1. Exploitation of Patchy Nutrients via AMF Hyphal Network
4.3.2. Organic Matter Mineralization and Redistribution in the Soil Matrix
4.3.3. Homogenization of Patchy Nutrients via Enzymatic Activities
5. Evolutionary Advantages of Microbially Mediated Nutrient Acquisition in a Heterogeneous Environment
5.1. Ecologically Cost-Effective
5.2. The Wider Proliferation of Mycelial Networks
5.3. Transport of Organic P Solubilizing Bacteria (PSB)
5.4. Plant-Mycorrhizal Fungi Symbioses
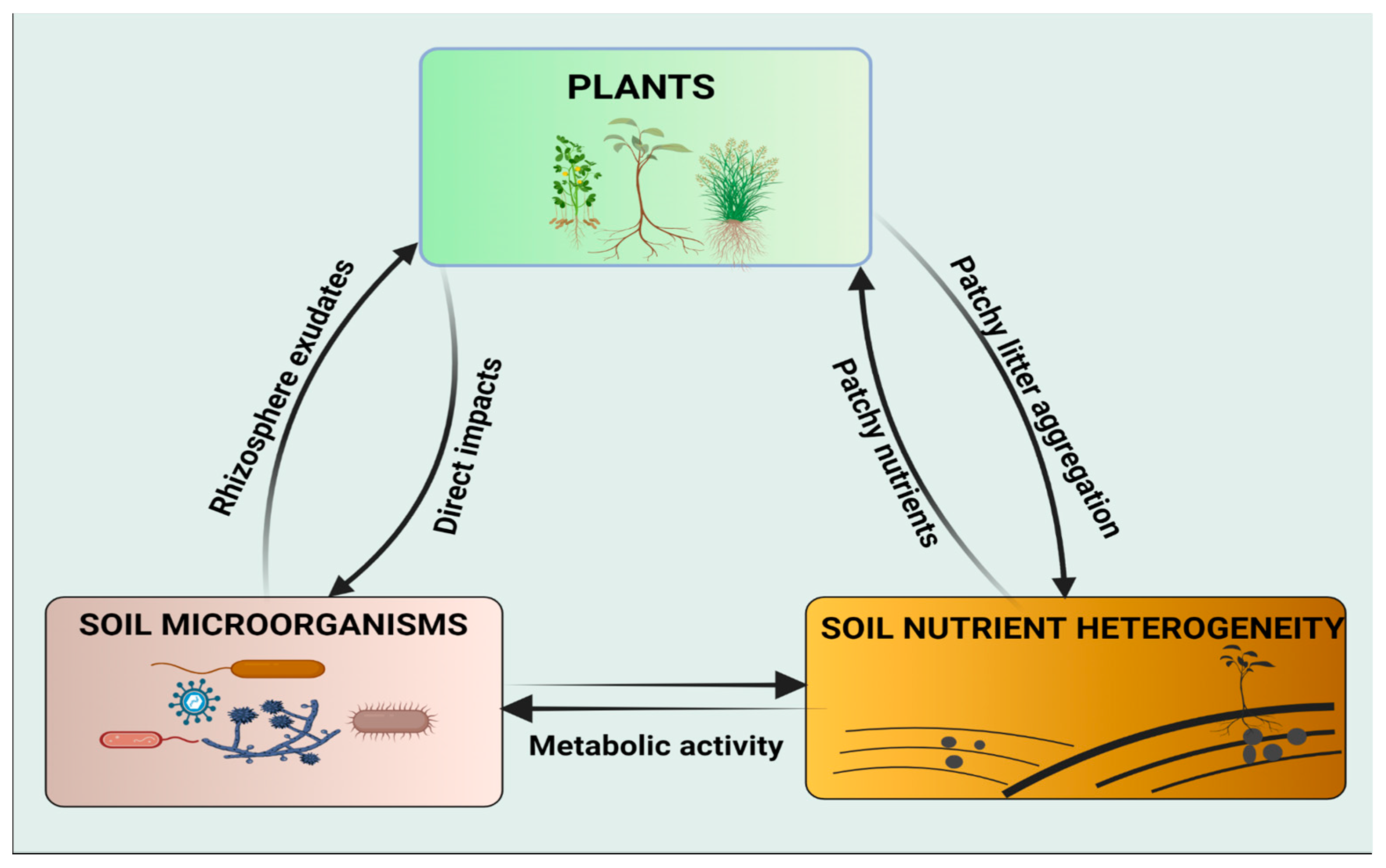
6. Plants—Soil Microorganisms—Soil Nutrient Heterogeneity Loop
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitter, B.; Pfaffenbichler, N.; Sessitsch, A. Plant–microbe partnerships in 2020. Microb. Biotechnol. 2016, 9, 635–640. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Harris, J. Soil microbial communities and restoration ecology: Facilitators or followers? Science 2009, 325, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; IJdo, M.; Genney, D.R.; Anderson, I.C.; Alexander, I.J. How do plants regulate the function, community structure, and diversity of mycorrhizal fungi? J. Exp. Bot. 2005, 56, 1751–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P. Soil–microbes–plants: Interactions and ecological diversity. In Plant Microbe Interface; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Read, D.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Cahill, J.F.; McNickle, G.G.; Haag, J.J.; Lamb, E.G.; Nyanumba, S.M.; St. Clair, C.C. Plants integrate information about nutrients and neighbors. Science 2010, 328, 1657. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, M.; Minamisawa, K. Plant–microbe communications for symbiosis. Plant Cell Physiol. 2010, 51, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, F.-H.; Alpert, P.; Dong, M. Arbuscular mycorrhizal fungi reduce effects of physiological integration in Trifolium repens. Ann. Bot. 2009, 104, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adomako, M.O.; Xue, W.; Du, D.-L.; Yu, F.-H. Soil biota and soil substrates influence responses of the rhizomatous clonal grass Leymus chinensis to nutrient heterogeneity. Plant Soil 2021, 465, 19–29. [Google Scholar] [CrossRef]
- Hodge, A. Plastic plants and patchy soils. J. Exp. Bot. 2005, 57, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Stark, J. Causes of soil nutrient heterogeneity at different scales. In Exploitation of Environmental Heterogeneity by Plants; Caldwell, M., Pearcy, R., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 255–284. [Google Scholar]
- Cai, A.; Chang, N.; Zhang, W.; Liang, G.; Zhang, X.; Hou, E.; Jiang, L.; Chen, X.; Xu, M.; Luo, Y. The spatial patterns of litter turnover time in Chinese terrestrial ecosystems. Eur. J. Soil Sci. 2019, 71, 856–867. [Google Scholar] [CrossRef]
- Loydi, A.; Lohse, K.; Otte, A.; Donath, T.W.; Eckstein, R.L. Distribution and effects of tree leaf litter on vegetation composition and biomass in a forest–grassland ecotone. J. Plant Ecol. 2013, 7, 264–275. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.-C.; Alpert, P.; Zhang, Q.; Yu, F.-H. Clonal integration in homogeneous environments increases performance in Alternanthera philoxeroides. Oecologia 2015, 179, 393–400. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, B.C.; Alpert, P.; Li, H.-L.; Zhang, M.-X.; Lei, G.-C.; Yu, F.-H. Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides. Ann. Bot. 2012, 109, 813–818. [Google Scholar] [CrossRef] [Green Version]
- Day, K.J.; Hutchings, M.J.; John, E.A. The effects of spatial pattern of nutrient supply on the early stages of growth in plant populations. J. Ecol. 2003, 91, 305–315. [Google Scholar] [CrossRef]
- Birch, C.P.D.; Hutchings, M.J. Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. J. Ecol. 1994, 82, 653–664. [Google Scholar] [CrossRef]
- Adomako, M.O.; Gao, F.-L.; Li, J.-M.; Du, D.-L.; Xue, W.; Yu, F.-H. Effects of soil nutrient heterogeneity and parasitic plant infection on an experimental grassland community. Flora 2020, 271, 151666. [Google Scholar] [CrossRef]
- Xue, W.; Huang, L.; Yu, F.-H.; Bezemer, T.M. Intraspecific aggregation and soil heterogeneity: Competitive interactions of two clonal plants with contrasting spatial architecture. Plant Soil 2018, 425, 231–240. [Google Scholar] [CrossRef]
- de Kroon, H.; Hendriks, M.; van Ruijven, J.; Ravenek, J.; Padilla, F.M.; Jongejans, E.; Visser, E.J.W.; Mommer, L. Root responses to nutrients and soil biota: Drivers of species coexistence and ecosystem productivity. J. Ecol. 2012, 100, 6–15. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Alpert, P.; Yu, F.-H. Nutrient foraging ability promotes intraspecific competitiveness in the clonal plant Hydrocotyle vulgaris. Ecol. Indic. 2022, 138, 108862. [Google Scholar] [CrossRef]
- Hutchings, M.J.; de Kroon, H.D. Foraging in plants: The role of morphological plasticity in resource acquisition. Adv. Ecol. Res. 1994, 25, 159–238. [Google Scholar]
- Jackson, R.B.; Caldwell, M.M. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 1993, 74, 612–614. [Google Scholar] [CrossRef]
- Wang, P.; Alpert, P.; Yu, F.-H. Clonal integration increases relative competitive ability in an invasive aquatic plant. Am. J. Bot. 2016, 103, 2079–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, M.; Wijesinghe, D. Performance of a clonal species in patchy environments: Effects of environmental context on yield at local and whole-plant scales. Evol. Ecol. 2008, 22, 313–324. [Google Scholar] [CrossRef]
- Bliss, K.M.; Jones, R.H.; Mitchell, R.J.; Mou, P.P. Are competitive interactions influenced by spatial nutrient heterogeneity and root foraging behavior? New Phytol. 2002, 154, 409–417. [Google Scholar] [CrossRef]
- Chen, D.; Ali, A.; Yong, X.H.; Lin, C.G.; Niu, X.H.; Cai, A.M.; Dong, B.C.; Zhou, Z.X.; Wang, Y.J.; Yu, F.H. A multi-species comparison of selective placement patterns of ramets in invasive alien and native clonal plants to light, soil nutrient and water heterogeneity. Sci. Total Environ. 2019, 657, 1568–1577. [Google Scholar] [CrossRef]
- Fransen, B.; de Kroon, H.; Berendse, F. Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 1998, 115, 351–358. [Google Scholar] [CrossRef]
- Qian, Y.-Q.; Luo, D.; Gong, G.; Han, L.; Ju, G.-S.; Sun, Z.-Y. Effects of spatial scale of soil heterogeneity on the growth of a clonal plant producing both spreading and clumping ramets. J. Plant Growth Regul. 2014, 33, 214–221. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Haubensak, K.A. Soil fertility, heterogeneity, and microbes: Towards an integrated understanding of grassland structure and dynamics. Appl. Veg. Sci. 2009, 12, 33–44. [Google Scholar] [CrossRef]
- Georgiou, K.; Abramoff, R.Z.; Harte, J.; Riley, W.J.; Torn, M.S. Microbial community-level regulation explains soil carbon responses to long-term litter manipulations. Nat. Commun. 2017, 8, 1223. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.; Robinson, D.; Fitter, A.H. An arbuscular mycorrhizal inoculum enhances root proliferation in, but not nitrogen capture from, nutrient-rich patches in soil. New Phytol. 2000, 145, 575–584. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Pajares, S.; Bohannan, B.J. Ecology of nitrogen fixing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. 2016, 7, 1045. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, C.C.; Townsend, A.R.; Schimel, D.S.; Fisher, H.; Howarth, R.W.; Hedin, L.O.; Perakis, S.S.; Latty, E.F.; Von Fischer, J.C.; Elseroad, A.; et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 1999, 13, 623–645. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heijden, M.G.A.; Streitwolf-Engel, R.; Riedl, R.; Siegrist, S.; Neudecker, A.; Ineichen, K.; Boller, T.; Wiemken, A.; Sanders, I.R. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 2006, 172, 739–752. [Google Scholar] [CrossRef]
- Benson, D.R.; Silvester, W.B. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 1993, 57, 293–319. [Google Scholar] [CrossRef]
- Maestre, F.T.; Reynolds, J.F. Spatial heterogeneity in soil nutrient supply modulates nutrient and biomass responses to multiple global change drivers in model grassland communities. Glob. Chang. Biol. 2006, 12, 2431–2441. [Google Scholar] [CrossRef]
- García-Palacios, P.; Maestre, F.T.; Gallardo, A. Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. J. Ecol. 2011, 99, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P.; Holzapfel, C.; Slominski, C. Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. J. Ecol. 2003, 91, 27–35. [Google Scholar] [CrossRef]
- Weiser, M.; Koubek, T.; Herben, T. Root foraging performance and life-history traits. Front. Plant Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Alpert, P.; Dong, B.-C.; Yu, F.-H. Modification by earthworms of effects of soil heterogeneity and root foraging in eight species of grass. Sci. Total Environ. 2020, 708, 134941. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.D.; Grime, J.P.; Mackey, J.M.L. A trade-off between scale and precision in resource foraging. Oecologia 1991, 87, 532–538. [Google Scholar] [CrossRef]
- Wijesinghe, D.; Hutchings, M. The effects of spatial scale of environmental heterogeneity on the growth of a clonal plant: An experimental study with Glechoma hederacea. J. Ecol. 1997, 85, 17–28. [Google Scholar] [CrossRef]
- Lamb, E.G.; Haag, J.J.; Cahill Jr, J.F. Patch–background contrast and patch density have limited effects on root proliferation and plant performance in Abutilon theophrasti. Funct. Ecol. 2004, 18, 836–843. [Google Scholar] [CrossRef]
- Gersani, M.; Brown, J.s.; O’Brien, E.E.; Maina, G.M.; Abramsky, Z. Tragedy of the commons as a result of root competition. J. Ecol. 2001, 89, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Maestre, F.; Roynolds, J. Amount or pattern? Grassland responses to the hetergeneity and availability of two key resources. Ecology 2007, 88, 501–511. [Google Scholar] [CrossRef]
- Wagg, C.; Jansa, J.; Schmid, B.; van der Heijden, M.G. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 2011, 14, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Schimel, J.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Hobara, S.; Osono, T.; Hirose, D.; Noro, K.; Hirota, M.; Benner, R. The roles of microorganisms in litter decomposition and soil formation. Biogeochemistry 2014, 118, 471–486. [Google Scholar] [CrossRef]
- Pellitier, P.T.; Zak, D.R. Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: Why evolutionary history matters. New Phytol. 2018, 217, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Nicolás, C.; Martin-Bertelsen, T.; Floudas, D.; Bentzer, J.; Smits, M.; Johansson, T.; Troein, C.; Persson, P.; Tunlid, A. The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 2019, 13, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Hannula, S.E.; Träger, S. Soil fungal guilds as important drivers of the plant richness–productivity relationship. New Phytol. 2020, 226, 947–949. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yuan, Y.; Liu, Q.; Yin, H. Plant nitrogen acquisition from inorganic and organic sources via root and mycelia pathways in ectomycorrhizal alpine forests. Soil Biol. Biochem. 2019, 136, 107517. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Matías, L.; Castro, J.; Zamora, R. Soil-nutrient availability under a global-change scenario in a Mediterranean mountain ecosystem. Glob. Chang. Biol. 2011, 17, 1646–1657. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Pan, F.; Jiang, Z.; Li, Q.; Pu, J.; Liu, K. Accumulation in nutrient acquisition strategies of arbuscular mycorrhizal fungi and plant roots in poor and heterogeneous soils of karst shrub ecosystems. BMC Plant Biol. 2022, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef]
- Bano, S.; Iqbal, S.M. Biological nitrogen fixation to improve plant growth and productivity. Int. J. Agric. Innov. Res. 2016, 4, 597–599. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B.; et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 2002, 57, 1–45. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W.M.; Elliott, G.N.; Bontemps, C.; Estrada-de Los Santos, P.; Gross, E.; Dos Reis, F.B.; Sprent, J.I.; et al. Legume-nodulating betaproteobacteria: Diversity, host range, and future prospects. Mol. Plant Microbe Interact. 2011, 24, 1276–1288. [Google Scholar] [CrossRef] [Green Version]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.C.; Vitousek, P.M.; Cleveland, C.C. Are patterns in nutrient limitation belowground consistent with those aboveground: Results from a 4 million year chronosequence. Biogeochemistry 2011, 106, 323–336. [Google Scholar] [CrossRef]
- Semenov, A.M.; Đukić, D.A. The role of microbial communities in soil formation and soil ecosystem health. Paleontol. J. 2020, 54, 843–852. [Google Scholar] [CrossRef]
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 2013, 10, 3983–3996. [Google Scholar] [CrossRef] [Green Version]
- Taunton, A.E.; Welch, S.A.; Banfield, J.F. Microbial controls on phosphate and lanthanide distributions during granite weathering and soil formation. Chem. Geol. 2000, 169, 371–382. [Google Scholar] [CrossRef]
- Croft, S.A.; Hodge, A.; Pitchford, J.W. Optimal root proliferation strategies: The roles of nutrient heterogeneity, competition and mycorrhizal networks. Plant Soil 2012, 351, 191–206. [Google Scholar] [CrossRef]
- de Kroon, H.; Mommer, L. Root foraging theory put to the test. Trends Ecol. Evol. 2006, 21, 113–116. [Google Scholar] [CrossRef]
- Ostonen, I.; Helmisaari, H.-S.; Borken, W.; Tedersoo, L.; Kukumägi, M.; Bahram, M.; Lindroos, A.-J.; Nöjd, P.; Uri, V.; Merilä, P.; et al. Fine root foraging strategies in Norway spruce forests across a European climate gradient. Glob. Chang. Biol. 2011, 17, 3620–3632. [Google Scholar] [CrossRef]
- Rajaniemi, T.K. Root allocation and foraging precision in heterogeneous soils. Basic Appl. Ecol. 2022, 60, 25–33. [Google Scholar] [CrossRef]
- Keser, L.H.; Visser, E.J.W.; Dawson, W.; Song, Y.-B.; Yu, F.-H.; Fischer, M.; Dong, M.; van Kleunen, M. Herbaceous plant species invading natural areas tend to have stronger adaptive root foraging than other naturalized species. Front. Plant Sci. 2015, 6, 273. [Google Scholar] [CrossRef] [Green Version]
- Kembel, S.W.; Cahill, J.F., Jr. Plant phenotypic plasticity belowground: A phylogenetic perspective on root foraging trade-offs. Am. Nat. 2005, 166, 216–230. [Google Scholar] [CrossRef]
- Oborny, B. The plant body as a network of semi-autonomous agents: A review. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SR, R.; Retuerto, R. Small-scale heterogeneity in soil quality influences photosynthetic efficiency and habitat selection in a clonal plant. Ann. Bot. 2006, 98, 1043–1052. [Google Scholar]
- Alpert, P. Clonal integration in Fragaria chiloensis differs between populations: Ramets from grassland are selfish. Oecologia 1999, 120, 69–76. [Google Scholar] [CrossRef]
- Cabal, C.; Martínez-García, R.; Aguilar, A.d.C.; Valladares, F.; Pacala, S.W. The exploitative segregation of plant roots. Science 2020, 370, 1197–1199. [Google Scholar] [CrossRef]
- Oborny, B.; Kun, Á.; Czárán, T.; Bokros, S. The effect of clonal integration on plant competition for mosaic habitat space. Ecology 2000, 81, 3291–3304. [Google Scholar] [CrossRef]
- Pennings, S.; Callaway, R. The advantages of clonal integration under different ecological conditions: A community-wide test. Ecology 2000, 81, 709–716. [Google Scholar] [CrossRef]
- Hutchings, M.; Wijesinghe, D. Patchy habitats, division of labour and growth dividends in clonal plants. Trends Ecol. Evol. 1997, 12, 390–394. [Google Scholar] [CrossRef]
- Alpert, P.; Stuefer, J.F. Division of labour in clonal plants. In The Ecology and Physiology of Clonal Plants; de Kroon, H., van Groenendael, J., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1997; pp. 137–154. [Google Scholar]
- Van Kleunen, M.; Fischer, M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 2005, 166, 49–60. [Google Scholar] [CrossRef]
- Stuefer, J.F.; Gómez, S.; Mölken, T.v. Clonal integration beyond resource sharing: Implications for defence signalling and disease transmission in clonal plant networks. Evol. Ecol. 2004, 18, 647–667. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Retuerto, R. Clonal integration in Fragaria vesca growing in metal-polluted soils: Parents face penalties for establishing their offspring in unsuitable environments. Ecol. Res. 2012, 27, 95–106. [Google Scholar] [CrossRef]
- Bicharanloo, B.; Bagheri Shirvan, M.; Keitel, C.; Dijkstra, F.A. Rhizodeposition mediates the effect of nitrogen and phosphorous availability on microbial carbon use efficiency and turnover rate. Soil Biol. Biochem. 2020, 142, 107705. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Zhang, L.; Zhou, J.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021, 230, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef]
- Li, Q.; Umer, M.; Guo, Y.; Shen, K.; Xia, T.; Xu, X.; Han, X.; Ren, W.; Sun, Y.; Wu, B.; et al. Karst soil patch heterogeneity with gravels promotes plant root development and nutrient utilization associated with arbuscular mycorrhizal fungi. Agronomy 2022, 12, 1063. [Google Scholar] [CrossRef]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytol. 1998, 139, 479–494. [Google Scholar] [CrossRef]
- Kucey, R.M.N. Phosphate-solubilizing bacteria and fungi in various cultivated and Virgin Alberta soils. Can. J. Soil Sci. 1983, 63, 671–678. [Google Scholar] [CrossRef]
- Barrett, G.; Campbell, C.D.; Fitter, A.H.; Hodge, A. The arbuscular mycorrhizal fungus Glomus hoi can capture and transfer nitrogen from organic patches to its associated host plant at low temperature. Appl. Soil Ecol. 2011, 48, 102–105. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Cameron, D.D.; Hodge, A. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ. 2016, 39, 1683–1690. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facelli, E.; Facelli, J.M. Soil phosphorus heterogeneity and mycorrhizal symbiosis regulate plant intra-specific competition and size distribution. Oecologia 2002, 133, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, T.R.; Smith, F.A.; Smith, S.E.; Jakobsen, I. Functional diversity in arbuscular mycorrhizas: Exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell Environ. 2005, 28, 642–650. [Google Scholar] [CrossRef]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Plant, soil fauna and microbial responses to N-rich organic patches of contrasting temporal availability. Soil Biol. Biochem. 1999, 31, 1517–1530. [Google Scholar] [CrossRef]
- Chen, W.; Koide, R.T.; Adams, T.S.; DeForest, J.L.; Cheng, L.; Eissenstat, D.M. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. USA 2016, 113, 8741–8746. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Chen, W.; Adams, T.S.; Wei, X.; Li, L.; McCormack, M.L.; DeForest, J.L.; Koide, R.T.; Eissenstat, D.M. Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology 2016, 97, 2815–2823. [Google Scholar] [CrossRef]
- Tibbett, M.; Sanders, F.E. Ectomycorrhizal symbiosis can enhance plant nutrition through improved access to discrete organic nutrient patches of high resource quality. Ann. Bot. 2002, 89, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Felderer, B.; Jansa, J.; Schulin, R. Interaction between root growth allocation and mycorrhizal fungi in soil with patchy P distribution. Plant Soil 2013, 373, 569–582. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L.; Magthab, E.A.; Gleeson, D.B.; Hill, P.W.; Sánchez-Rodríguez, A.R.; Roberts, P.; Ge, T.; Murphy, D.V. Microbial competition for nitrogen and carbon is as intense in the subsoil as in the topsoil. Soil Biol. Biochem. 2018, 117, 72–82. [Google Scholar] [CrossRef]
- Čapek, P.; Manzoni, S.; Kaštovská, E.; Wild, B.; Diáková, K.; Bárta, J.; Schnecker, J.; Biasi, C.; Martikainen, P.J.; Alves, R.J.E.; et al. A plant–microbe interaction framework explaining nutrient effects on primary production. Nat. Ecol. Evol. 2018, 2, 1588–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, D.J.; Firestone, M.K.; Nuccio, E.; Hodge, A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 2012, 80, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The role of microbial community in the decomposition of leaf litter and deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E. Below-ground frontiers in trait-based plant ecology. New Phytol. 2017, 213, 1597–1603. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Miransari, M. Soil microbes and the availability of soil nutrients. Acta Physiol. Plant. 2013, 35, 3075–3084. [Google Scholar] [CrossRef]
- Wang, R.; Lu, J.; Jiang, Y.; Dijkstra, F.A. Carbon efficiency for nutrient acquisition (CENA) by plants: Role of nutrient availability and microbial symbionts. Plant Soil 2022, 476, 289–300. [Google Scholar] [CrossRef]
- Fitter, A.H. Costs and benefits of mycorrhizas: Implications for functioning under natural conditions. Experientia 1991, 47, 350–355. [Google Scholar] [CrossRef]
- Snellgrove, R.C.; Splittstoesser, W.E.; Stribley, D.P.; Tinker, P.B. The distribution of carbon and the demand of the fungal symbiont in leek plants with vesicular-arbuscular mycorrhizas. New Phytol. 1982, 92, 75–87. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Johnson, C.R.; Koch, K.E. Carbon cost of the fungal symbiont relative to net leaf p accumulation in a split-root va mycorrhizal symbiosis 1. Plant Physiol. 1988, 86, 491–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Fan, J.; Ding, X.; He, X.; Zhang, F.; Feng, G. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol. Biochem. 2014, 74, 177–183. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Wehner, J.; Antunes, P.M.; Powell, J.R.; Mazukatow, J.; Rillig, M.C. Plant pathogen protection by arbuscular mycorrhizas: A role for fungal diversity? Pedobiologia 2010, 53, 197–201. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef] [Green Version]
- Kakouridis, A.; Hagen, J.A.; Kan, M.P.; Mambelli, S.; Feldman, L.J.; Herman, D.J.; Weber, P.K.; Pett-Ridge, J.; Firestone, M.K. Routes to roots: Direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytol. 2022, 236, 210–221. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Franson, R.L.; Brown, M.S.; Bethlenfalvay, G.J. The Glycine-Glomus-Bradyrhizobiun symbiosis. XI. Nodule gas exchange and efficiency as a function of soil and root water status in mycorrhizal soybean. Physiol. Plant. 1991, 83, 476–482. [Google Scholar] [CrossRef]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.O.; Xue, W.; Du, D.-L.; Yu, F.-H. Soil microbe-mediated N:P stoichiometric effects on Solidago canadensis performance depend on nutrient levels. Microb. Ecol. 2021, 83, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.O.; Xue, W.; Tang, M.; Du, D.-L.; Yu, F.-H. Synergistic effects of soil microbes on Solidago canadensis depend on water and nutrient availability. Microb. Ecol. 2020, 80, 837–845. [Google Scholar]


| Identity of Soil Microorganism | Substrate Type | Host Plant | Effects of Soil Microorganism | References |
|---|---|---|---|---|
| Glomus etunicatum | Karst soil patches | Biden pilosa | Increased overall plant performance | [98] |
| Soil biota | Field, potting, and ceramsite-quartz. | Leymus chinensis | Decreased plant growth in heterogeneous but increased it in homogeneous soil. | [13] |
| Soil microorganisms | N-rich patches | Lolium perenne | Reduced N in microbial biomass. | [104] |
| Gigaspora margarita | Heterogeneous P. | Trifolium subterraneum | Increased total plant biomass and competitive intensity. | [105] |
| AM fungi species | Heterogeneous P. | Linum usitatissimum | Variable response among AM fungi species. | [106] |
| AM fungi | N-organic patches | Lolium perenne | No visible effects detected. | [107] |
| AM vs. EM fungi | Nutrient-rich patches | Multiple tree species | AM fungi increased root proliferation but EM decreased it. | [108] |
| AM vs. EM fungi | Nutrient-rich patches | Multiple tree species | High root and foraging precision and AM fungi proliferation. | [109] |
| Hebeloma syrjense | Nutrient-rich patches | Salix hybrid | Strong N-uptake from organic patches. | [110] |
| Effect | df | Total Mass | Aboveground Mass | Belowground Mass | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Substrate (S) | 2,60 | 21.3 | <0.001 | 16.5 | <0.001 | 5.3 | 0.007 |
| Heterogeneity (H) | 1,60 | 1.0 | 0.301 | 3.5 | 0.065 | 1.3 | 0.250 |
| Soil biota (B) | 1,60 | <0.1 | 0.924 | <0.1 | 0.948 | 0.2 | 0.614 |
| S × H | 2,60 | 1.4 | 0.239 | 0.5 | 0.608 | 3.2 | 0.046 |
| S × B | 2,60 | 0.3 | 0.730 | 0.8 | 0.444 | 0.2 | 0.768 |
| H × B | 1,60 | 21.2 | <0.001 | 11.0 | 0.001 | 13.6 | <0.001 |
| S × H × B | 2,60 | 0.5 | 0.557 | <0.1 | 0.980 | 1.1 | 0.312 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adomako, M.O.; Roiloa, S.; Yu, F.-H. Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance. Microorganisms 2022, 10, 2399. https://doi.org/10.3390/microorganisms10122399
Adomako MO, Roiloa S, Yu F-H. Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance. Microorganisms. 2022; 10(12):2399. https://doi.org/10.3390/microorganisms10122399
Chicago/Turabian StyleAdomako, Michael Opoku, Sergio Roiloa, and Fei-Hai Yu. 2022. "Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance" Microorganisms 10, no. 12: 2399. https://doi.org/10.3390/microorganisms10122399
APA StyleAdomako, M. O., Roiloa, S., & Yu, F.-H. (2022). Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance. Microorganisms, 10(12), 2399. https://doi.org/10.3390/microorganisms10122399









