Surveillance of Legionella pneumophila: Detection in Public Swimming Pool Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Measurement of Legionella pneumophila in Pool Water
2.3. Physicochemical Parameter Measurements in Pool Water
2.4. Statistical Analysis
3. Results and Discussion
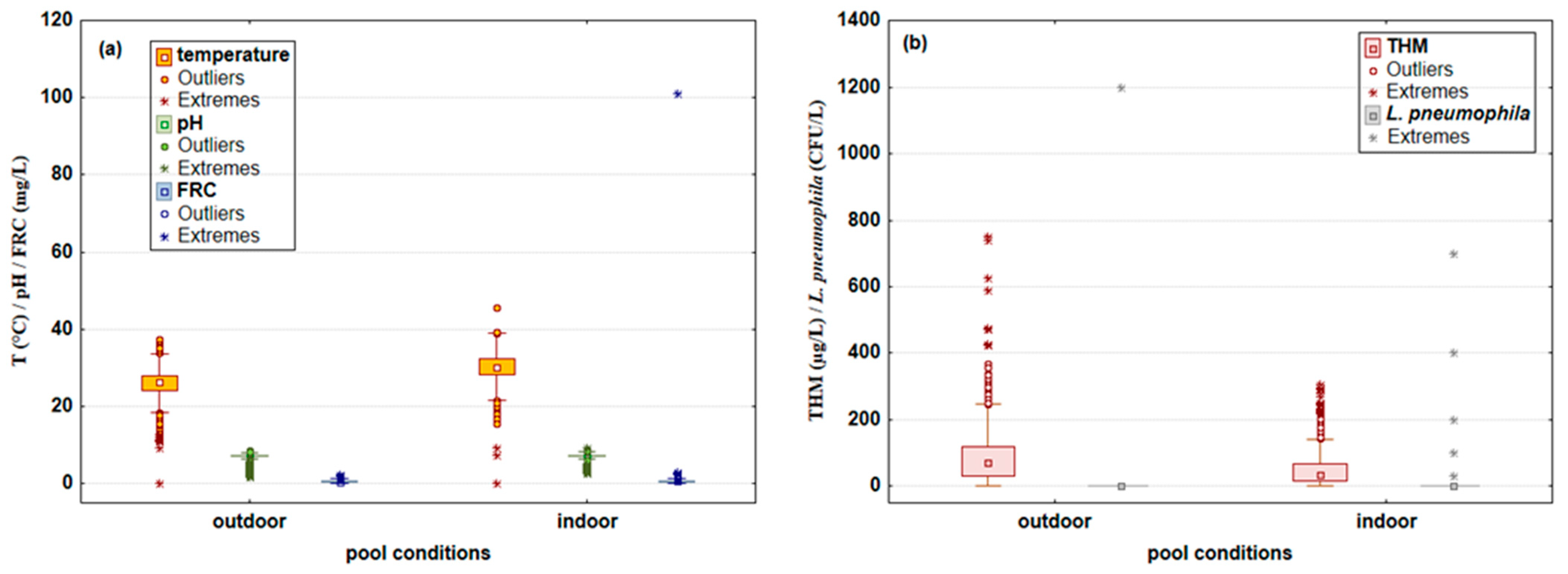
3.1. Microbiological and PhysicoChemical Parameters Measured in Swimming Pool Water Samples
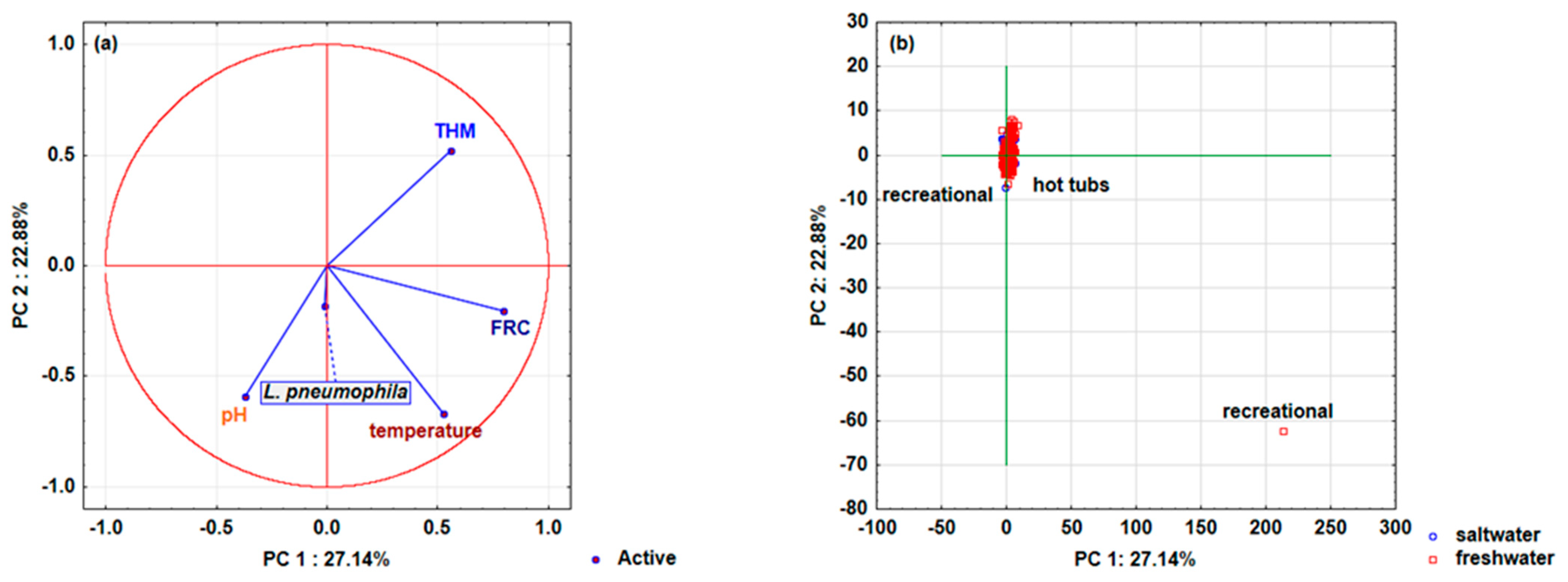
3.2. Predictive Models for the Incidence of Legionella pneumophila
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leoni, E.; Catalani, F.; Marini, S.; Dallolio, L. Legionellosis Associated with Recreational Waters: A Systematic Review of Cases and Outbreaks in Swimming Pools, Spa Pools, and Similar Environments. Int. J. Environ. Res. Public Health 2018, 15, 1612. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.T.; Slow, S.; Scott-Thomas, A.; Murdoch, D.R. Legionellosis Caused by Non-Legionella Pneumophila Species, with a Focus on Legionella Longbeachae. Microorganisms 2021, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Legionnaires’ disease. In Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_Legionnaires.pdf (accessed on 30 October 2022).
- Del Piano, M.; La Palombara, P.; Nicosia, R.; Picchiotti, R. The legionellosis. Boll Ist Sieroter Milan. 1984, 63, 87–99. [Google Scholar] [PubMed]
- Ryu, S.; Yang, K.; Chun, B.C. Community-acquired Legionnaires’ Disease in a Newly Constructed Apartment Building. Prev. Med. Public Health 2017, 50, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Tomić Linšak, D.; Keše, D.; Broznić, D.; Vukić, L.D.; Cenov, A.; Morić, M.; Gobin, I. Sea water whirlpool spa as a source of Legionella infection. J. Water Health 2021, 19, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Assaidi, A.; Ellouali, M.; Latrache, H.; Zahir, H.; Mliji, E.M. Role of Biofilms in the Survival of Legionella Pneumophila to Sodium Chloride Treatment. Iran J. Microbiol. 2021, 13, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Iliadi, V.; Staykova, J.; Iliadis, S.; Konstantinidou, I.; Sivykh, P.; Romanidou, G.; Vardikov, D.F.; Cassimos, D.; Konstantinidis, T.G. Legionella pneumophila: The Journey from the Environment to the Blood. J. Clin. Med. 2022, 11, 6126. [Google Scholar] [CrossRef] [PubMed]
- Sigler Zekanović, M.; Begić, G.; Medić, A.; Gobin, I.; Tomić Linšak, D. Effects of a Combined Disinfection Method on Pseudomonas aeruginosa Biofilm in Freshwater Swimming Pool. Environments 2022, 9, 103. [Google Scholar] [CrossRef]
- Giampaoli, S.; Spica, V.R. Health and safety in recreational waters. Bull. World Health Organ. 2014, 92, 79. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Gentile, M.; Moiraghi, A.R.; Zotti, C.M. Effective environmental sampling strategies for monitoring Legionella spp contamination in hot water systems. Am. J. Infect. Control 2010, 38, 344–349. [Google Scholar] [CrossRef]
- Bédard, E.; Fey, S.; Charron, D.; Lalancette, C.; Cantin, P.; Dolcé, P.; Laferrière, C.; Déziel, E.; Prévost, M. Temperature diagnostic to identify high risk areas and optimize Legionella pneumophila surveillance in hot water distribution systems. Water Res. 2015, 71, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.R.; Centers for Medicare & Medicaid Services. Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease (LD). In S&C 17-30-Hospitals/CAHs/NHs; Department of Health & Human Services: Baltimore, MD, USA, 2017. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Developing a Water Management Program to Reduce Legionella Growth & Spread in Buildings—A Practical Guide to Implementing Industry Standards; US Department of Health and Human Services: Atlanta, GA, USA, 2017.
- Borella, P.; Montagna, M.T.; Romano-Spica, V.; Stampi, S.; Stancanelli, G.; Triassi, M.; Neglia, R.; Marchesi, I.; Fantuzzi, G.; Tato, D.; et al. Legionella infection risk from domestic hot water. Emerg. Infect. Dis. 2004, 10, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Rakic, A.; Vukic Lušic, D.; Jurčev Savicević, A. Influence of Metal Concentration and Plumbing Materials on Legionella Contamination. Microorganisms 2022, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Roque, C.M.; Hazen, T.C. Abundance and distribution of Legionellaceae in Puerto Rican waters. Appl. Environ. Microbiol. 1987, 53, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.J.; Tsai, Y.L.; Paszko-Kolva, C.; Mayer, C.; Sangermano, L.G. Detection of Legionella species in sewage and ocean water by polymerase chain reaction direct fluorescent antibody and plate culture methods. Appl. Environ. Microbiol. 1993, 59, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.; Höller, C.; Süssmuth, R.; Gundermann, K.O. Effect of salt concentration and temperature on survival of Legionella pneumophila. Lett. Appl. Microbiol. 1998, 26, 64–68. [Google Scholar] [CrossRef]
- Parr, A.; Whitney, E.A.; Berkelman, R.L. Legionellosis on the Rise: A Review of Guidelines for Prevention in the United States. J. Public Health Manag. Pract. 2015, 21, E17–E26. [Google Scholar] [CrossRef]
- Van Kenhove, E.; Dinne, K.; Janssens, A.; Laverge, J. Overview and comparison of Legionella regulations worldwide. Am. J. Infect. Control 2019, 47, 968–978. [Google Scholar] [CrossRef]
- EUR-Lex-32020L2184-EN; Directive (EU) 2020/2184 of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast). European Commission: Bruxelles, Belgium, 2020. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 2 November 2022).
- ISO 11731:2017; Water Quality—Enumeration of Legionella. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Glažar Ivče, D.; Rončević, D.; Šantić, M.; Cenov, A.; Tomić Linšak, D.; Mićović, V.; Lušić, D.; Glad, M.; Ljubas, D.; Vukić Lušić, D. Is a Proactive Approach to Controlling Legionella in the Environment Justified? Food Technol. Biotechnol. 2021, 59, 314–324. [Google Scholar] [CrossRef]
- Standard Method 2550 B. Temperature of Water. Standard Methods for the Examination of Water and Waste Water, 23rd ed.; American Public Health Association (APHA): Washington, DC, USA, 2017.
- ISO 7393-2:2018; Water Quality—Determination of Free Chlorine and Total Chlorine—Part 2: Colorimetric Method Using N,N-Diethyl-1,4-Phenylenediamine, for Routine Control Purposes. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- ISO 10523:2008; Water Quality—Determination of pH. International Organization for Standardization (ISO): Geneva, Switzerland, 2008.
- ISO 10301:1997; Water Quality—Determination of Highly Volatile Halogenated Hydrocarbons—Gas-Chromatographic Methods. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Pierre, D.; Baron, J.L.; Ma, X.; Sidari III, F.P.; Wagener, M.M.; Stout, J.E. Water quality as a predictor of Legionella positivity of building water systems. Pathogens 2018, 8, 295. [Google Scholar] [CrossRef]
- Bargellini, A.; Marchesi, I.; Righi, E.; Ferrari, A.; Cencetti, S.; Borella, P.; Rovesti, S. Parameters predictive of Legionella contamination in hot water systems: Association with trace elements and heterotrophic plate counts. Water Res. 2011, 45, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C.; Richardson, S.D.; DeMarini, D.M.; Grummt, T.; Glauner, T.; Frimmel, F. Drowning in disinfection by-products? Assessing swimming pool water. Environ. Sci. Technol. 2007, 41, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, M.M. Assessment of water quality of some swimming pools: A case study in Alexandria, Egypt. Environ. Monit. Assess. 2012, 184, 7395–7406. [Google Scholar] [CrossRef] [PubMed]
- Leoni, E.; Legnani, P.; Guberti, E.; Masotti, A. Risk of infection associated with microbiological quality of public swimming pools in Bologna, Italy. Public Health 1999, 113, 227–232. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Swimming pools and similar environments. In Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2006; Volume 2. [Google Scholar]
- Kuroki, T.; Amemura-Maekawa, J.; Ohya, H.; Furukawa, I.; Suzuki, M.; Masaoka, T.; Kura, F. Outbreak of Legionnaire’s Disease Caused by Legionella pneumophila Serogroups 1 and 13. Emerg. Infect. Dis. 2017, 23, 349–351. [Google Scholar] [CrossRef]
- Euser, S.M.; Pelgrim, M.; den Boer, J.W. Legionnaires’ disease and Pontiac fever after using a private outdoor whirlpool spa. Scand. J. Infect. Dis. 2010, 42, 910–916. [Google Scholar] [CrossRef]
- Silva, Z.I.; Rebelo, M.H.; Silva, M.M.; Alves, A.M.; Cabral Mda, C.; Almeida, A.C.; Aguiar, F.R.; de Oliveira, A.L.; Nogueira, A.C.; Pinhal, H.R.; et al. Trihalomethanes in Lisbon indoor swimming pools: Occurrence, determining factors, and health risk classification. J. Toxicol. Environ. Health Part A 2012, 75, 878–892. [Google Scholar] [CrossRef]
- Simard, S.; Tardif, R.; Rodriguez, M.J. Variability of chlorination by-product occurrence in water of indoor and outdoor swimming pools. Water Res. 2013, 47, 1763–1772. [Google Scholar] [CrossRef]
- Kudlek, E.; Lempart, A.; Dudziak, M.; Bujak, M. Impact of the UV lamp power on the formation of swimming pool water treatment by-products. Archit. Civ. Eng. Environ. 2018, 11, 131–138. [Google Scholar] [CrossRef]
- Marco, E.; Lourencetti, C.; Grimalt, J.O.; Gari, M.; Fernández, P.; Font-Ribera, L.; Villanueva, C.M.; Kogevinas, M. Influence of physical activity in the intake of trihalomethanes in indoor swimming pools. Environ. Res. 2015, 140, 292–299. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Schijven, J.F.; Schalk, J.A.; de Roda Husman, A.M. Quantitative risk estimation for a Legionella pneumophila infection due to whirlpool use. Risk Anal. 2013, 33, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Official Gazette of the Republic of Croatia. Regulation on Sanitary-Technical and Hygienic Conditions of Swimming Pools and on the Health Safety of Pool Waters; Ministry of Health: Zagreb, Croatia, 2020.
- Armstrong, T.W.; Haas, C.N. Quantitative microbial risk assessment model for Legionnaires’ disease: Assessment of human exposures for selected spa outbreaks. J. Occup. Environ. Hyg. 2007, 4, 634–646. [Google Scholar] [CrossRef] [PubMed]



| Pool Characteristics | Variable | Temperature (°C) | pH | THM (μg/L) | FRC (mg/L) |
|---|---|---|---|---|---|
| Outdoor swimming pools (N = 2233) | Legionella pneumophila (CFU/L) | 0.03 | −0.01 | −0.03 | −0.01 |
| Indoor swimming pools (N = 2354) | 0.02 | 0.01 | −0.01 | 0.01 | |
| Recreational (N = 2833) | 0.03 | −0.01 | −0.03 | −0.01 | |
| Hot tubs (N = 966) | −0.02 | 0.01 | −0.02 | 0.02 | |
| Saltwater (N = 1478) | 0.03 | −0.02 | −0.03 | −0.01 | |
| Freshwater (N = 3109) | 0.04 | 0.01 | −0.01 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukić Lušić, D.; Piškur, V.; Cenov, A.; Tomić Linšak, D.; Broznić, D.; Glad, M.; Linšak, Ž. Surveillance of Legionella pneumophila: Detection in Public Swimming Pool Environment. Microorganisms 2022, 10, 2429. https://doi.org/10.3390/microorganisms10122429
Vukić Lušić D, Piškur V, Cenov A, Tomić Linšak D, Broznić D, Glad M, Linšak Ž. Surveillance of Legionella pneumophila: Detection in Public Swimming Pool Environment. Microorganisms. 2022; 10(12):2429. https://doi.org/10.3390/microorganisms10122429
Chicago/Turabian StyleVukić Lušić, Darija, Vanda Piškur, Arijana Cenov, Dijana Tomić Linšak, Dalibor Broznić, Marin Glad, and Željko Linšak. 2022. "Surveillance of Legionella pneumophila: Detection in Public Swimming Pool Environment" Microorganisms 10, no. 12: 2429. https://doi.org/10.3390/microorganisms10122429
APA StyleVukić Lušić, D., Piškur, V., Cenov, A., Tomić Linšak, D., Broznić, D., Glad, M., & Linšak, Ž. (2022). Surveillance of Legionella pneumophila: Detection in Public Swimming Pool Environment. Microorganisms, 10(12), 2429. https://doi.org/10.3390/microorganisms10122429







