Leishmania Vesicle-Depleted Exoproteome: What, Why, and How?
Abstract
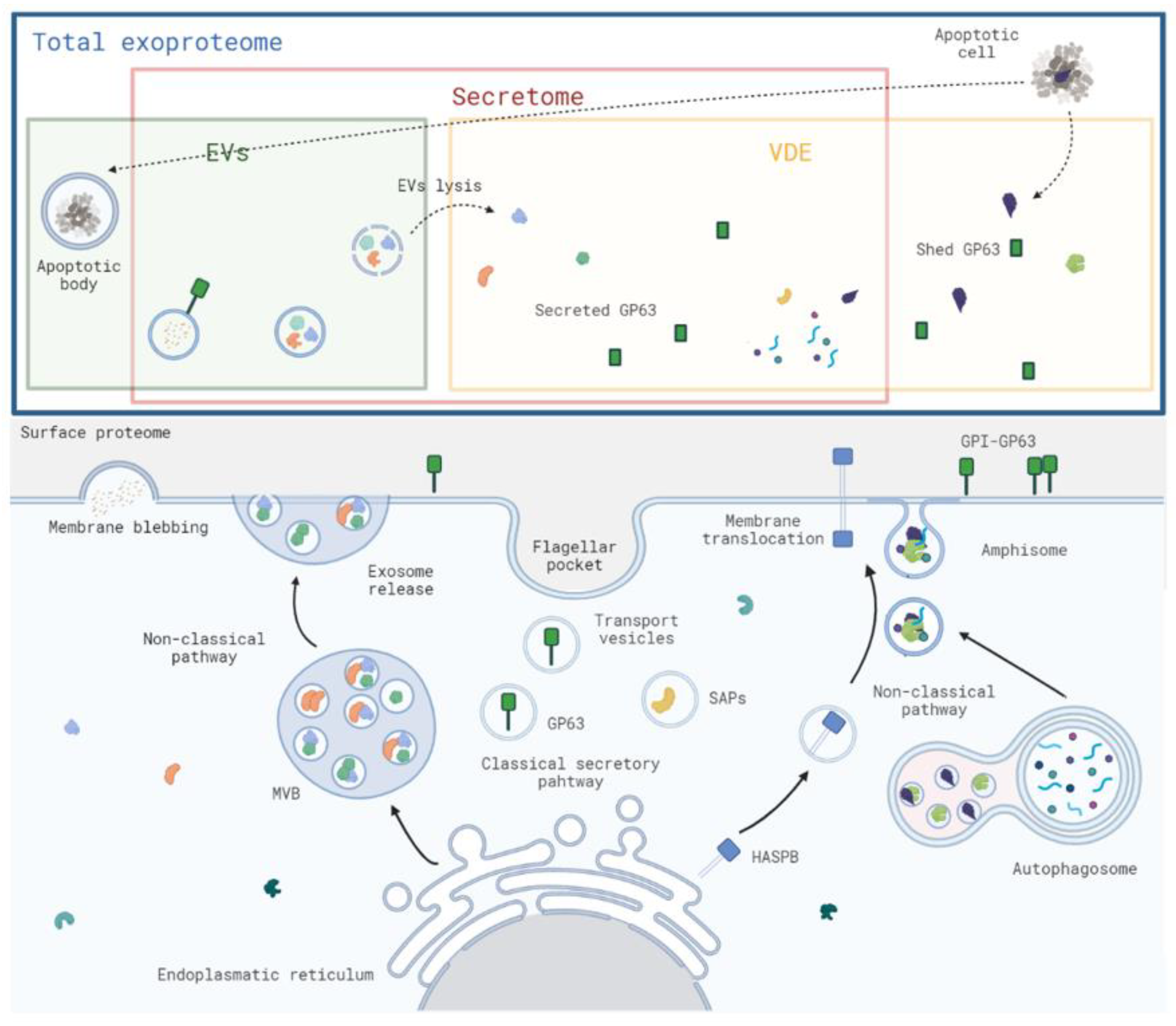
1. Introduction
2. Exoproteome
2.1. Definition
2.2. Biological Sources of Exoproteome Components in Leishmania spp.
2.2.1. Conventional Secretory Pathway
2.2.2. Unconventional Secretory Pathway
2.2.3. Other Contributions to the Exoproteome Composition
3. The Vesicle-Depleted Exoproteome
3.1. Available Proteomic Information on Leishmania spp. VDE
3.2. VDE Recovery Approaches
3.2.1. Cultivation Method
3.2.2. Vesicle-Depleted Exoproteome Recovery
3.3. VDE Research Potential
3.3.1. Diagnostics
3.3.2. Vaccine Development
3.3.3. Understanding of Basic Parasite Biology and Host–Parasite Interactions
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Leishmaniasis. 2022. Available online: http://www.who.int/leishmaniasis/en/ (accessed on 2 October 2022).
- Vuitika, L.; Prates-Syed, W.A.; Silva, J.D.Q.; Crema, K.P.; Cortes, N.; Lira, A.; Lima, J.B.M.; Camara, N.O.S.; Schimke, L.F.; Cabral-Marques, O.; et al. Vaccines against Emerging and Neglected Infectious Diseases: An Overview. Vaccines 2022, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; de Souza, M.V.N. Current leishmaniasis drug discovery. RSC Med. Chem. 2022, 13, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Meneses, A.V.; Corbeil, A.; Wagner, V.; Onwuchekwa, C.; Fernandez-Prada, C. Identification of asymptomatic Leishmania infections: A scoping review. Parasit Vectors 2022, 15, 5. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Miro, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine Leishmaniasis Control in the Context of One Health. Emerg. Infect. Dis. 2019, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, J.; Christie-Oleza, J.A.; Clair, G.; Malard, V.; Duport, C. Exoproteomics: Exploring the world around biological systems. Expert Rev. Proteom. 2012, 9, 561–575. [Google Scholar] [CrossRef]
- Garg, G.; Singh, K.; Ali, V. Proteomic approaches unravel the intricacy of secreted proteins of Leishmania: An updated review. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Clos, J.; de’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 2010, 123, 842–852. [Google Scholar] [CrossRef]
- Dong, G.; Filho, A.L.; Olivier, M. Modulation of Host-Pathogen Communication by Extracellular Vesicles (EVs) of the Protozoan Parasite Leishmania. Front. Cell. Infect. Microbiol. 2019, 9, 100. [Google Scholar] [CrossRef]
- Dong, G.; Wagner, V.; Minguez-Menendez, A.; Fernandez-Prada, C.; Olivier, M. Extracellular vesicles and leishmaniasis: Current knowledge and promising avenues for future development. Mol. Immunol. 2021, 135, 73–83. [Google Scholar] [CrossRef]
- Torrecilhas, A.C.; Soares, R.P.; Schenkman, S.; Fernandez-Prada, C.; Olivier, M. Extracellular Vesicles in Trypanosomatids: Host Cell Communication. Front. Cell. Infect. Microbiol. 2020, 10, 602502. [Google Scholar] [CrossRef]
- Marti, M.; Johnson, P.J. Emerging roles for extracellular vesicles in parasitic infections. Curr. Opin. Microbiol. 2016, 32, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Rossi, I.V.; Ferreira Nunes, M.A.; Vargas-Otalora, S.; da Silva Ferreira, T.C.; Cortez, M.; Ramirez, M.I. Extracellular Vesicles during TriTryps infection: Complexity and future challenges. Mol. Immunol. 2021, 132, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Gavinho, B.; Rossi, I.V.; Evans-Osses, I.; Inal, J.; Ramirez, M.I. A new landscape of host-protozoa interactions involving the extracellular vesicles world. Parasitology 2018, 145, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Szempruch, A.J.; Dennison, L.; Kieft, R.; Harrington, J.M.; Hajduk, S.L. Sending a message: Extracellular vesicles of pathogenic protozoan parasites. Nat. Rev. Microbiol. 2016, 14, 669–675. [Google Scholar] [CrossRef]
- De Souza, W.; Barrias, E.S. Membrane-bound extracellular vesicles secreted by parasitic protozoa: Cellular structures involved in the communication between cells. Parasitol. Res. 2020, 119, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Fernandez-Prada, C. Leishmania and its exosomal pathway: A novel direction for vaccine development. Future Microbiol. 2019, 14, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Peysselon, F.; Launay, G.; Lisacek, F.; Duclos, B.; Ricard-Blum, S. Comparative analysis of Leishmania exoproteomes: Implication for host-pathogen interactions. Biochim. Biophys. Acta 2013, 1834, 2653–2662. [Google Scholar] [CrossRef]
- Cavanagh, J.P.; Askarian, F.; Pain, M.; Bruun, J.A.; Urbarova, I.; Wai, S.N.; Schmidt, F.; Johannessen, M. Proteome profiling of secreted and membrane vesicle associated proteins of an invasive and a commensal Staphylococcus haemolyticus isolate. Data Brief 2019, 22, 914–919. [Google Scholar] [CrossRef]
- Couto, N.; Schooling, S.R.; Dutcher, J.R.; Barber, J. Proteome Profiles of Outer Membrane Vesicles and Extracellular Matrix of Pseudomonas aeruginosa Biofilms. J. Proteome Res. 2015, 14, 4207–4222. [Google Scholar] [CrossRef]
- Santarem, N.; Racine, G.; Silvestre, R.; Cordeiro-da-Silva, A.; Ouellette, M. Exoproteome dynamics in Leishmania infantum. J. Proteom. 2013, 84, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Reales-Calderon, J.A.; Corona, F.; Monteoliva, L.; Gil, C.; Martinez, J.L. Quantitative proteomics unravels that the post-transcriptional regulator Crc modulates the generation of vesicles and secreted virulence determinants of Pseudomonas aeruginosa. J. Proteom. 2015, 127, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Monteoliva, L.; Gil, C. Global Proteomic Profiling of the Secretome of Candida albicans ecm33 Cell Wall Mutant Reveals the Involvement of Ecm33 in Sap2 Secretion. J. Proteome Res. 2015, 14, 4270–4281. [Google Scholar] [CrossRef]
- Ribeiro, K.S.; Vasconcellos, C.I.; Soares, R.P.; Mendes, M.T.; Ellis, C.C.; Aguilera-Flores, M.; de Almeida, I.C.; Schenkman, S.; Iwai, L.K.; Torrecilhas, A.C. Proteomic analysis reveals different composition of extracellular vesicles released by two Trypanosoma cruzi strains associated with their distinct interaction with host cells. J. Extracell. Vesicles 2018, 7, 1463779. [Google Scholar] [CrossRef]
- Goncalves, D.S.; Ferreira, M.D.S.; Liedke, S.C.; Gomes, K.X.; de Oliveira, G.A.; Leao, P.E.L.; Cesar, G.V.; Seabra, S.H.; Cortines, J.R.; Casadevall, A.; et al. Extracellular vesicles and vesicle-free secretome of the protozoa Acanthamoeba castellanii under homeostasis and nutritional stress and their damaging potential to host cells. Virulence 2018, 9, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Olajide, J.S.; Xiong, L.; Yang, S.; Qu, Z.; Xu, X.; Yang, B.; Wang, J.; Liu, B.; Ma, X.; Cai, J. Eimeria falciformis secretes extracellular vesicles to modulate proinflammatory response during interaction with mouse intestinal epithelial cells. Parasit Vectors 2022, 15, 245. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Aguilar-Bonavides, C.; Rodrigues, S.P.; Cordero, E.M.; Marques, A.F.; Varela-Ramirez, A.; Choi, H.; Yoshida, N.; da Silveira, J.F.; Almeida, I.C. Proteomic analysis of Trypanosoma cruzi secretome: Characterization of two populations of extracellular vesicles and soluble proteins. J. Proteome Res. 2013, 12, 883–897. [Google Scholar] [CrossRef]
- Papait, A.; Ragni, E.; Cargnoni, A.; Vertua, E.; Romele, P.; Masserdotti, A.; Perucca Orfei, C.; Signoroni, P.B.; Magatti, M.; Silini, A.R.; et al. Comparison of EV-free fraction, EVs, and total secretome of amniotic mesenchymal stromal cells for their immunomodulatory potential: A translational perspective. Front. Immunol. 2022, 13, 960909. [Google Scholar] [CrossRef]
- Hathout, Y. Approaches to the study of the cell secretome. Expert Rev. Proteom. 2007, 4, 239–248. [Google Scholar] [CrossRef]
- Corrales, R.M.; Sereno, D.; Mathieu-Daude, F. Deciphering the Leishmania exoproteome: What we know and what we can learn. FEMS Immunol. Med. Microbiol. 2010, 58, 27–38. [Google Scholar] [CrossRef]
- Schatz, G.; Dobberstein, B. Common principles of protein translocation across membranes. Science 1996, 271, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- McConville, M.J.; Mullin, K.A.; Ilgoutz, S.C.; Teasdale, R.D. Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 122–154. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Natesan, S.K.; Gabernet-Castello, C.; Koumandou, V.L. Intracellular trafficking in the trypanosomatids. Traffic 2007, 8, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Landfear, S.M.; Ignatushchenko, M. The flagellum and flagellar pocket of trypanosomatids. Mol. Biochem. Parasitol. 2001, 115, 1–17. [Google Scholar] [CrossRef]
- Coppens, I.; Opperdoes, F.R.; Courtoy, P.J.; Baudhuin, P. Receptor-mediated endocytosis in the bloodstream form of Trypanosoma brucei. J. Protozool. 1987, 34, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Overath, P.; Stierhof, Y.D.; Wiese, M. Endocytosis and secretion in trypanosomatid parasites—Tumultuous traffic in a pocket. Trends Cell Biol. 1997, 7, 27–33. [Google Scholar] [CrossRef]
- Duszenko, M.; Ivanov, I.E.; Ferguson, M.A.; Plesken, H.; Cross, G.A. Intracellular transport of a variant surface glycoprotein in Trypanosoma brucei. J. Cell Biol. 1988, 106, 77–86. [Google Scholar] [CrossRef]
- Rogers, M.E.; Chance, M.L.; Bates, P.A. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology 2002, 124, 495–507. [Google Scholar] [CrossRef]
- Rogers, M.E.; Ilg, T.; Nikolaev, A.V.; Ferguson, M.A.; Bates, P.A. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature 2004, 430, 463–467. [Google Scholar] [CrossRef]
- Rogers, M.E.; Bates, P.A. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog. 2007, 3, e91. [Google Scholar] [CrossRef]
- Secundino, N.; Kimblin, N.; Peters, N.C.; Lawyer, P.; Capul, A.A.; Beverley, S.M.; Turco, S.J.; Sacks, D. Proteophosphoglycan confers resistance of Leishmania major to midgut digestive enzymes induced by blood feeding in vector sand flies. Cell. Microbiol. 2010, 12, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.E.; Corware, K.; Muller, I.; Bates, P.A. Leishmania infantum proteophosphoglycans regurgitated by the bite of its natural sand fly vector, Lutzomyia longipalpis, promote parasite establishment in mouse skin and skin-distant tissues. Microbes Infect. 2010, 12, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Dwyer, D.M. Identification and partial characterization of an extracellular acid phosphatase activity of Leishmania donovani promastigotes. Mol. Cell. Biol. 1982, 2, 76–81. [Google Scholar]
- Singla, N.; Khuller, G.K.; Vinayak, V.K. Acid phosphatase activity of promastigotes of Leishmania donovani: A marker of virulence. FEMS Microbiol. Lett. 1992, 73, 221–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katakura, K.; Kobayashi, A. Acid phosphatase activity of virulent and avirulent clones of Leishmania donovani promastigotes. Infect. Immun. 1988, 56, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Ilg, T.; Stierhof, Y.D.; Etges, R.; Adrian, M.; Harbecke, D.; Overath, P. Secreted acid phosphatase of Leishmania mexicana: A filamentous phosphoglycoprotein polymer. Proc. Natl. Acad. Sci. USA 1991, 88, 8774–8778. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.B.; Mallinson, D.J.; Dwyer, D.M. The human pathogen Leishmania donovani secretes a histidine acid phosphatase activity that is resistant to proteolytic degradation. J. Eukaryot. Microbiol. 2004, 51, 108–112. [Google Scholar] [CrossRef]
- Peters, C.; Stierhof, Y.D.; Ilg, T. Proteophosphoglycan secreted by Leishmania mexicana amastigotes causes vacuole formation in macrophages. Infect. Immun. 1997, 65, 783–786. [Google Scholar] [CrossRef]
- Peters, C.; Kawakami, M.; Kaul, M.; Ilg, T.; Overath, P.; Aebischer, T. Secreted proteophosphoglycan of Leishmania mexicana amastigotes activates complement by triggering the mannan binding lectin pathway. Eur. J. Immunol. 1997, 27, 2666–2672. [Google Scholar] [CrossRef]
- Schlein, Y.; Jacobson, R.L.; Shlomai, J. Chitinase secreted by Leishmania functions in the sandfly vector. Proc. Biol. Sci. 1991, 245, 121–126. [Google Scholar] [CrossRef]
- Rogers, M.E.; Hajmova, M.; Joshi, M.B.; Sadlova, J.; Dwyer, D.M.; Volf, P.; Bates, P.A. Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice. Cell Microbiol. 2008, 10, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.B.; Rogers, M.E.; Shakarian, A.M.; Yamage, M.; Al-Harthi, S.A.; Bates, P.A.; Dwyer, D.M. Molecular characterization, expression, and in vivo analysis of LmexCht1: The chitinase of the human pathogen, Leishmania mexicana. J. Biol. Chem. 2005, 280, 3847–3861. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.R.; Cooper, A.M.; Peacock, C.; Lane, R.P.; Blackwell, J.M. Expression of LPG and GP63 by different developmental stages of Leishmania major in the sandfly Phlebotomus papatasi. Parasitology 1990, 101, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Branquinha, M.H.; D’Avila-Levy, C.M. The ubiquitous gp63-like metalloprotease from lower trypanosomatids: In the search for a function. An. Acad. Bras. Ciências 2006, 78, 687–714. [Google Scholar] [CrossRef]
- Yao, C.; Donelson, J.E.; Wilson, M.E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 2003, 132, 1–16. [Google Scholar] [CrossRef]
- Joshi, P.B.; Kelly, B.L.; Kamhawi, S.; Sacks, D.L.; McMaster, W.R. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 2002, 120, 33–40. [Google Scholar] [CrossRef]
- Gomez, M.A.; Contreras, I.; Halle, M.; Tremblay, M.L.; McMaster, R.W.; Olivier, M. Leishmania GP63 alters host signaling through cleavage-activated protein tyrosine phosphatases. Sci. Signal 2009, 2, ra58. [Google Scholar] [CrossRef]
- Contreras, I.; Gomez, M.A.; Nguyen, O.; Shio, M.T.; McMaster, R.W.; Olivier, M. Leishmania-induced inactivation of the macrophage transcription factor AP-1 is mediated by the parasite metalloprotease GP63. PLoS Pathog. 2010, 6, e1001148. [Google Scholar] [CrossRef]
- Nickel, W.; Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell. Biol. 2009, 10, 148–155. [Google Scholar] [CrossRef]
- Silverman, J.M.; Chan, S.K.; Robinson, D.P.; Dwyer, D.M.; Nandan, D.; Foster, L.J.; Reiner, N.E. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 2008, 9, R35. [Google Scholar] [CrossRef]
- Figueiredo, R.C.; Soares, M.J. The Golgi complex of Trypanosoma cruzi epimastigote forms. J. Submicrosc. Cytol. Pathol. 1995, 27, 209–215. [Google Scholar] [PubMed]
- Nickel, W. Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 2010, 21, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Stegmayer, C.; Kehlenbach, A.; Tournaviti, S.; Wegehingel, S.; Zehe, C.; Denny, P.; Smith, D.F.; Schwappach, B.; Nickel, W. Direct transport across the plasma membrane of mammalian cells of Leishmania HASPB as revealed by a CHO export mutant. J. Cell. Sci. 2005, 118, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Flinn, H.M.; Rangarajan, D.; Smith, D.F. Expression of a hydrophilic surface protein in infective stages of Leishmania major. Mol. Biochem. Parasitol. 1994, 65, 259–270. [Google Scholar] [CrossRef]
- Sadlova, J.; Price, H.P.; Smith, B.A.; Votypka, J.; Volf, P.; Smith, D.F. The stage-regulated HASPB and SHERP proteins are essential for differentiation of the protozoan parasite Leishmania major in its sand fly vector, Phlebotomus papatasi. Cell Microbiol. 2010, 12, 1765–1779. [Google Scholar] [CrossRef]
- Tournaviti, S.; Pietro, E.S.; Terjung, S.; Schafmeier, T.; Wegehingel, S.; Ritzerfeld, J.; Schulz, J.; Smith, D.F.; Pepperkok, R.; Nickel, W. Reversible phosphorylation as a molecular switch to regulate plasma membrane targeting of acylated SH4 domain proteins. Traffic 2009, 10, 1047–1060. [Google Scholar] [CrossRef]
- Denny, P.W.; Gokool, S.; Russell, D.G.; Field, M.C.; Smith, D.F. Acylation-dependent protein export in Leishmania. J. Biol. Chem. 2000, 275, 11017–11025. [Google Scholar] [CrossRef]
- Tournaviti, S.; Hannemann, S.; Terjung, S.; Kitzing, T.M.; Stegmayer, C.; Ritzerfeld, J.; Walther, P.; Grosse, R.; Nickel, W.; Fackler, O.T. SH4-domain-induced plasma membrane dynamization promotes bleb-associated cell motility. J. Cell. Sci. 2007, 120, 3820–3829. [Google Scholar] [CrossRef]
- Qu, Y.; Franchi, L.; Nunez, G.; Dubyak, G.R. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 2007, 179, 1913–1925. [Google Scholar] [CrossRef]
- Ganesan, D.; Cai, Q. Understanding amphisomes. Biochem. J. 2021, 478, 1959–1976. [Google Scholar] [CrossRef]
- Schuck, S. Microautophagy—Distinct molecular mechanisms handle cargoes of many sizes. J. Cell. Sci. 2020, 133, jcs246322. [Google Scholar] [CrossRef] [PubMed]
- esteiro, S.; Williams, R.A.; Morrison, L.S.; Coombs, G.H.; Mottram, J.C. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J. Biol. Chem. 2006, 281, 11384–11396. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.; Perez-Morga, D.; Schtickzelle, N.; Michels, P.A. Turnover of glycosomes during life-cycle differentiation of Trypanosoma brucei. Autophagy 2008, 4, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Michels, P.A.; Bringaud, F.; Herman, M.; Hannaert, V. Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta 2006, 1763, 1463–1477. [Google Scholar] [CrossRef]
- Perez-Cabezas, B.; Santarem, N.; Cecilio, P.; Silva, C.; Silvestre, R.; J, A.M.C.; Cordeiro da Silva, A. More than just exosomes: Distinct Leishmania infantum extracellular products potentiate the establishment of infection. J. Extracell. Vesicles 2019, 8, 1541708. [Google Scholar] [CrossRef]
- Vucetic, A.; Filho, A.; Dong, G.; Olivier, M. Isolation of Extracellular Vesicles from Leishmania spp. Methods Mol. Biol. 2020, 2116, 555–574. [Google Scholar] [CrossRef]
- Gabriel, A.M.; Galue-Parra, A.; Pereira, W.L.A.; Pedersen, K.W.; da Silva, E.O. Leishmania 360 degrees: Guidelines for Exosomal Research. Microorganisms 2021, 9, 2081. [Google Scholar] [CrossRef]
- Schneider, P.; Rosat, J.P.; Bouvier, J.; Louis, J.; Bordier, C. Leishmania major: Differential regulation of the surface metalloprotease in amastigote and promastigote stages. Exp. Parasitol. 1992, 75, 196–206. [Google Scholar] [CrossRef]
- Yao, C.; Donelson, J.E.; Wilson, M.E. Internal and surface-localized major surface proteases of Leishmania spp. and their differential release from promastigotes. Eukaryot Cell 2007, 6, 1905–1912. [Google Scholar] [CrossRef][Green Version]
- Chang, K.P.; Reed, S.G.; McGwire, B.S.; Soong, L. Leishmania model for microbial virulence: The relevance of parasite multiplication and pathoantigenicity. Acta Trop. 2003, 85, 375–390. [Google Scholar] [CrossRef]
- Santarem, N.; Silvestre, R.; Tavares, J.; Silva, M.; Cabral, S.; Maciel, J.; Cordeiro-da-Silva, A. Immune response regulation by leishmania secreted and nonsecreted antigens. J. Biomed. Biotechnol. 2007, 2007, 85154. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vega, A.; Losada-Barragan, M.; Berbert, L.R.; Mesquita-Rodrigues, C.; Bombaca, A.C.S.; Menna-Barreto, R.; Aquino, P.; Carvalho, P.C.; Padron, G.; de Jesus, J.B.; et al. Quantitative analysis of proteins secreted by Leishmania (Viannia) braziliensis strains associated to distinct clinical manifestations of American Tegumentary Leishmaniasis. J. Proteom. 2021, 232, 104077. [Google Scholar] [CrossRef] [PubMed]
- Pissarra, J.; Pagniez, J.; Petitdidier, E.; Seveno, M.; Vigy, O.; Bras-Goncalves, R.; Lemesre, J.L.; Holzmuller, P. Proteomic Analysis of the Promastigote Secretome of Seven Leishmania Species. J. Proteome Res. 2022, 21, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, P.; De Jesus, J.B.; Saboia-Vahia, L.; Mendonca-Lima, L.; Domont, G.B.; Cupolillo, E. Proteomic characterization of the released/secreted proteins of Leishmania (Viannia) braziliensis promastigotes. J. Proteom. 2009, 73, 79–92. [Google Scholar] [CrossRef]
- Braga, M.S.; Neves, L.X.; Campos, J.M.; Roatt, B.M.; de Oliveira Aguiar Soares, R.D.; Braga, S.L.; de Melo Resende, D.; Reis, A.B.; Castro-Borges, W. Shotgun proteomics to unravel the complexity of the Leishmania infantum exoproteome and the relative abundance of its constituents. Mol. Biochem. Parasitol. 2014, 195, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Douanne, N.; Dong, G.; Douanne, M.; Olivier, M.; Fernandez-Prada, C. Unravelling the proteomic signature of extracellular vesicles released by drug-resistant Leishmania infantum parasites. PLoS Negl. Trop. Dis. 2020, 14, e0008439. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; Aslan, H.; Townsend, S.; Hassani, K.; Kamhawi, S.; Olivier, M. Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut. Cell Rep. 2015, 13, 957–967. [Google Scholar] [CrossRef]
- Forrest, D.M.; Batista, M.; Marchini, F.K.; Tempone, A.J.; Traub-Cseko, Y.M. Proteomic analysis of exosomes derived from procyclic and metacyclic-like cultured Leishmania infantum chagasi. J. Proteom. 2020, 227, 103902. [Google Scholar] [CrossRef]
- da Silva Lira Filho, A.; Fajardo, E.F.; Chang, K.P.; Clement, P.; Olivier, M. Leishmania Exosomes/Extracellular Vesicles Containing GP63 Are Essential for Enhance Cutaneous Leishmaniasis Development Upon Co-Inoculation of Leishmania amazonensis and Its Exosomes. Front. Cell. Infect. Microbiol. 2021, 11, 709258. [Google Scholar] [CrossRef]
- Hassani, K.; Antoniak, E.; Jardim, A.; Olivier, M. Temperature-induced protein secretion by Leishmania mexicana modulates macrophage signalling and function. PLoS ONE 2011, 6, e18724. [Google Scholar] [CrossRef]
- Belo, R.; Santarem, N.; Pereira, C.; Perez-Cabezas, B.; Macedo, F.; Leite-de-Moraes, M.; Cordeiro-da-Silva, A. Leishmania infantum Exoproducts Inhibit Human Invariant NKT Cell Expansion and Activation. Front. Immunol. 2017, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Toth, E.A.; Turiak, L.; Visnovitz, T.; Cserep, C.; Mazlo, A.; Sodar, B.W.; Forsonits, A.I.; Petovari, G.; Sebestyen, A.; Komlosi, Z.; et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell. Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.S. Mass spectrometry-based proteomics in the life sciences. Cell Mol. Life Sci. 2005, 62, 848–869. [Google Scholar] [CrossRef] [PubMed]
- Santarem, N.; Cunha, J.; Silvestre, R.; Silva, C.; Moreira, D.; Ouellette, M.; Cordeiro, D.A.S.A. The impact of distinct culture media in Leishmania infantum biology and infectivity. Parasitology 2014, 141, 192–205. [Google Scholar] [CrossRef]
- Lyda, T.A.; Joshi, M.B.; Andersen, J.F.; Kelada, A.Y.; Owings, J.P.; Bates, P.A.; Dwyer, D.M. A unique, highly conserved secretory invertase is differentially expressed by promastigote developmental forms of all species of the human pathogen, Leishmania. Mol. Cell. Biochem. 2015, 404, 53–77. [Google Scholar] [CrossRef]
- Blum, J.J.; Opperdoes, F.R. Secretion of sucrase by Leishmania donovani. J. Eukaryot. Microbiol. 1994, 41, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Kornilov, R.; Puhka, M.; Mannerstrom, B.; Hiidenmaa, H.; Peltoniemi, H.; Siljander, P.; Seppanen-Kaijansinkko, R.; Kaur, S. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2018, 7, 1422674. [Google Scholar] [CrossRef]
- Gil, C.; Solano, C.; Burgui, S.; Latasa, C.; Garcia, B.; Toledo-Arana, A.; Lasa, I.; Valle, J. Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infect. Immun. 2014, 82, 1017–1029. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Wang, G.; Yin, H.; Wang, M. Immunization with excreted-secreted antigens reduces tissue cyst formation in pigs. Parasitol. Res. 2013, 112, 3835–3842. [Google Scholar] [CrossRef]
- Pacheco-Fernandez, T.; Volpedo, G.; Gannavaram, S.; Bhattacharya, P.; Dey, R.; Satoskar, A.; Matlashewski, G.; Nakhasi, H.L. Revival of Leishmanization and Leishmanin. Front. Cell. Infect. Microbiol. 2021, 11, 639801. [Google Scholar] [CrossRef]
- Tonui, W.K.; Mejia, J.S.; Hochberg, L.; Mbow, M.L.; Ryan, J.R.; Chan, A.S.; Martin, S.K.; Titus, R.G. Immunization with Leishmania major exogenous antigens protects susceptible BALB/c mice against challenge infection with L. major. Infect. Immun. 2004, 72, 5654–5661. [Google Scholar] [CrossRef]
- Tonui, W.K.; Titus, R.G. Cross-protection against Leishmania donovani but not L. Braziliensis caused by vaccination with L. Major soluble promastigote exogenous antigens in BALB/c mice. Am. J. Trop. Med. Hyg. 2007, 76, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Lemesre, J.L.; Holzmuller, P.; Goncalves, R.B.; Bourdoiseau, G.; Hugnet, C.; Cavaleyra, M.; Papierok, G. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: Double-blind randomised efficacy field trial. Vaccine 2007, 25, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Lemesre, J.L.; Holzmuller, P.; Cavaleyra, M.; Goncalves, R.B.; Hottin, G.; Papierok, G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine 2005, 23, 2825–2840. [Google Scholar] [CrossRef] [PubMed]
- Velez, R.; Domenech, E.; Rodriguez-Cortes, A.; Barrios, D.; Tebar, S.; Fernandez-Arevalo, A.; Aguilar, R.; Dobano, C.; Alberola, J.; Cairo, J.; et al. Evaluation of canine leishmaniosis vaccine CaniLeish(R) under field conditions in native dog populations from an endemic Mediterranean area-A randomized controlled trial. Acta Trop. 2020, 205, 105387. [Google Scholar] [CrossRef]
- Calzetta, L.; Pistocchini, E.; Ritondo, B.L.; Roncada, P.; Palma, E.; di Cave, D.; Mattei, M.; Britti, D. Immunoprophylaxis pharmacotherapy against canine leishmaniosis: A systematic review and meta-analysis on the efficacy of vaccines approved in European Union. Vaccine 2020, 38, 6695–6703. [Google Scholar] [CrossRef]
- Kumar, A.; Samant, M.; Misra, P.; Khare, P.; Sundar, S.; Garg, R.; Dube, A. Immunostimulatory potential and proteome profiling of Leishmania donovani soluble exogenous antigens. Parasite Immunol. 2015, 37, 368–375. [Google Scholar] [CrossRef]
- Coler, R.N.; Goto, Y.; Bogatzki, L.; Raman, V.; Reed, S.G. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect. Immun. 2007, 75, 4648–4654. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.; Abbehusen, M.; Netto, E.M.; Nakatani, M.; Pedral-Sampaio, G.; de Jesus, R.S.; Goto, Y.; Guderian, J.; Howard, R.F.; Reed, S.G. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine 2010, 28, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.; Fernandes, D.F.; Vieira, E.P.; Campos-Neto, A.; Ashman, J.A.; Alves, F.P.; Coler, R.N.; Bogatzki, L.Y.; Kahn, S.J.; Beckmann, A.M.; et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine 2010, 28, 6581–6587. [Google Scholar] [CrossRef]
- Tabatabaee, P.A.; Abolhassani, M.; Mahdavi, M.; Nahrevanian, H.; Azadmanesh, K. Leishmania major: Secreted antigens of Leishmania major promastigotes shift the immune response of the C57BL/6 mice toward Th2 in vitro. Exp. Parasitol. 2011, 127, 46–51. [Google Scholar] [CrossRef]
- Nandan, D.; Yi, T.; Lopez, M.; Lai, C.; Reiner, N.E. Leishmania EF-1alpha activates the Src homology 2 domain containing tyrosine phosphatase SHP-1 leading to macrophage deactivation. J. Biol. Chem. 2002, 277, 50190–50197. [Google Scholar] [CrossRef] [PubMed]
- Douanne, N.; Dong, G.; Amin, A.; Bernardo, L.; Blanchette, M.; Langlais, D.; Olivier, M.; Fernandez-Prada, C. Leishmania parasites exchange drug-resistance genes through extracellular vesicles. Cell Rep. 2022, 40, 111121. [Google Scholar] [CrossRef] [PubMed]
- Bhavya, G.; Hiremath, K.Y.; Jogaiah, S.; Geetha, N. Heavy metal-induced oxidative stress and alteration in secretory proteins in yeast isolates. Arch. Microbiol. 2022, 204, 172. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, S.A.; Palma Medina, L.M.; Glasner, C.; Tsompanidou, E.; de Jong, A.; Grasso, S.; Schaffer, M.; Mader, U.; Larsen, A.R.; Gumpert, H.; et al. Signatures of cytoplasmic proteins in the exoproteome distinguish community- and hospital-associated methicillin-resistant Staphylococcus aureus USA300 lineages. Virulence 2017, 8, 891–907. [Google Scholar] [CrossRef]
- Garg, G.; Ali, V.; Singh, K.; Gupta, P.; Ganguly, A.; Sahasrabuddhe, A.A.; Das, P. Quantitative secretome analysis unravels new secreted proteins in Amphotericin B resistant Leishmania donovani. J. Proteom. 2019, 207, 103464. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]

| Species | Total Exoproteome | EVs | VDE |
|---|---|---|---|
| Leishmania braziliensis | [83,84,85] | ||
| Leishmania donovani | [62,86,87,88] | [8] | |
| Leishmania infantum | [84,86] | [22,87,89] | [22] |
| Leishmania major | [84] | [88] | |
| Leishmania tropica | [84] | ||
| Leishmania amazonensis | [84] | [90] | |
| Leishmania tarentolae | [84] | ||
| Leishmania mexicana | [91] |
| Most Abundant Core Exoproteome Proteins 1 | Detected in VDE 2 |
|---|---|
| 14-3-3 protein-like protein | Yes |
| Adenosylhomocysteinase | |
| Cytoplasmic tryparedoxin peroxidase | Yes |
| Elongation factor 1-alpha | Yes |
| Elongation factor 2 | Yes |
| Enolase | Yes |
| Heat-shock protein 83-1 | Yes |
| Histone H4 | |
| Leishmanolysin (GP63) | Yes |
| Nucleoside diphosphate kinase | Yes |
| Peptidyl-prolyl cis-trans isomerase | |
| Peroxidoxin 2 | |
| Probable eukaryotic initiation factor 4A | Yes |
| Prostaglandin f2-alpha synthase (Fragment) | |
| Prostaglandin f2-alpha synthase/D-arabinose dehydrogenase | Yes |
| Putative 2,4-dihydroxyhept-2-ene-1,7-dioic acid aldolase | Yes |
| Putative beta-fructofuranosidase | Yes |
| Putative calpain-like cysteine peptidase | Yes |
| Putative heat-shock protein hsp70 | Yes |
| Putative small myristoylated protein-1 | |
| Putative small myristoylated protein-3 | |
| Thiol specific antioxidant | |
| Tryparedoxin peroxidase | Yes |
| Tubulin alpha chain | Yes |
| Tubulin beta chain | Yes |
| Ubiquitin-60S ribosomal protein L40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, S.; Costa, I.; Luelmo, S.; Santarém, N.; Cordeiro-da-Silva, A. Leishmania Vesicle-Depleted Exoproteome: What, Why, and How? Microorganisms 2022, 10, 2435. https://doi.org/10.3390/microorganisms10122435
Esteves S, Costa I, Luelmo S, Santarém N, Cordeiro-da-Silva A. Leishmania Vesicle-Depleted Exoproteome: What, Why, and How? Microorganisms. 2022; 10(12):2435. https://doi.org/10.3390/microorganisms10122435
Chicago/Turabian StyleEsteves, Sofia, Inês Costa, Sara Luelmo, Nuno Santarém, and Anabela Cordeiro-da-Silva. 2022. "Leishmania Vesicle-Depleted Exoproteome: What, Why, and How?" Microorganisms 10, no. 12: 2435. https://doi.org/10.3390/microorganisms10122435
APA StyleEsteves, S., Costa, I., Luelmo, S., Santarém, N., & Cordeiro-da-Silva, A. (2022). Leishmania Vesicle-Depleted Exoproteome: What, Why, and How? Microorganisms, 10(12), 2435. https://doi.org/10.3390/microorganisms10122435








