Bacillus velezensis BY6 Promotes Growth of Poplar and Improves Resistance Contributing to the Biocontrol of Armillaria solidipes
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Strains and Plant Material
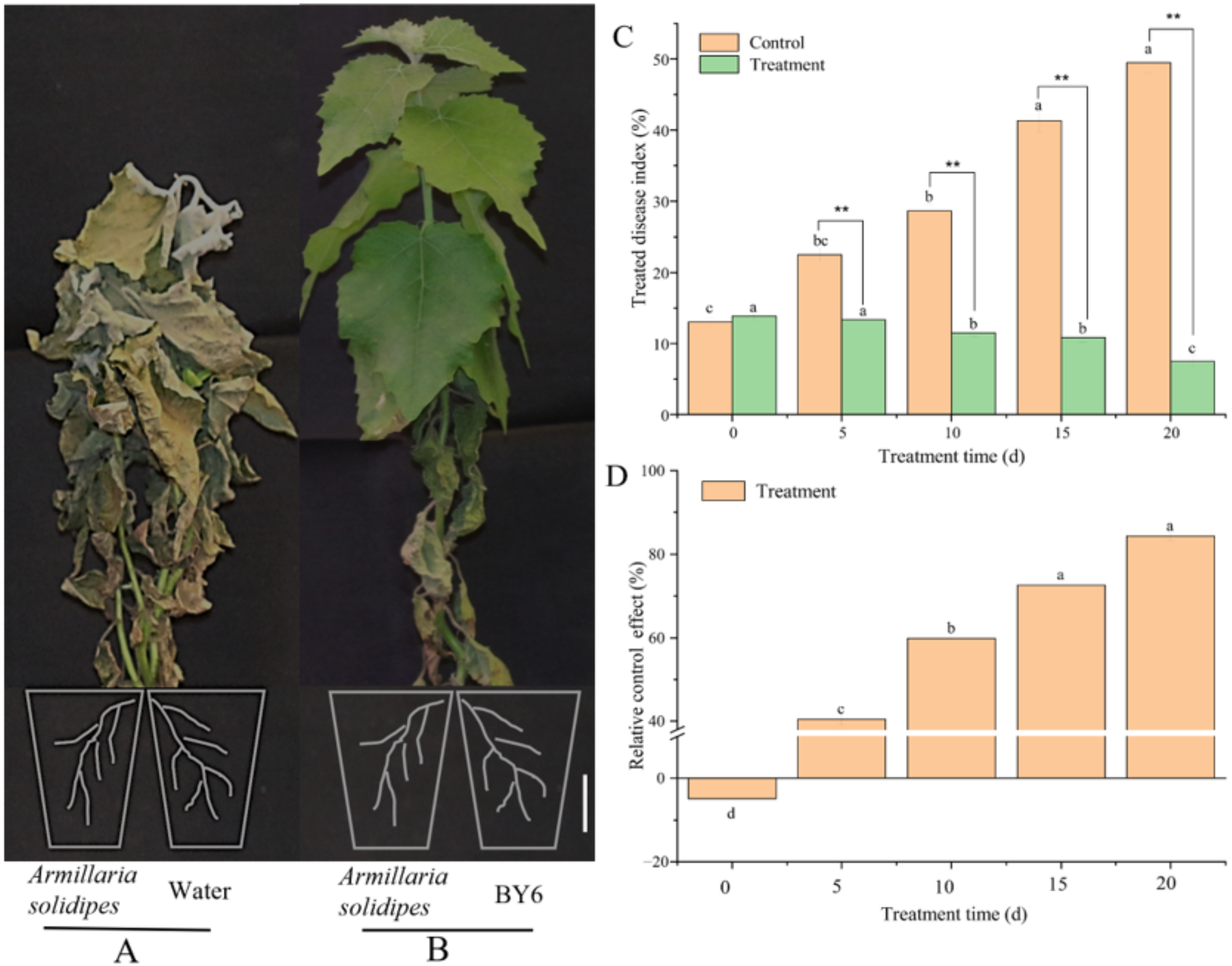
2.2. Determination of Treated Disease Index and Relative Control Effect on Pdpap Poplar ARR after BY6 Induction
2.3. Determination of Pdpap Poplar Enzyme Activity after BY6 Induction
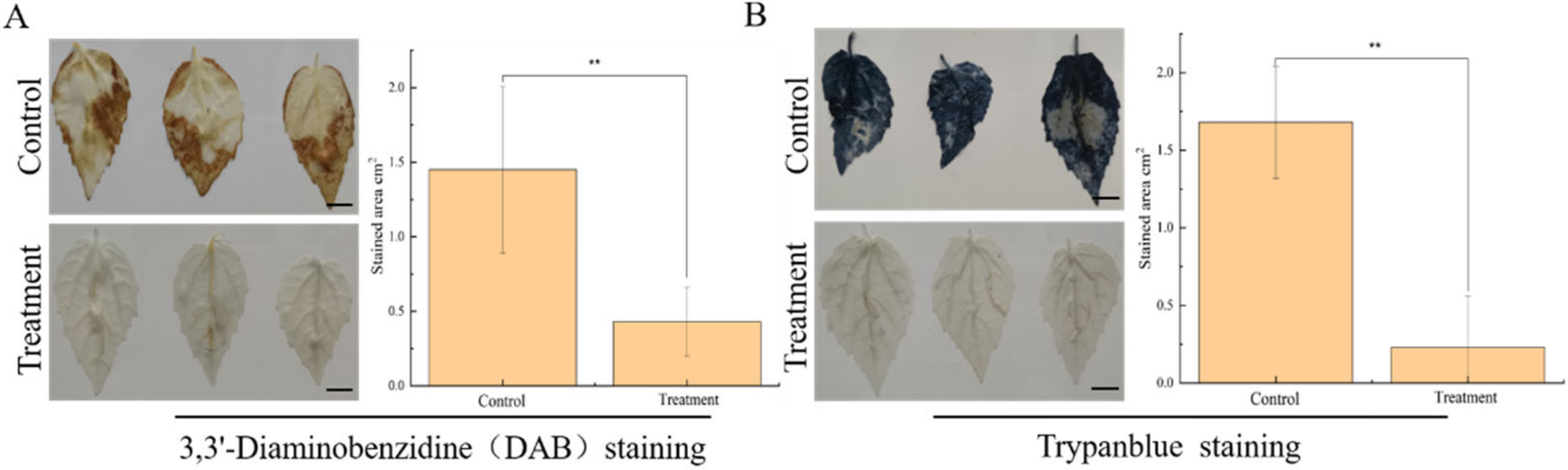
2.4. Detection of Reactive Oxygen Species and Cell Death in Pdpap Poplar Leaves after BY6 Induction
2.5. Determination of Pdpap Poplar Seedling Growth after BY6 Induction
2.6. Determination of Soluble Sugar and Soluble Protein in Pdpap Poplar Seedlings after BY6 Induction
2.7. Determination of Disease Resistance and Auxin Gene Expression in Pdpap Poplar after BY6 Induction
2.8. Statistical Analysis
3. Results
3.1. Treated Disease Index and Relative Control on Pdpap Poplar Root Rot after BY6 Induction
3.2. Effects of BY6 Induction on the Activity of Defense Enzymes in Pdpap Poplar
3.3. Staining Results of Reactive Oxygen Species and Cell Death in Pdpap Poplar Leaves after BY6 Induction
3.4. Effects of BY6 Induction on Pdpap Poplar Growth
3.5. Determination of Soluble Protein and Soluble Sugar in Pdpap Poplar after BY6 Induction
3.6. Determination of Expression Levels of Disease Resistance and Growth-Related Genes in Pdpap Poplar after BY6 Induction
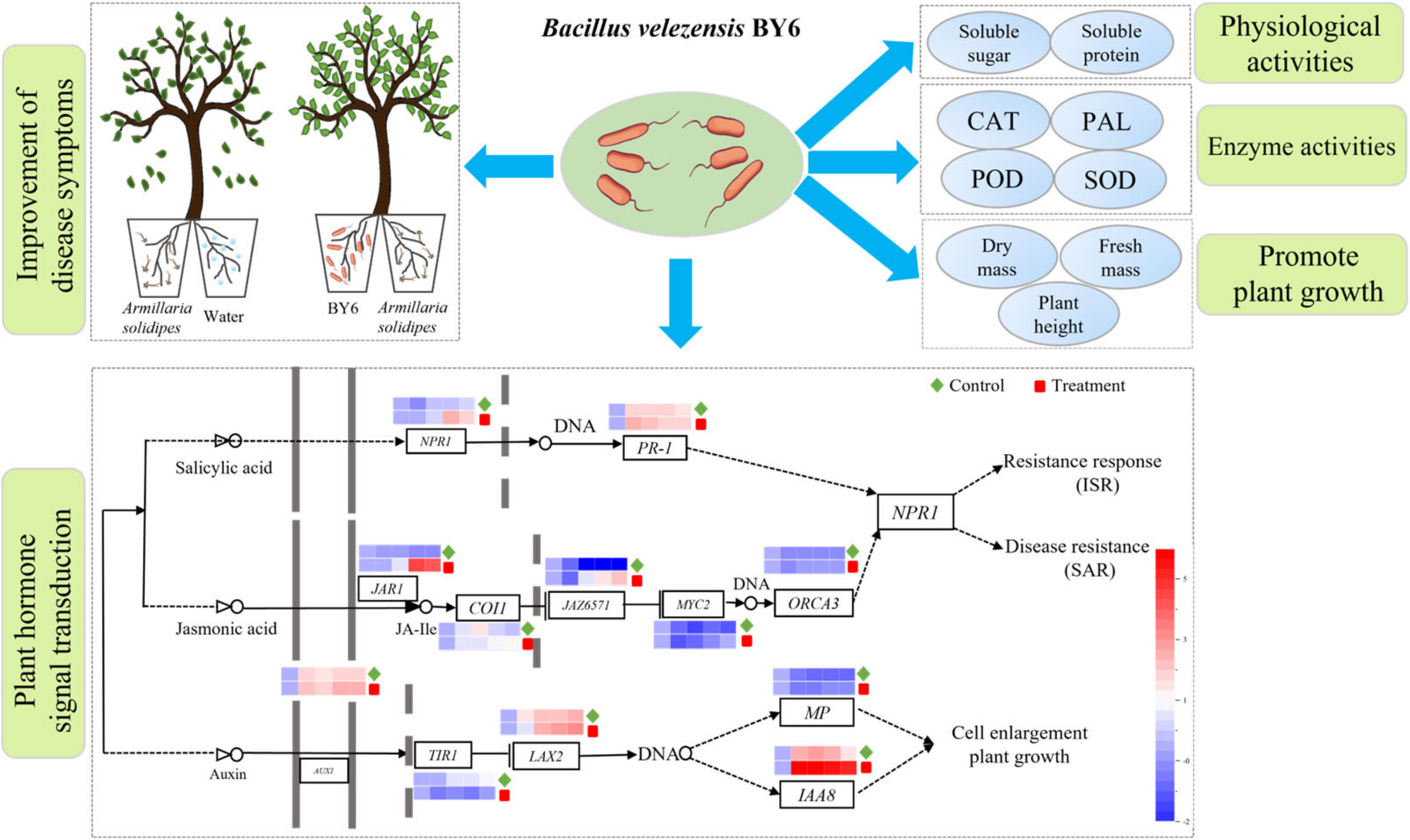
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, S.D.; Liu, Z.H.; Liu, B.; Wang, Y.C. Comparative analysis of biocontrol agent Trichoderma asperellum ACCC30536 transcriptome during its interaction with Populus davidiana × P. alba var. pyramidalis. Microbiol. Res. 2019, 227, 126294. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Li, M.R.; Zhang, P.; Zong, C.; Ma, W.; Ma, L. Overexpression of the Pdpap poplar ERF109 gene enhances resistance of Populus davidiana × P. alba var. pyramidalis to Fusarium oxysporum infection. J. For. Res. 2022, 33, 1925–1937. [Google Scholar] [CrossRef]
- Sun, Y.S.; Chen, S.; Huang, H.J.; Jiang, J.; Bai, S.; Liu, G.F. Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii. J. For. Res. 2014, 25, 813–818. [Google Scholar] [CrossRef]
- Cleary, M.R.; van der Kamp, B.J.; Morrison, D.J. Effects of wounding and fungal infection with Armillaria ostoyae in three conifer species. II. Host response to the pathogen. Forest Pathol. 2012, 42, 109–123. [Google Scholar] [CrossRef]
- Teklu, B.M.; Haileslassie, A.; Mekuria, W. Pesticides as water pollutants and level of risks to environment and people: An example from central rift valley of Ethiopia. Environ. Dev. Sustain. 2022, 24, 5275–5294. [Google Scholar] [CrossRef]
- Munnecke, D.E.; Wilbur, W.D.; Kolbezen, M.J. Dosage response of Armillaria-mellea to methyl bromide. Phytopathology 1970, 60, 992. [Google Scholar] [CrossRef]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.; Mahgoub, H.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative study between exogenously applied plant growth hormones versus metabolites of microbial endophytes as plant growth-promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef]
- Ren, Z.H.; Xie, L.; Okyere, S.K.; Wen, J.; Ran, Y.A.; Nong, X.; Hu, Y.C. Antibacterial activity of two metabolites isolated from endophytic bacteria Bacillus velezensis Ea73 in Ageratina adenophora. Front. Microbiol. 2022, 13, 9. [Google Scholar] [CrossRef]
- Nanjani, S.; Soni, R.; Paul, D.; Keharia, H. Genome analysis uncovers the prolific antagonistic and plant growth-promoting potential of endophyte Bacillus velezensis K1. Gene 2022, 836, 146671. [Google Scholar] [CrossRef]
- Ji, C.; Wang, X.H.; Song, X.; Zhou, Q.S.; Li, C.H.; Chen, Z.Z.; Gao, Q.X.; Li, H.Y.; Li, J.T.; Zhang, P.C.; et al. Effect of Bacillus velezensis JC-K3 on endophytic bacterial and fungal diversity in wheat under salt stress. Front. Microbiol. 2021, 12, 802054. [Google Scholar] [CrossRef]
- Doan, H.V.; Hoseinifar, S.H.; Khanongnuch, C.; Kanpiengjai, A.; Unban, K.; Kim, V.V.; Srichaiyo, S. Host-associated probiotics boosted mucosal and serum immunity, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 491, 94–100. [Google Scholar] [CrossRef]
- Han, V.C.; Yu, N.H.; Yoon, H.; Ahn, N.H.; Son, Y.K.; Lee, B.H.; Kim, J.C. Identification, characterization, and efficacy evaluation of Bacillus velezensis for shot-hole disease biocontrol in flowering Cherry. Plant Pathol. J. 2022, 38, 115–130. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.C.; Hou, Y.; Li, G.Q.; Yang, J.Y.; Li, D.W.; Ye, J.R. Bacillus velezensis strain HYEB5-6 as a potential biocontrol agent against anthracnose on Euonymus japonicus. Biocontrol Sci. Technol. 2017, 27, 636–653. [Google Scholar] [CrossRef]
- Meng, Q.X.; Jiang, H.; Hao, J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Constantin, M.E.; de Lamo, F.J.; Vlieger, B.V.; Rep, M.; Takken, F. Endophyte-mediated resistance in tomato to Fusarium oxysporum is independent of ET, JA, and SA. Front. Plant Sci. 2019, 10, 979. [Google Scholar] [CrossRef]
- Zhang, P.; Diao, J.; Xie, G.Q.; Ma, L.; Wang, L.H. A complete genome sequence of the wood stem endophyte Bacillus velezensis BY6 strain possessing plant growth-promoting and antifungal activities. Biomed. Res. Int. 2021, 2021, 3904120. [Google Scholar] [CrossRef]
- Hernandez, M.; Davila, A.R.; de Algaba, A.P.; Lopez, M.; Casas, A.T. Occurrence and etiology of death of young olive trees in southern Spain. Eur. J. Plant Pathol. 1998, 104, 347–357. [Google Scholar] [CrossRef]
- Tang, Y.; Li, R.; Jiang, Z.C.; Cheng, Z.Y.; Li, W.; Shao, Y.Z. Combined effect of Debaryomyces hansenii and Bacillus atrophaeus on the physicochemical attributes, defense-related enzyme activity, and transcriptomic profile of stored litchi fruit. Biol. Control 2022, 172, 104975. [Google Scholar] [CrossRef]
- Lin, C.W.; Chang, H.B.; Huang, H.J. Zinc induces mitogen-activated protein kinase activation mediated by reactive oxygen species in rice roots. Plant Physiol. Biochem. 2005, 43, 963–968. [Google Scholar] [CrossRef]
- Hung, W.C.; Huang, D.D.; Chien, P.S.; Yeh, C.M.; Chen, P.Y.; Chi, W.C.; Huang, H.J. Protein tyrosine dephosphorylation during copper-induced cell death in rice roots. Chemosphere 2007, 69, 55–62. [Google Scholar] [CrossRef]
- Goicoechea, N.; Aguirreolea, J.; Cenoz, S.; Garcia-Mina, J.M. Verticillium dahliae modifies the concentrations of proline, soluble sugars, starch, soluble protein and abscisic acid in pepper plants. Eur. J. Plant. Pathol. 2000, 106, 19–25. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hong, S.R.; Kim, T.Y.; Won, S.J.; Moon, J.H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef]
- Hong, P.; Hao, W.N.; Luo, J.J.; Chen, S.H.; Hu, M.Y.; Zhong, G.H. Combination of hot water, Bacillus amyloliquefaciens HF-01 and sodium bicarbonate treatments to control postharvest decay of mandarin fruit. Postharvest Biol. Technol. 2014, 88, 96–102. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Li, X.P.; Wei, J.; Wu, B. Effect of nitrous oxide against Botrytis cinerea and phenylpropanoid pathway metabolism in table grapes. Sci. Hortic. 2019, 254, 99–105. [Google Scholar] [CrossRef]
- Zhang, X.T.; Kong, W.L.; Wu, X.Q.; Ye, J.R. Bacillus velezensis JK-XZ8 prevents and controls crown gall disease on Prunus subhirtella by colonizing and inducing resistance. J. For. Res. 2022, 33, 1019–1031. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Manzar, N.; Nebapure, S.M.; Rajawat, M.; Deo, M.M.; Singh, J.P.; Kesharwani, A.K.; Singh, R.P.; Dubey, S.C.; Singh, D. Unraveling microbial volatile elicitors using a transparent methodology for induction of systemic resistance and regulation of antioxidant genes at expression levels in chili against bacterial wilt disease. Antioxidants 2022, 11, 404. [Google Scholar] [CrossRef]
- Zhang, P.; Hao, H.T.; Wang, L.H.; Liu, Z.H.; Ma, L. Endophytes Bacillus amyloliquefaciens AW3 (CGMCC1.16683) improves the growth of Populus davidiana × Populus bolleana (Pdpap poplar) and induces its resistance to wilt disease by Fusarium oxysporum Fox68 (CFCC86068). Eur. J. Plant Pathol. 2022, 162, 1–17. [Google Scholar] [CrossRef]
- Wang, X.; Liang, L.Q.; Shao, H.; Ye, X.X.; Yang, X.B.; Chen, X.Y.; Shi, Y.; Zhang, L.H.; Xu, L.H.; Wang, J.X. Isolation of the Novel strain Bacillus amyloliquefaciens F9 and identification of lipopeptide extract components responsible for activity against Xanthomonas citri subsp. citri. Plants 2022, 11, 457. [Google Scholar] [CrossRef]
- Ji, C.L.; Zhang, M.L.; Kong, Z.R.; Chen, X.; Wang, X.; Ding, W.; Lai, H.X.; Guo, Q. Genomic analysis reveals potential mechanisms underlying promotion of tomato plant growth and antagonism of soilborne pathogens by Bacillus amyloliquefaciens Ba13. Microbiol. Spectr. 2021, 9, e01615-21. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, B.X.; Narisawa, K.; Nong, Q.; Qin, L.P.; Xie, L. The dark septate endophyte, Phialocephala fortinii J2PC4, Mitigates southern rice black-streaked dwarf disease and impacts the mortality of white back planthopper. Biol. Control. 2022, 170, 104911. [Google Scholar] [CrossRef]
- Jin, W.; Peng, L.; Zhang, X.G.; Sun, H.J.; Yuan, Z.L. Effects of endophytic and ectomycorrhizal basidiomycetes on Quercus virginiana seedling growth and nutrient absorption. J. Sustain. For. 2019, 38, 457–470. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, S.H.; Khaine, I.; Kwak, M.J.; Lee, H.K.; Lee, T.Y.; Lee, W.Y.; Woo, S.Y. Physiological changes and growth promotion induced in poplar seedlings by the plant growth-promoting rhizobacteria Bacillus subtilis JS. Photosynthetica 2018, 56, 1188–1203. [Google Scholar] [CrossRef]
- Chen, Z.X.; Klessig, D.F. Identification of a soluble salicylic acid-binding protein that may function in signal transduction in the plant disease-resistance response. Proc. Natl. Acad. Sci. USA 1991, 88, 8179–8183. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.F.; Zhang, C.L.; You, X.Q.; Zhao, T.T.; Jiang, J.B.; Chen, X.L.; Zhang, H.; Yang, H.H.; Zhang, D.Y.; et al. Physiological and RNA-seq analyses provide insights into the response mechanism of the Cf-10-mediated resistance to Cladosporium fulvum infection in tomato. Plant Mol. Biol. 2018, 96, 403–416. [Google Scholar] [CrossRef]
- Yang, Y.N.; Qi, M.; Mei, C.S. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004, 40, 909–919. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Gheysen, G.; Hofte, M. Hormone defense networking in rice: Tales from a different world. Trends Plant Sci. 2013, 18, 555–565. [Google Scholar] [CrossRef]
- Kalsi, H.S.; Karkhanis, A.A.; Natarajan, B.; Bhide, A.J.; Banerjee, A.K. AUXIN RESPONSE FACTOR 16 (StARF16) regulates defense gene StNPR1 upon infection with necrotrophic pathogen in potato. Plant Mol. Biol. 2022, 109, 13–28. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Schwarzenberg, A.; Nguema-Ona, E.; Clement, C.; Baillieul, F.; Aziz, A. Bacillus subtilis and Pseudomonas fluorescens trigger common and distinct systemic immune responses in Arabidopsis thaliana depending on the pathogen lifestyle. Vaccines 2020, 8, 503. [Google Scholar] [CrossRef]



| Target Gene | Primer Sequence (5′–3′) | Predicted Product Size (bp) |
|---|---|---|
| PR1 | ACACCACCGTGCAACCTATG | 240 |
| CGAGCAGAGTTCGCCAAACCA | ||
| NPR1 | GGCCGACGATATTCCCTAGTT | 261 |
| TGCCCTCATAGTTTCTGAGCT | ||
| AUX1 | CAGTCGGTGCTCTTCGGTCA | 230 |
| GTCCACCTGCCTACGAAGT | ||
| LAX2 | TCATTCGGCCCTTCACCAAGT | 212 |
| GCCATGATTGCATCACACCTT | ||
| MP | GCACATGAACGGCAGGGTTT | 213 |
| ACCTGACCATGACGACACTT | ||
| TIR1 | GTTGGTACGCAAAGGTAGAGA | 235 |
| GGCTGACCATGCAATACTAGC | ||
| IAA8 | CGGAGACTGGATGTTGTTGGT | 226 |
| ACCTGAAACCTGATCGTGCTC | ||
| JAR1 | AGTGGTGACCAAGCGAGGAG | 210 |
| GGATTTGTGCACCTTGCTGTT | ||
| JAZ6571 | GGAAGCTCATTGGCACAGATG | 221 |
| CCGGAGTGGGTTGTTTGTCTG | ||
| MYC2 | ACGAAGCGCAATCTGCTGAGT | 223 |
| CCAGGCTCTCAAAGCCGACAT | ||
| COI | GAGGTATTGTGGGTGCATGGT | 245 |
| ACGCGAACCTACCCTCGCT | ||
| ORCA3 | AGAGGATGAGGCAAAGACCCT | 231 |
| TCCGGCTCTCGCGCTTAGT | ||
| Actin(P) | AGCTGATCGAATAGCAAG | 196 |
| CTAGAAGCACTCCTGTG | ||
| β-tubulin (P) | TACCGAGGCTGAGATAACAT | 210 |
| GGACCCACAACTCATCACAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Xie, G.; Wang, L.; Xing, Y. Bacillus velezensis BY6 Promotes Growth of Poplar and Improves Resistance Contributing to the Biocontrol of Armillaria solidipes. Microorganisms 2022, 10, 2472. https://doi.org/10.3390/microorganisms10122472
Zhang P, Xie G, Wang L, Xing Y. Bacillus velezensis BY6 Promotes Growth of Poplar and Improves Resistance Contributing to the Biocontrol of Armillaria solidipes. Microorganisms. 2022; 10(12):2472. https://doi.org/10.3390/microorganisms10122472
Chicago/Turabian StyleZhang, Ping, Guangqiang Xie, Lihai Wang, and Yanqiu Xing. 2022. "Bacillus velezensis BY6 Promotes Growth of Poplar and Improves Resistance Contributing to the Biocontrol of Armillaria solidipes" Microorganisms 10, no. 12: 2472. https://doi.org/10.3390/microorganisms10122472
APA StyleZhang, P., Xie, G., Wang, L., & Xing, Y. (2022). Bacillus velezensis BY6 Promotes Growth of Poplar and Improves Resistance Contributing to the Biocontrol of Armillaria solidipes. Microorganisms, 10(12), 2472. https://doi.org/10.3390/microorganisms10122472










