Lactic Acid Bacteria Improve the Photoprotective Effect via MAPK/AP-1/MMP Signaling Pathway on Skin Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cell-Free Supernatant (CFS) from Lactobacillus, Streptococcus, and Bifidobacterium Strains
2.2. 2,2′-Azino-Bis-(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS+) Radical Scavenging Activity
2.3. Cell Culture and UVB Treatment
2.4. Cytotoxicity
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Antioxidant Activity of LAB Strains by ABTS+ Radical Scavenging Activity
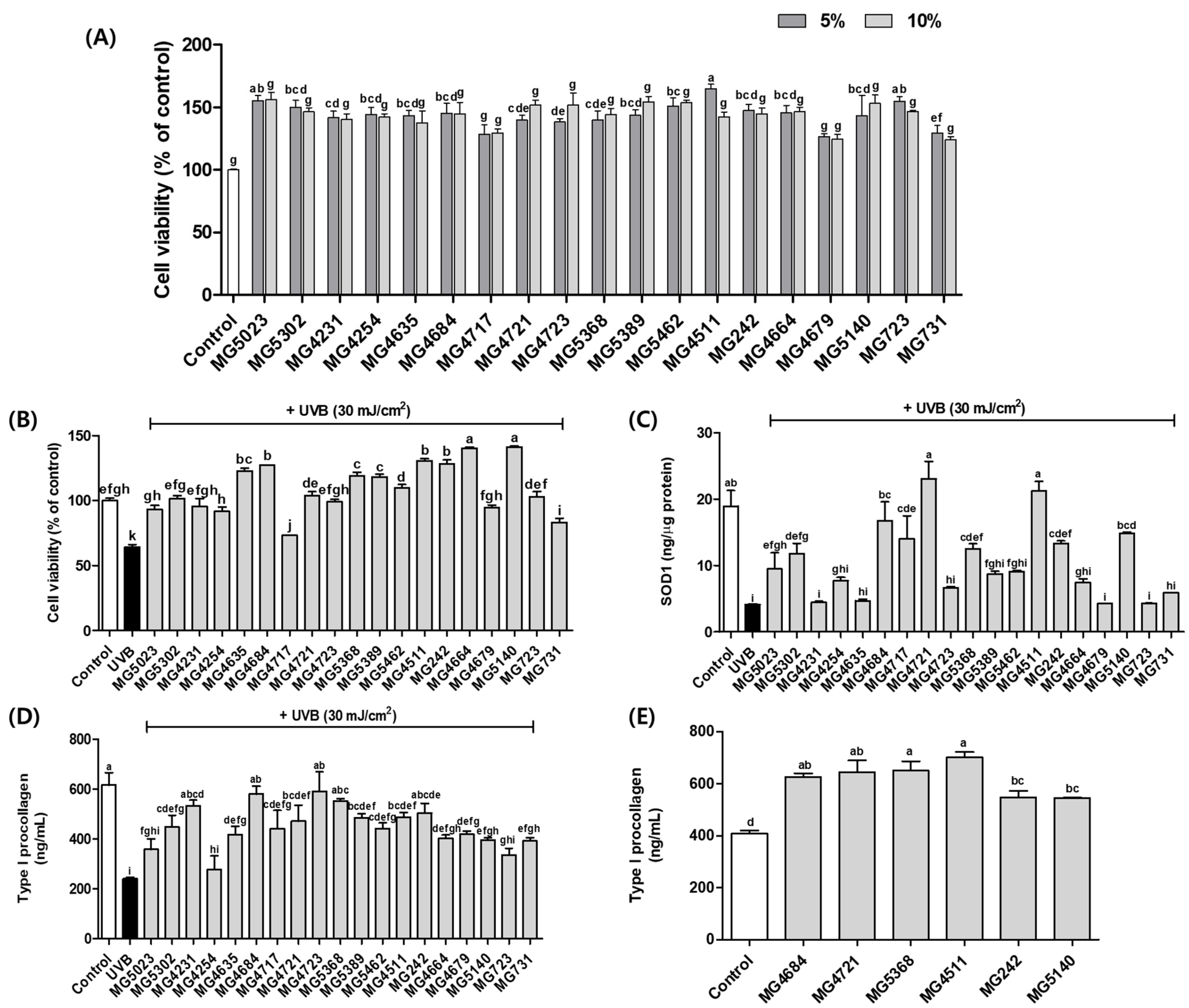
3.2. CFS of LAB Improves SOD Enzyme and Type I Procollagen Levels in UVB-Exposed Hs68 Cells
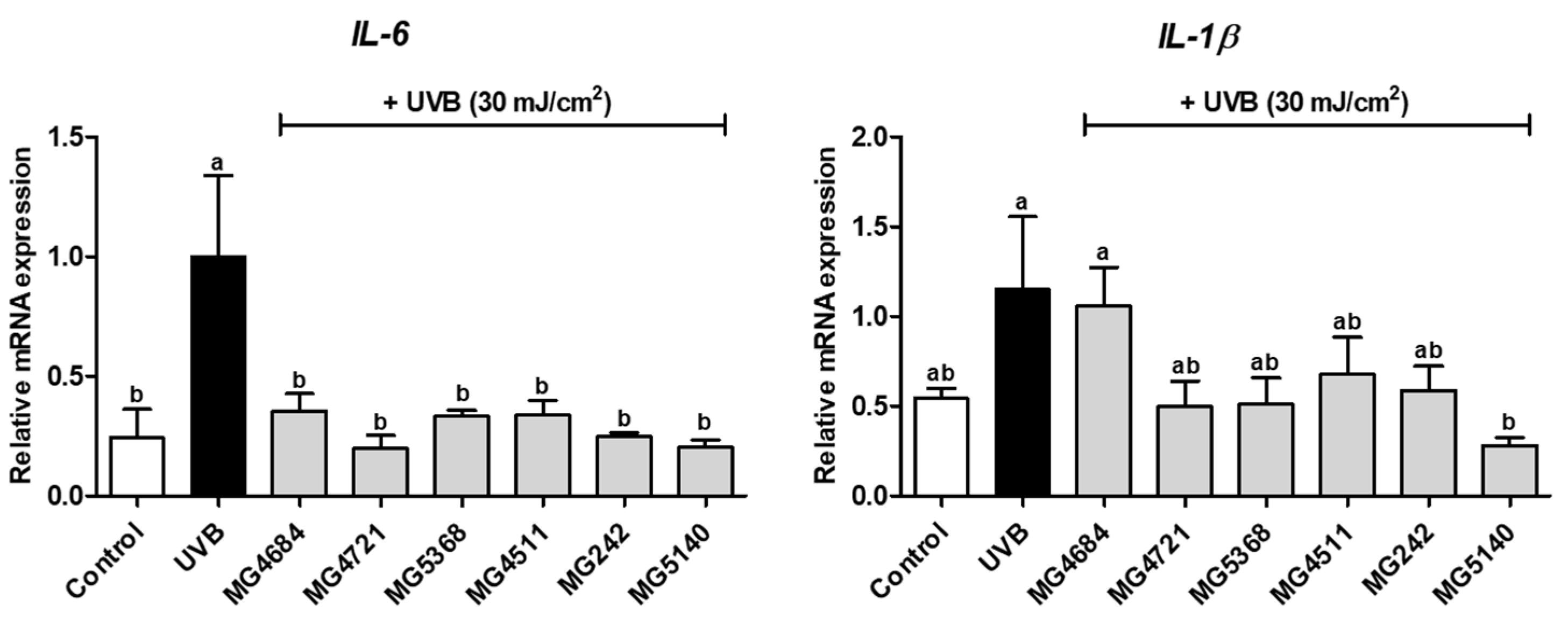
3.3. CFS of LAB Inhibits mRNA Expression of Proinflammatory Cytokines in UVB-Exposed Hs68 Cells
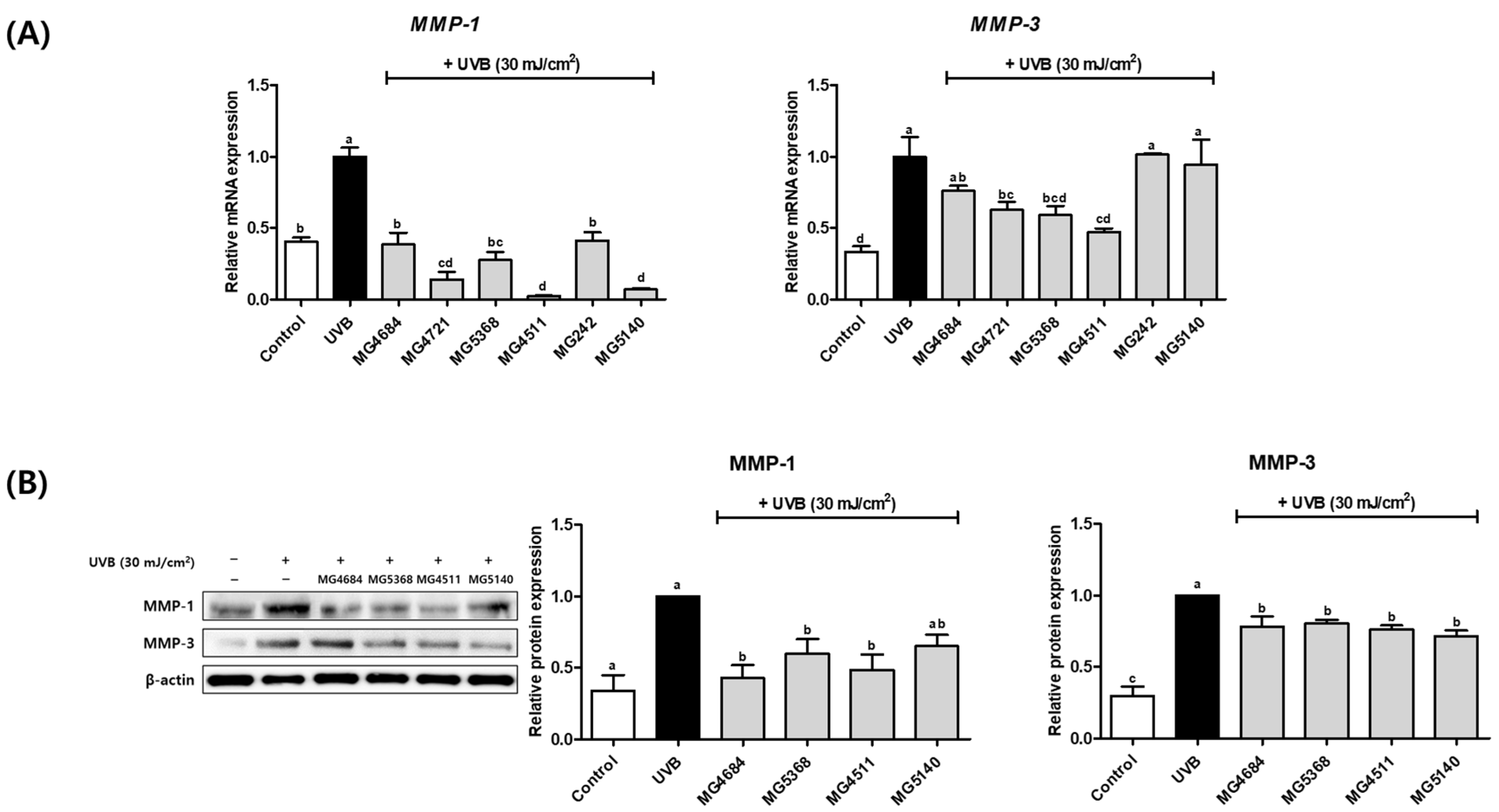
3.4. CFS of LAB Inhibits mRNA and Protein Expression of MMP-1 and MMP-3 in UVB-Exposed Hs68 Cells
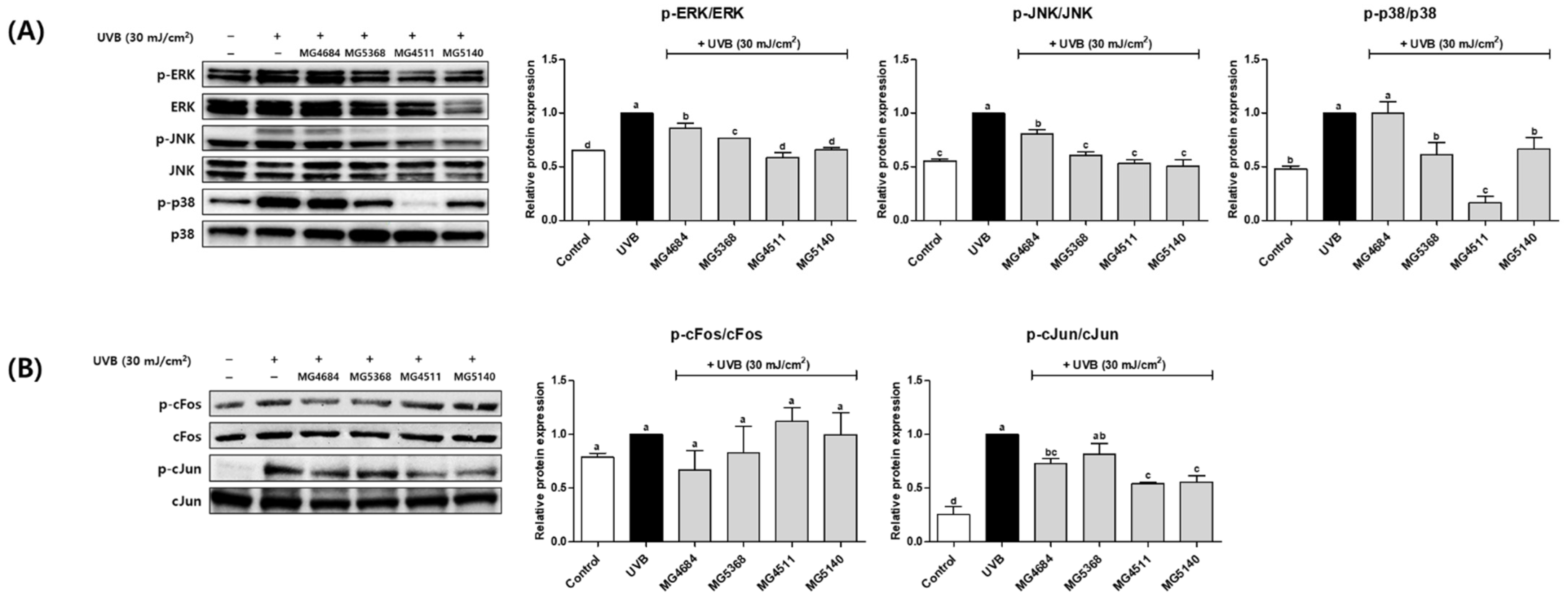
3.5. CFS of LAB Inhibit Protein Expression of MAPK and AP-1 Signaling Pathway in UVB-Exposed Hs68 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, B. Aging-related structural change in 3D extracellular matrix affects its mechanics. Med. Eng. Phys. 2022, 106, 103843. [Google Scholar] [CrossRef] [PubMed]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.-R.; Bulanin, D. Galactose-induced skin aging: The role of oxidative stress. Oxid. Med. Cell. Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Choi, H.-C.; Bata-Csorgo, Z.; Shao, Y.; Datta, S.; Wang, Z.-Q.; Kang, S.; Voorhees, J. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J. Investig. Dermatol. 2001, 117, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.j.; Lee, J.O.; Kim, S.Y.; Lee, J.M.; Lee, E.; Na, J.; Yoo, K.H.; Park, S.J.; Kim, B.J. Effect of A. polygama APEE (Actinidia polygama ethanol extract) or APWE (Actinidia polygama water extract) on wrinkle formation in UVB-irradiated hairless mice. J. Cosmet. Dermatol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jung, H.-J.; Choi, J.-S.; Nam, T.-J. Anti-wrinkle effects of a tuna heart H2O fraction on Hs27 human fibroblasts. Int. J. Mol. Med. 2016, 37, 92–98. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chen, H.-C.; Chiu, H.-H.; Chen, C.-W.; Wang, S.-M.; Wen, K.-C. Neonauclea reticulata (Havil.) Merr stimulates skin regeneration after UVB exposure via ROS scavenging and modulation of the MAPK/MMPs/collagen pathway. eCAM 2013, 2013, 324864. [Google Scholar] [CrossRef]
- Talwar, H.S.; Griffiths, C.E.; Fisher, G.J.; Hamilton, T.A.; Voorhees, J.J. Reduced Type I and Type III Procollagens in Photodamaged Adult Human Skin. J. Investig. Dermatol. 1995, 105, 285–290. [Google Scholar] [CrossRef]
- Iddamalgoda, A.; Le, Q.T.; Ito, K.; Tanaka, K.; Kojima, H.; Kido, H. Mast cell tryptase and photoaging: Possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Arch. Dermatol. 2008, 300, 69–76. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Song, Y.; Zheng, B.; Wen, Z.; Gong, M.; Meng, L. Heat-Killed Lacticaseibacillus paracasei Ameliorated UVB-Induced Oxidative Damage and Photoaging and Its Underlying Mechanisms. Antioxidants 2022, 11, 1875. [Google Scholar] [CrossRef]
- Holick, M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef]
- Akhter, K.F.; Mumin, M.A.; Lui, E.M.; Charpentier, P.A. Transdermal nanotherapeutics: Panax quinquefolium polysaccharide nanoparticles attenuate UVB-induced skin cancer. Int. J. Biol. Macromol. 2021, 181, 221–231. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Wu, P.-Y.; Hou, C.-W.; Chien, T.-Y.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protective effects of sesamin against UVB-induced skin inflammation and photodamage in vitro and in vivo. Biomolecules 2019, 9, 479. [Google Scholar] [CrossRef]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.-B. An anthocyanin-enriched extract from Vaccinium uliginosum improves signs of skin aging in UVB-induced photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef]
- Yuksel Egrilmez, M.; Kocturk, S.; Aktan, S.; Oktay, G.; Resmi, H.; Simsek Keskin, H.; Guner Akdogan, G.; Ozkan, S. Melatonin Prevents UVB-Induced Skin Photoaging by Inhibiting Oxidative Damage and MMP Expression through JNK/AP-1 Signaling Pathway in Human Dermal Fibroblasts. Life 2022, 12, 950. [Google Scholar] [CrossRef]
- Fang, M.; Lee, H.-M.; Oh, S.; Zheng, S.; Bellere, A.; Kim, M.; Choi, J.; Kim, M.; Yu, D.; Yi, T.-H. Rosa davurica inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 signaling in UVB-irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.K.; Bae, S.; Kim, H.; Park, Y.; Chu, N.K.; Kim, S.G.; Kim, H.-R.; Hwang, Y.-i.; Kang, J.S. The anti-inflammatory effect of alloferon on UVB-induced skin inflammation through the down-regulation of pro-inflammatory cytokines. Immunol. Lett. 2013, 149, 110–118. [Google Scholar] [CrossRef]
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.-W.; Ku, H.K.; Kim, T.-Y.; Choi, I.-D.; Jeung, W.; Sim, J.-H.; Ahn, Y.-T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.E.; Park, S.D.; Kim, Y.-T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.-T.; Sim, J.-H.; Huh, C.-S. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Lolou, V.; Panayiotidis, M.I. Functional role of probiotics and prebiotics on skin health and disease. Fermentation 2019, 5, 41. [Google Scholar] [CrossRef]
- Gwon, G.Y.; Park, G.G. Protective Effects of Bifidobacterium bifidum Culture Supernatants and Intracellular Cell-Free Extracts on Human Dermal Fibroblasts against UV-B Irradiation. J. Korean Soc. Food Sci. Nutr. 2017, 46, 801–808. [Google Scholar]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.J.; Kim, S.A.; Park, S.D.; Shim, J.J.; Lee, J.L. Exopolysaccharide from Lactobacillus plantarum HY7714 protects against skin aging through skin–gut axis communication. Molecules 2021, 26, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Choi, D.; Park, T. Decanal protects against UVB-induced photoaging in human dermal fibroblasts via the cAMP pathway. Nutrients 2020, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Han, H.-S.; Kim, J.-M.; Park, G.; Jang, Y.-P.; Shin, Y.-K.; Ahn, H.-S.; Lee, S.-H.; Lee, K.-T. Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Mar. Drugs 2021, 19, 693. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Liu, C.; Tseng, Y.P.; Chan, L.P.; Liang, C.H. The potential of Streptococcus thermophiles (TCI633) in the anti-aging. J. Cosmet. Dermatol. 2022, 21, 2635–2647. [Google Scholar] [CrossRef]
- Saito, Y.; Mihara, T.; Maruyama, K.; Saito, J.; Ikeda, M.; Tomonaga, A.; Kumagai, T. Effects of intake of Lactobacillus casei subsp. casei 327 (lactobacillus K-1) on skin conditions: A randomized, double-blind, placebo-controlled, parallel-group study in women. Biosci. Microbiota Food Health 2017, 36, 111–120. [Google Scholar] [CrossRef]
- Satoh, T.; Murata, M.; Iwabuchi, N.; Odamaki, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Xiao, J. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Benef. Microbes 2015, 6, 497–504. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, E.; Park, Y.M.; Hong, S.-J. Microbiome in the gut-skin axis in atopic dermatitis. Asthma Allergy Immunol. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Lew, L.C.; Liong, M.T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.; Moyá, M.L.; Troncoso, A.; García-Parrilla, M. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Mahendra, C.K.; Yow, Y.Y.; Chan, K.G.; Khan, T.M.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM273b: A mangrove-derived potential source for antioxidant and UVB radiation protectants. MicrobiologyOpen 2019, 8, e859. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Terra, V.; Souza-Neto, F.; Pereira, R.; Silva, T.; Costa, A.; Luiz, R.; Cecchini, R.; Cecchini, A. Time-dependent reactive species formation and oxidative stress damage in the skin after UVB irradiation. J. Photochem. Photobiol. B Biol. 2012, 109, 34–41. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Poorahong, W.; Innajak, S.; Ungsurungsie, M.; Watanapokasin, R. Purple Corn Silk Extract Attenuates UVB-Induced Inflammation in Human Keratinocyte Cells. Sci. Pharm. 2022, 90, 18. [Google Scholar] [CrossRef]
- Han, H.-S.; Shin, J.-S.; Myung, D.-B.; Ahn, H.S.; Lee, S.H.; Kim, H.J.; Lee, K.-T. Hydrangea serrata (Thunb.) Ser. extract attenuate UVB-induced photoaging through MAPK/AP-1 inactivation in human skin fibroblasts and hairless mice. Nutrients 2019, 11, 533. [Google Scholar] [CrossRef]
- Quan, T.; He, T.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J. Biol. Chem. 2005, 280, 8079–8085. [Google Scholar] [CrossRef]
- Hachiya, A.; Sriwiriyanont, P.; Fujimura, T.; Ohuchi, A.; Kitahara, T.; Takema, Y.; Kitzmiller, W.J.; Visscher, M.O.; Tsuboi, R.; Boissy, R.E. Mechanistic effects of long-term ultraviolet B irradiation induce epidermal and dermal changes in human skin xenografts. Am. J. Clin. Pathol. 2009, 174, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, N.; Westermarck, J.; Hakkinen, L.; Holmstrom, T.H.; Elo, I.; Eriksson, J.E.; Kahari, V.-M. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J. Biol. Chem. 1998, 273, 5137–5145. [Google Scholar] [CrossRef] [PubMed]
| LAB | Strain | NCBI Accession Number | Origin |
|---|---|---|---|
| Lactiplantibacillus plantarum | MG5023 | OP102478.1 | Food |
| MG5372 | ON631286.1 | ||
| MG5302 | MN833016.1 | Fermented food | |
| MG5433 | ON631843.1 | ||
| Limosilactobacillus fermentum | MG4231 | MW947163.1 | Human |
| MG4254 | MW947155.1 | ||
| MG4635 | ON631318.1 | ||
| MG4684 | OP035518.1 | ||
| MG4698 | OP077101.1 | ||
| MG4717 | OP035525.1 | ||
| MG4721 | OP035529.1 | ||
| MG4723 | OP035531.1 | ||
| MG5368 | ON631268.1 | Food | |
| MG5389 | ON668175.1 | ||
| Limosilactobacillus reuteri | MG4658 | OP077269.1 | Human |
| MG5460 | ON619506.1 | Food | |
| MG5462 | ON619508.1 | ||
| Lacticaseibacillus rhamnosus | MG4511 | MN368054.1 | Human |
| MG5437 | ON631847.1 | Food | |
| MG5511 | ON631745.1 | ||
| Ligilactobacillus salivarius | MG242 | ON631745.1 | Human |
| MG4664 | OP077274.1 | ||
| MG4679 | OP035513.1 | ||
| Streptococcus thermophilus | MG5140 | MN055727.1 | Fermented food |
| MG5475 | ON619521.1 | ||
| MG5481 | ON619527.1 | ||
| Bifidobacterium longum | MG723 | MN055725.1 | Human |
| MG4669 | OP035506.1 | ||
| Bifidobacterium bifidum | MG731 | MN055710.1 | Human |
| Gene | Primer Sequence (5′ → 3′) | |
|---|---|---|
| MMP-1 | F 1 | GCATATCGATGCTGCTCTTTC |
| R 2 | GATAACCTGGATCCATAGATCGTT | |
| MMP-3 | F | CAAAACATATTTCTTTGTAGAGGACAA |
| R | TTCAGCTATTTGCTTGGGAAA | |
| IL-6 | F | AAGCCAGAGCTGTGCAGATGAGTA |
| R | TGTCCTGCAGCCACTGGTTC | |
| IL-1β | F | AGAAGTACCTGAGCTCGCCA |
| R | CCTGAAGCCCTTGCTGTAGT | |
| GAPDH | F | ACCCACTCCTCCACCTTTG |
| R | ACCCACTCCTCCACCTTTG | |
| Species | Strain | Inhibition Rate (%) |
|---|---|---|
| (1 × 108 CFU/mL) | ||
| Lactiplantibacillus plantarum | MG5023 | 69.9 ± 3.0 fg |
| MG5372 | 23.3 ± 0.6 o | |
| MG5302 | 64.6 ± 3.4 i | |
| MG5433 | 23.3 ± 1.0 o | |
| Limosilactobacillus fermentum | MG4231 | 75.7 ± 0.1 e |
| MG4254 | 64.6 ± 2.0 i | |
| MG4684 | 68.6 ± 1.3 gh | |
| MG4698 | 32.5 ± 0.2 kl | |
| MG4717 | 64.7 ± 2.4 i | |
| MG4721 | 77.2 ± 1.9 de | |
| MG4723 | 86.9 ± 1.5 c | |
| MG4635 | 93.1 ± 0.4 a | |
| MG5368 | 72.1 ± 2.6 f | |
| MG5389 | 79.2 ± 1.8 d | |
| Limosilactobacillus reuteri | MG4658 | 41.9 ± 0.9 j |
| MG5460 | 35.0 ± 0.6 k | |
| MG5462 | 64.7 ± 2.4 i | |
| Lacticaseibacillus rhamnosus | MG4511 | 64.9 ± 1.1 i |
| MG5437 | 27.0 ± 1.5 n | |
| MG5511 | 27.1 ± 0.7n | |
| Ligilactobacillus salivarius | MG242 | 92.8 ± 0.7 a |
| MG4664 | 92.7 ± 0.8 a | |
| MG4679 | 78.3 ± 0.3 de | |
| Streptococcus thermophilus | MG5140 | 65.9 ± 2.3 hi |
| MG5475 | 30.6 ± 3.9 lm | |
| MG5481 | 28.9 ± 2.0 mn | |
| Bifidobacterium longum | MG723 | 89.2 ± 0.3 bc |
| MG4669 | 27.9 ± 0.1 mn | |
| Bifidobacterium bifidum | MG731 | 90.6 ± 0.2 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Lactic Acid Bacteria Improve the Photoprotective Effect via MAPK/AP-1/MMP Signaling Pathway on Skin Fibroblasts. Microorganisms 2022, 10, 2481. https://doi.org/10.3390/microorganisms10122481
Park J-Y, Lee JY, Kim Y, Kang C-H. Lactic Acid Bacteria Improve the Photoprotective Effect via MAPK/AP-1/MMP Signaling Pathway on Skin Fibroblasts. Microorganisms. 2022; 10(12):2481. https://doi.org/10.3390/microorganisms10122481
Chicago/Turabian StylePark, Jeong-Yong, Ji Yeon Lee, YongGyeong Kim, and Chang-Ho Kang. 2022. "Lactic Acid Bacteria Improve the Photoprotective Effect via MAPK/AP-1/MMP Signaling Pathway on Skin Fibroblasts" Microorganisms 10, no. 12: 2481. https://doi.org/10.3390/microorganisms10122481
APA StylePark, J.-Y., Lee, J. Y., Kim, Y., & Kang, C.-H. (2022). Lactic Acid Bacteria Improve the Photoprotective Effect via MAPK/AP-1/MMP Signaling Pathway on Skin Fibroblasts. Microorganisms, 10(12), 2481. https://doi.org/10.3390/microorganisms10122481





