Safety Evaluation of Bacillus subtilis IDCC1101, Newly Isolated from Cheonggukjang, for Industrial Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
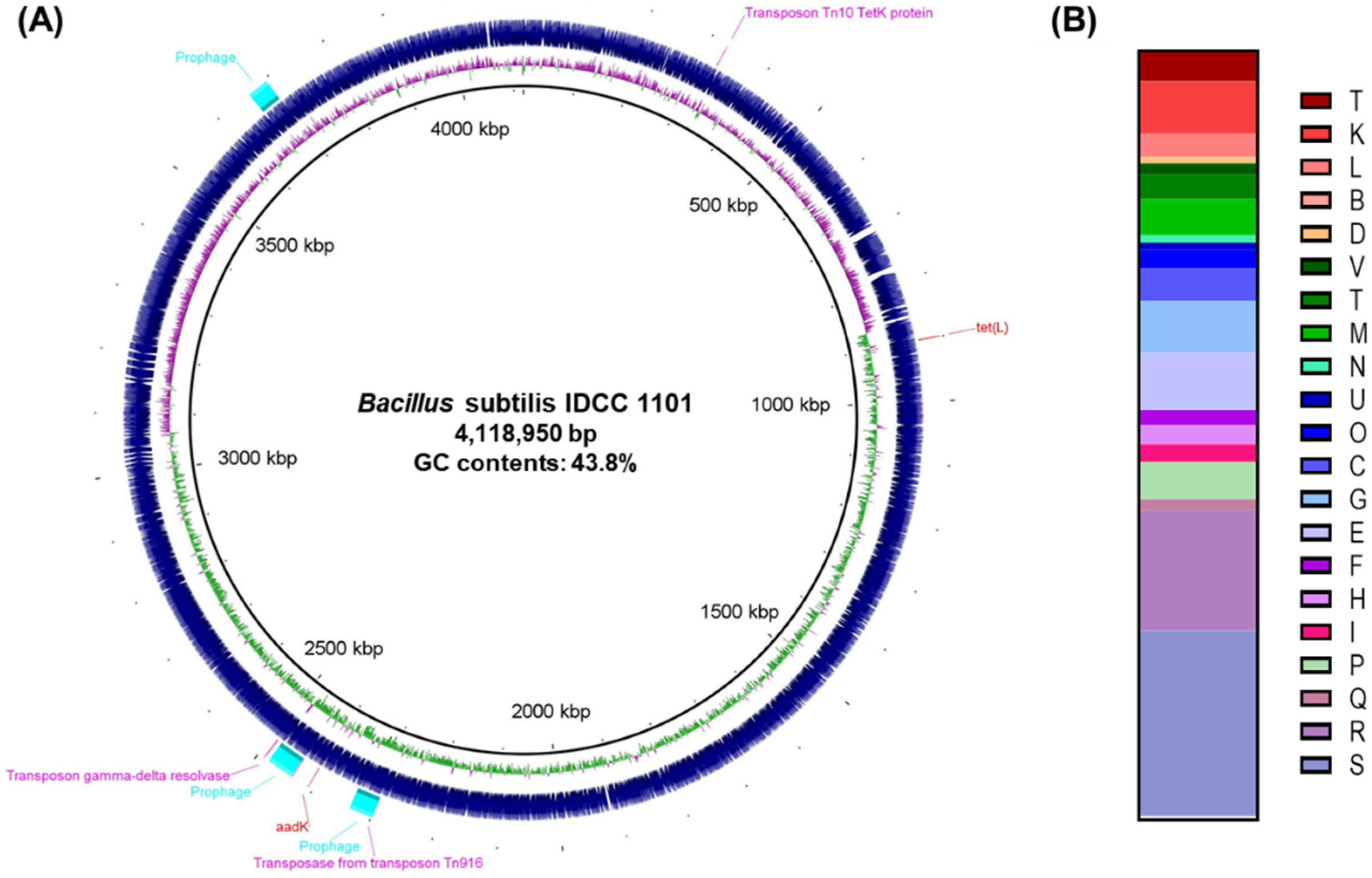
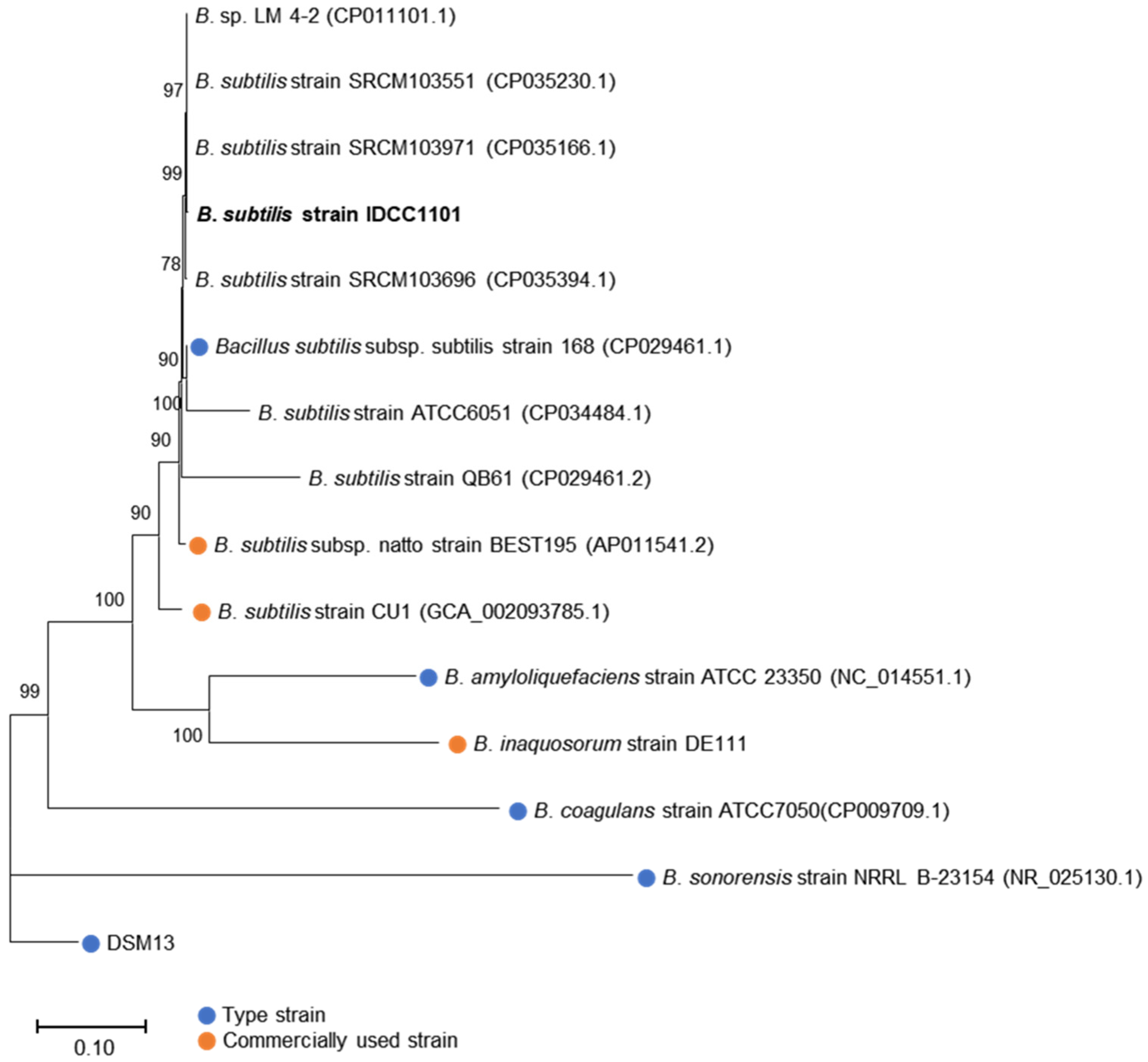
2.2. Genome Sequencing and Taxonomic Classification of BS IDCC1101
2.3. In Silico Identification of Secondary Metabolites via antiSMASH
2.4. Safety Assessment of BS IDCC1101
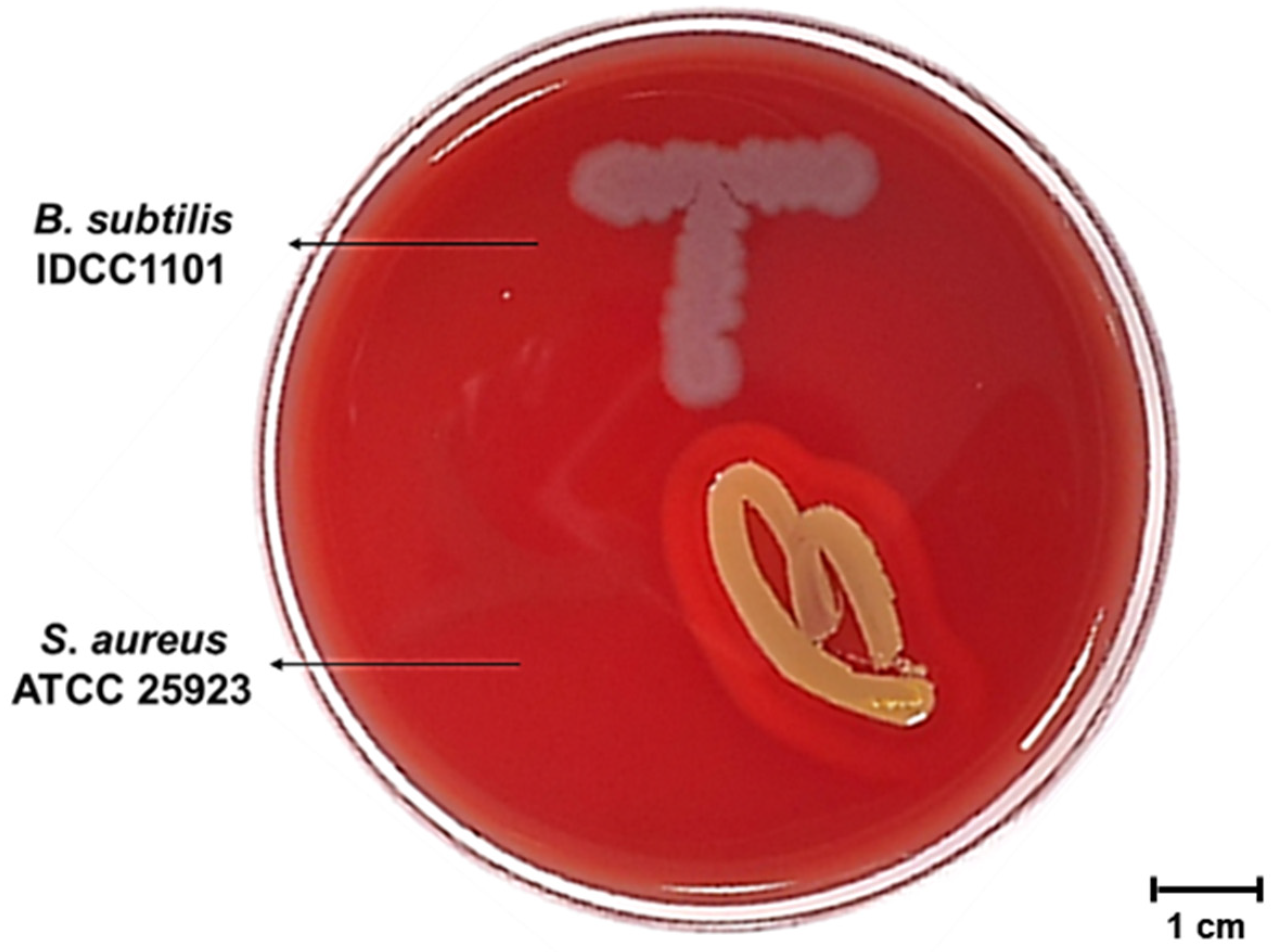
2.5. Hemolysis Activity of BS IDCC1101
2.6. Genotypic and Phenotypic Antibiotic Susceptibility of BS IDCC1101
2.7. Enzyme Activities and Carbohydrate Utilization of BS IDCC1101
2.8. Biogenic Amines Production of BS IDCC1101
2.9. Proportion of D-/L-Lactate of BS IDCC1101
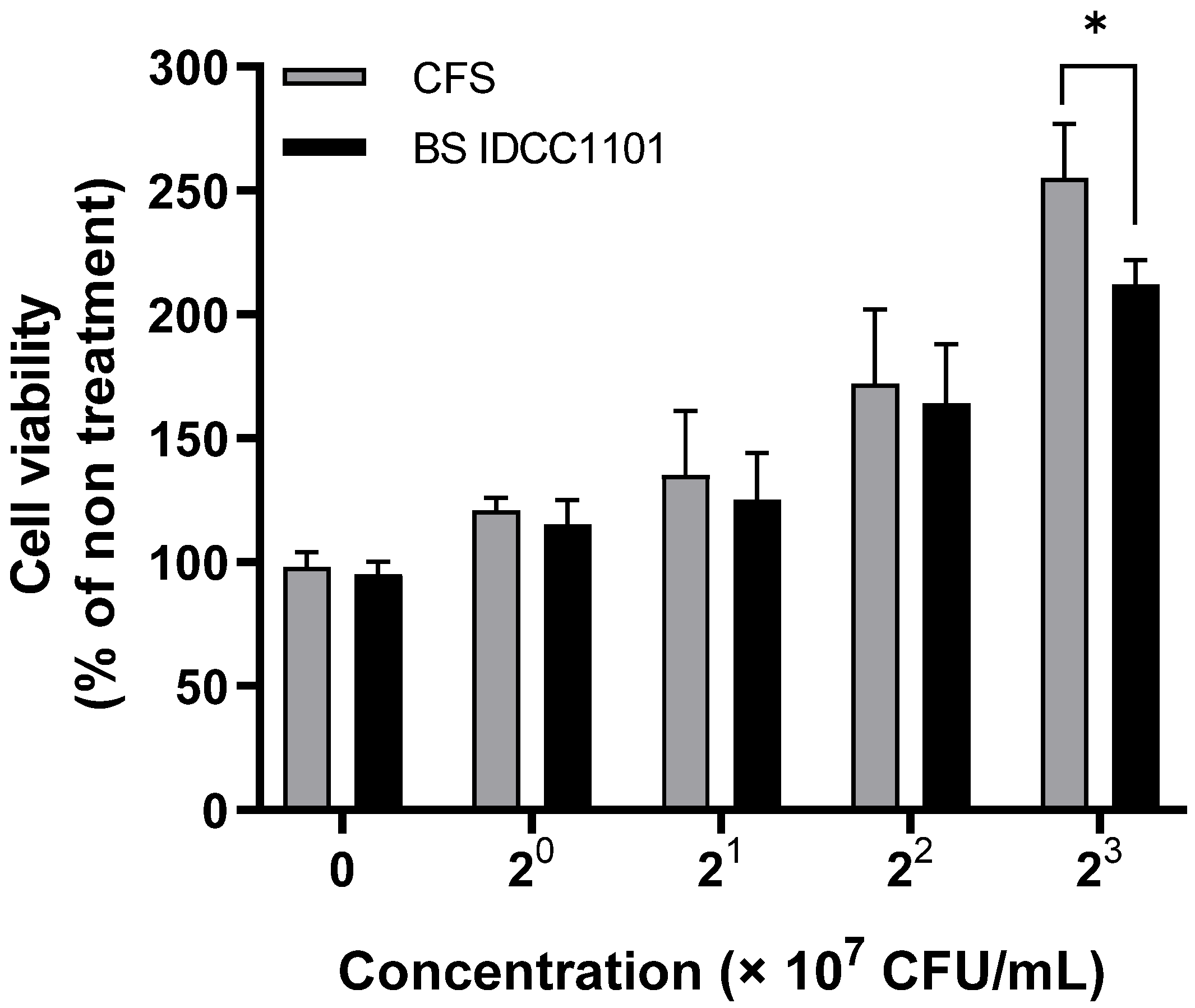
2.10. Evaluation of the Cytotoxicity of BS IDCC1101
2.11. Acute Oral Toxicity Study of BS IDCC1101 in Rats
3. Results and Discussion
3.1. Genomic Characteristics and Taxonomic Classification of BS IDCC1101
3.2. In Silico Identification of Secondary Metabolites in the BS IDCC1101 Genome
3.3. Safety Assessment of BS IDCC1101
3.4. Hemolysis Activity of B. subtilis IDCC1101
3.5. Genotypic and Phenotypic Antibiotic Susceptibility of BS IDCC1101
3.6. Enzyme Activities and Carbohydrate Utilization of BS IDCC1101
3.7. Production of Biogenic Amine and Lactate by BS IDCC1101
3.8. Cytotoxicity of BS IDCC1101
3.9. Acute Oral Toxicity of BS IDCC1101 in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fortune Business Insights. Probiotics Market Size, Share and COVID-19 Impact Analysis, By Microbial Genus (Lactobacillus, Bifidobacterium, and Yeast), By Application (Functional Foods and Beverages, Dietary Supplements, and Animal Feed), Distribution Channel (Supermarkets/Hypermarkets, Pharmacies/Health Stores, Convenience Stores, Online Retail, and Others), and Regional Forecast, 2020–2027; FBI: Pune, India, 2021; FBI100083. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Shin, H.S. Antimicrobial and Immunomodulatory Effects of Bifidobacterium Strains: A Review. J. Microbiol. Biotechnol. 2020, 30, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Yang, S.; Lee, H.H.; Choe, D.; Johnston, T.V.; Ji, G.E.; Park, M.S. Biosafety assessment of Bifidobacterium animalis subsp. lactis AD011 used for human consumption as a probiotic microorganism. Food Control 2019, 117, 106985. [Google Scholar] [CrossRef]
- O’Shea, E.F.; Cotter, P.D.; Stanton, C.; Ross, R.P.; Hill, C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: Bacteriocins and conjugated linoleic acid. Int. J. Food Microbiol. 2012, 152, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Adimpong, D.B.; Sørensen, K.I.; Thorsen, L.; Stuer-Lauridsen, B.; Abdelgadir, W.S.; Nielsen, D.S.; Derkx, P.M.F.; Jespersen, L. Antimicrobial Susceptibility of Bacillus Strains Isolated from Primary Starters for African Traditional Bread Production and Characterization of the Bacitracin Operon and Bacitracin Biosynthesis. Appl. Environ. Microbiol. 2012, 78, 7903–7914. [Google Scholar] [CrossRef]
- Kubo, Y.; Rooney, A.P.; Tsukakoshi, Y.; Nakagawa, R.; Hasegawa, H.; Kimura, K. Phylogenetic Analysis of Bacillus subtilis Strains Applicable to Natto (Fermented Soybean) Production. Appl. Environ. Microbiol. 2011, 77, 6463–6469. [Google Scholar] [CrossRef]
- Jeon, H.-L.; Lee, N.-K.; Yang, S.-J.; Kim, W.-S.; Paik, H.-D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef]
- Kotb, E. Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK-07 isolated from kishk, a traditional Egyptian fermented food. Appl. Biochem. Microbiol. 2014, 51, 34–43. [Google Scholar] [CrossRef]
- Inatsu, Y.; Nakamura, N.; Yuriko, Y.; Fushimi, T.; Watanasiritum, L.; Kawamoto, S. Characterization of Bacillus subtilis strains in Thua nao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 2006, 43, 237–242. [Google Scholar] [CrossRef]
- Sorokulova, I. Modern Status and Perspectives of Bacillus Bacteria as Probiotics. J. Probiotics Health 2013, 1, 2. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Sella, S.R.B.R.; Bueno, T.; de Oliveira, A.A.B.; Karp, S.G.; Soccol, C.R. Bacillus subtilis natto as a potential probiotic in animal nutrition. Crit. Rev. Biotechnol. 2021, 41, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; To, E.; Fakhry, S.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Defining the natural habitat of Bacillus spore-formers. Res. Microbiol. 2009, 160, 375–379. [Google Scholar] [CrossRef]
- Hazards, E.P.O.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA J. 2020, 18, e05966. [Google Scholar]
- Damgaard, P.H.; Granum, P.E.; Bresciani, J.; Torregrossa, M.V.; Eilenberg, J.; Valentino, L. Characterization of Bacillus thuringiensis isolated from infections in burn wounds. FEMS Immunol. Med. Microbiol. 1997, 18, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.; Ramisse, F.; Ducoureau, J.P.; Cruel, T.; Cavallo, J.D. Bacillus thuringiensis subsp. konkukian (serotype H34) superinfection: Case report and experimental evidence of pathogenicity in immunosuppressed mice. J. Clin. Microbiol. 1998, 36, 2138–2139. [Google Scholar] [CrossRef] [PubMed]
- Little, S.F.; Ivins, E.B. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1999, 1, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kotiranta, A.; Lounatmaa, K.; Haapasalo, M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000, 2, 189–198. [Google Scholar] [CrossRef]
- Raymond, B.; Johnston, P.R.; Nielsen-LeRoux, C.; Lereclus, D.; Crickmore, N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010, 18, 189–194. [Google Scholar] [CrossRef]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef]
- Schoeni, J.L.; Lee Wong, A.C. Bacillus cereus food poisoning and its toxins. J. Food Prot. 2005, 68, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; Von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.-F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef]
- Lee, I.; Kim, Y.O.; Park, S.-C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinform. 2017, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2018, 47, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, H.J.; Jeong, M.; Koo, M. Enterotoxin Genes, Antibiotic Susceptibility, and Biofilm Formation of Low-Temperature-Tolerant Bacillus cereus Isolated from Green Leaf Lettuce in the Cold Chain. Foods 2020, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Abbas, B.A.; Khudor, M.H.; Saeed, B.M. Detection of hbl, nhe and bceT toxin genes in Bacillus cereus isolates by multiplex PCR. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1009–1016. [Google Scholar]
- Lee, B.S.; Ban, O.-H.; Bang, W.Y.; Chae, A.S.; Oh, S.; Park, C.; Lee, M.; Kim, S.-J.; Yang, J.; Jung, Y.H. Safety assessment of Lactobacillus reuteri IDCC 3701 based on phenotypic and genomic analysis. Ann. Microbiol. 2021, 71, 1–6. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation; FAO Food and Nutrition Paper; FAO: Rome, Italy, 2006; Volume 85, p. 2. [Google Scholar]
- Choi, I.Y.; Kim, J.; Kim, S.-H.; Ban, O.-H.; Yang, J.; Park, A.M.-K. Safety Evaluation of Bifidobacterium breve IDCC4401 Isolated from Infant Feces for Use as a Commercial Probiotic. J. Microbiol. Biotechnol. 2021, 31, 949–955. [Google Scholar] [CrossRef]
- Wang, G.; Shi, T.; Chen, T.; Wang, X.; Wang, Y.; Liu, D.; Guo, J.; Fu, J.; Feng, L.; Wang, Z.; et al. Integrated whole-genome and transcriptome sequence analysis reveals the genetic characteristics of a riboflavin-overproducing Bacillus subtilis. Metab. Eng. 2018, 48, 138–149. [Google Scholar] [CrossRef]
- Cui, S.; Xia, H.; Chen, T.; Gu, Y.; Lv, X.; Liu, Y.; Li, J.; Du, G.; Liu, L. Cell Membrane and Electron Transfer Engineering for Improved Synthesis of Menaquinone-7 in Bacillus subtilis. iScience 2020, 23, 100918. [Google Scholar] [CrossRef]
- Araya, M.; Morelli, L.; Reid, G.; Sanders, M.; Stanton, C.; Pineiro, M.; Ben Embarek, P. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization, Food and Agriculture Organization of the United Nations: London, ON, Canada, 2002. [Google Scholar]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Konstantinidis, K.T.; Tiedje, J.M. Genomic insights that advance the species definition for prokaryotes. Proc. Natl. Acad. Sci. USA 2005, 102, 2567–2572. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Vater, J.; Piel, J.; Franke, P.; Scholz, R.; Schneider, K.; Koumoutsi, A.; Hitzeroth, G.; Grammel, N.; Strittmatter, A.W.; et al. Structural and Functional Characterization of Three Polyketide Synthase Gene Clusters in Bacillus amyloliquefaciens FZB 42. J. Bacteriol. 2006, 188, 4024–4036. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, X.; Dong, Y.; Xu, S.; Chen, C.; Feng, Y.; Cui, Q.; Li, W. Bacillaenes: Decomposition Trigger Point and Biofilm Enhancement in Bacillus. ACS Omega 2021, 6, 1093–1098. [Google Scholar] [CrossRef]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H.; et al. The Surfactin-Like Lipopeptides from Bacillus spp.: Natural Biodiversity and Synthetic Biology for a Broader Application Range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.-S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Candela, T.; Fouet, A. Bacillus anthracis CapD, belonging to the γ-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol. 2005, 57, 717–726. [Google Scholar] [CrossRef]
- Kongklom, N.; Shi, Z.; Chisti, Y.; Sirisansaneeyakul, S. Enhanced Production of Poly-γ-glutamic Acid by Bacillus licheniformis TISTR 1010 with Environmental Controls. Appl. Biochem. Biotechnol. 2016, 182, 990–999. [Google Scholar] [CrossRef]
- Cao, M.; Geng, W.; Liu, L.; Song, C.; Xie, H.; Guo, W.; Jin, Y.; Wang, S. Glutamic acid independent production of poly-γ-glutamic acid by Bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Bioresour. Technol. 2011, 102, 4251–4257. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, J.; Wu, X.; Huang, J.; Huang, L.; Zhu, J.; Zheng, Q.; Cen, P.; Xu, Z. The main byproducts and metabolic flux profiling of γ-PGA-producing strain B. subtilis ZJU-7 under different pH values. J. Biotechnol. 2013, 164, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, F.; Chen, Y.; Jin, C.; Guo, J.-H.; Chai, Y. Poly-γ-Glutamic Acids Contribute to Biofilm Formation and Plant Root Colonization in Selected Environmental Isolates of Bacillus subtilis. Front. Microbiol. 2016, 7, 1811. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-E.; Rhee, J.-H.; Park, C.; Sung, M.-H.; Lee, I.-H. Distribution of poly-γ-glutamate-PGA) producers in Korean fermented foods, Cheongkukjang, Doenjang, and Kochujang. Food Sci. Biotechnol. 2005, 14, 704–708. [Google Scholar]
- Yelin, I.; Flett, K.B.; Merakou, C.; Mehrotra, P.; Stam, J.; Snesrud, E.; Hinkle, M.; Lesho, E.; McGann, P.; McAdam, A.J.; et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 2019, 25, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Halder, D.; Mandal, M.; Chatterjee, S.S.; Pal, N.K.; Mandal, S. Indigenous Probiotic Lactobacillus Isolates Presenting Antibiotic like Activity against Human Pathogenic Bacteria. Biomedicines 2017, 5, 31. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougères, T.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2017, 83, 54–65. [Google Scholar] [CrossRef]
- Hong, H.; Huang, J.M.; Khaneja, R.; Hiep, L.; Urdaci, M.; Cutting, S. The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J. Appl. Microbiol. 2008, 105, 510–520. [Google Scholar] [CrossRef]
- Sorokulova, I.B.; Pinchuk, I.V.; Denayrolles, M.; Osipova, I.G.; Huang, J.M.; Cutting, S.M.; Urdaci, M.C. The Safety of Two Bacillus Probiotic Strains for Human Use. Dig. Dis. Sci. 2007, 53, 954–963. [Google Scholar] [CrossRef]
- Berkowitz, F.E. Hemolysis and Infection: Categories and Mechanisms of Their Interrelationship. Clin. Infect. Dis. 1991, 13, 1151–1162. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Borgmeier, C.; Garvey, S.M.; Spears, J.L. Preclinical Safety Assessment of Bacillus subtilis BS50 for Probiotic and Food Applications. Microorganisms 2022, 10, 1038. [Google Scholar] [CrossRef]
- Spears, J.; Kramer, R.; Nikiforov, A.; Rihner, M.; Lambert, E. Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements. Nutrients 2021, 13, 733. [Google Scholar] [CrossRef] [PubMed]
- AlGburi, A.; Volski, A.; Cugini, C.; Walsh, E.M.; Chistyakov, V.A.; Mazanko, M.S.; Bren, A.B.; Dicks, L.M.T.; Chikindas, M.L. Safety Properties and Probiotic Potential of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895. Adv. Microbiol. 2016, 06, 432–452. [Google Scholar] [CrossRef]
- Agersø, Y.; Bjerre, K.; Brockmann, E.; Johansen, E.; Nielsen, B.; Siezen, R.; Stuer-Lauridsen, B.; Wels, M.; Zeidan, A.A. Putative antibiotic resistance genes present in extant Bacillus licheniformis and Bacillus paralicheniformis strains are probably intrinsic and part of the ancient resistome. PLoS ONE 2019, 14, e0210363. [Google Scholar] [CrossRef]
- Cole, C.; Fuller, R. The effect of dietary fat and yoghurt on colonic bacterial enzymes (β-glucosidase and β-glucuronidase) associated with colon cancer. Food Microbiol. 1987, 4, 77–81. [Google Scholar] [CrossRef]
- Santos, M.S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Suzzi, G.; Torriani, S. Biogenic amines in foods. Front. Microbiol. 2015, 472, 1463–1474. [Google Scholar] [CrossRef]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef]
- Xue, B.; Sartori, P.; Leibler, S. Environment-to-phenotype mapping and adaptation strategies in varying environments. Proc. Natl. Acad. Sci. USA 2019, 116, 13847–13855. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Bang, W.Y.; Baek, K.-R.; Kim, G.-H.; Kang, M.-J.; Yang, J.; Seo, S.-O. Safety Evaluation by Phenotypic and Genomic Characterization of Four Lactobacilli Strains with Probiotic Properties. Microorganisms 2022, 10, 2218. [Google Scholar] [CrossRef]
- Eom, J.S.; Seo, B.Y.; Choi, H.S. Biogenic Amine Degradation by Bacillus Species Isolated from Traditional Fermented Soybean Food and Detection of Decarboxylase-Related Genes. J. Microbiol. Biotechnol. 2015, 25, 1519–1527. [Google Scholar] [CrossRef]
- Kim, H.; Chae, A.S.; Lee, M.; Yang, S.-Y.; Ban, O.-H.; Jung, Y.H.; Yang, J. Genomic and Toxicity Studies on Bifidobacterium bifidum IDCC 4201 and Bifidobacterium longum IDCC 4101 Isolated from Feces of Breast-Fed Infants. Food Suppl. Biomater. Health 2021, 1, e37. [Google Scholar] [CrossRef]
- Pohanka, M. D-Lactic Acid as a Metabolite: Toxicology, Diagnosis, and Detection. BioMed Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.W.; Simmons, L.A. Complete Genome Sequence of Bacillus subtilis Strain PY79. Genome Announc. 2013, 1, e01085-13. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primer Sequence (5’–3’) | Product (bp) | Reference | |
|---|---|---|---|---|
| Forward | Reverse | |||
| hblA | AAG CAA TGG AAT ACA ATG GG | AGA ATC TAA ATC ATG CCA CTG C | 1154 | [33] |
| hblC | GAT ACY AAT GTG GCA ACT GC | TTG AGA CTG CTC GYT AGT TG | 740 | [33] |
| hblD | ACC GGT AAC ACT ATT CAT GC | GAG TCC ATA TGC TTA GAT GC | 582 | [33] |
| nheA | TAC GCT AAG GAG GGG CA | GTT TTT ATT GCT TCA TCG GCT | 499 | [33] |
| nheB | CTA TCA GCA CTT ATG GCA G | ACT CCT AGC GGT GTT CC | 769 | [33] |
| nheC | CGG TAG TGA TTG CTG GG | CAG CAT TCG TAC TTG CCA A | 581 | [33] |
| cytK | GTA ACT TTC AAT GAT GAT CC | GAA TAC TAA ATA ATT GGT TTC C | 505 | [32] |
| bceT | CGT ATC GGT CGT TCA CTC GG | TTT CTT TCC CGC TTG CCT TT | 924 | [33] |
| entFM | ATG AAA AAA GTA ATT TGC AGG | TTA GTA TGC TTT TGT GTA ACC | 1269 | [32] |
| ces | GGT GAC ACA TTA TCA TAT AAG GTG | GTA AGC GAA CCT GTC TGT AAC AAC A | 1271 | [32] |
| Cluster Type * | Most Similar Cluster (Accession Number) | Location (nt) | Identity (%) | Functions |
|---|---|---|---|---|
| NRPS | Surfactin (AJ575642.1) | 450,863–514,298 | 82 | Antibacterial |
| RiPP | Sporulation killing factor (AL009126.3) | 644,154–666,225 | 100 | Antibacterial, inhibition of sporulating |
| NRPS | Bacilysin (CP000560.1) | 1,196,654–238,072 | 100 | Antibacterial, antifungal |
| RiPP | Subtilosin A (AJ430547.1) | 1,241,370–1,262,981 | 100 | Antibacterial, hemolytic activity |
| CDPS | – | 1,477,277–1,498,023 | – | – |
| NRPS | Bacillibactin (AL009126.3) | 1,783,015–1,829,009 | 100 | Antibacterial, antifungal |
| NRPS-like | Capsular polysaccharide (MH190222.1) | 2,340,772–2,382,008 | 10 | – |
| T3PKS | – | 2,804,413–2,845,306 | – | – |
| Terpene | – | 2,894,249–2,915,104 | – | – |
| NRPS | Fengycin (CP000560.1) | 2,991,749–3,068,802 | 100 | Antibacterial, antifungal |
| NRPS/PKS | Bacillaene (AJ634060.2) | 3,141,488–3,246,742 | 100 | Antibacterial, inhibition of sporulation |
| Terpene | – | 3,836,536–3,857,339 | – | – |
| Virulence Factor | Gene | Organisms | Accession | Identity (%) |
|---|---|---|---|---|
| Polyglutamic acid capsule | capA | B. subtilis subsp. subtilis str. 168 | NP_391469 | 99.7 |
| Polyglutamic acid capsule | capB | B. subtilis subsp. subtilis str. 168 | NP_391471 | 99.7 |
| Polyglutamic acid capsule | capC | B. subtilis subsp. subtilis str. 168 | NP_391470 | 100.0 |
| Polyglutamic acid capsule | capD | B. subtilis subsp. subtilis str. 168 | NP_389723 | 99.2 |
| Bacillibactin | dhbA | B. subtilis subsp. subtilis str. 168 | NP_391080.2 | 100.0 |
| Bacillibactin | dhbB | B. subtilis subsp. subtilis str. 168 | NP_391077.1 | 100.0 |
| Bacillibactin | dhbC | B. subtilis subsp. subtilis str. 168 | NP_391079.2 | 99.0 |
| Bacillibactin | dhbE | B. subtilis subsp. subtilis str. 168 | NP_832067.1 | 99.3 |
| Bacillibactin | dhbF | B. subtilis subsp. subtilis str. 168 | NP_391076.3 | 100.0 |
| Hemolysin III | hlyIII | B. subtilis subsp. subtilis str. 168 | NP_390062 | 99.53 |
| Bacteria | Enterotoxin Gene | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| hblA | hblC | hblD | nheA | nheB | nheC | cytK | bceT | entFM | ces | |
| B. cereus ATCC 14579T | + a | + | + | + | + | + | + | + | + | − |
| B. subtilis IDCC1101 | − b | − | − | − | − | − | − | − | − | − |
| Resistance Gene | Antibiotic | Location (nt) | Accession | Identity (%) | E-Value |
|---|---|---|---|---|---|
| aadK | Streptomysin | 2,407,895–2,408,747 | M26879 | 98.8 | 0 |
| tet(L) | Tetracycline | 898,343–899,719 | X08034 | 98.7 | 0 |
| Class | Antibiotic | MIC (µg/mL) | Assessment b | |
|---|---|---|---|---|
| Cutoff Values (µg/mL) | B. subtilis IDCC1101 | |||
| Aminopenicillins | Ampicillin | − a | <0.125 | – |
| Glycopeptides | Vancomycin | 4 | <0.125–0.25 | S |
| Aminoglycosides | Gentamicin | 4 | 2–4 | S |
| Aminoglycosides | Kanamycin | 8 | 4 | S |
| Aminoglycosides | Streptomycin | 8 | 64 | R |
| Macrolides | Erythromycin | 4 | <0.125 | S |
| Lincosamides | Clindamycin | 4 | 1–2 | S |
| Tetracyclines | Tetracycline | 8 | 1–2 | S |
| Amphenicols | Chloramphenicol | 8 | 2 | S |
| Gene | Location (nt) | Strand |
|---|---|---|
| Transposon Tn10 TetD protein | 327,982–328,854 | + |
| Transposase from transposon Tn916 | 2,298,050–2,299,207 | − |
| Tetracycline repressor protein class B from transposon Tn10 | 2,436,909–2,437,589 | + |
| Transposon gamma-delta resolvase | 2,488,579–2,490,081 | + |
| Enzyme | Activity | Enzyme | Activity |
|---|---|---|---|
| Alkaline phosphatase | + a | Naphthol-AS-BI-phosphohydrolase | + |
| Esterase | + | α-galactosidase | + |
| Esterase lipase | + | β-galactosidase | + |
| Acid phosphatase | + | α-glucosidase | + |
| Lipase | − b | β-glucosidase | + |
| Leucine arylamidase | − | β- glucuronidase | − |
| Valine arylamidase | − | N-acetyl-β-glucosaminidase | − |
| Cystine arylamidase | − | α-mannosidase | − |
| Trypsin | − | α-fucosidase | − |
| α-chymotrypsin | − |
| Substrate | Result a | Substrate | Result | Substrate | Result |
|---|---|---|---|---|---|
| Glycerol | + | Mannitol | + | D-Raffinose | + |
| Erythritol | − | Sorbitol | − | Amidon | + |
| D-Arabinose | − | α-Methyl-D-mannoside | + | Glycogen | − |
| L-Arabinose | + | α-Methyl-D-glucoside | + | Xylitol | + |
| Ribose | + | N-Acetyl-Glucosamine | + | Gentibiose | + |
| D-Xylose | + | Amygdaline | + | D-Turanose | − |
| L-Xylose | − | Arbutine | + | D-Lyxose | − |
| Adonitol | − | Esculine | + | D-Tagatose | − |
| β-Methyl-xylose | − | Salicine | + | D-Fucose | − |
| Galactose | + | Cellobiose | + | L-Fucose | − |
| D-Glucose | + | Maltose | − | D-Arabitol | − |
| D-Fructose | + | Lactose | + | L-Arabitol | + |
| D-Mannose | − | Melibiose | + | Gluconate | − |
| L-Sorbose | − | Sucrose | + | 2-keto-gluconate | − |
| Rhamnose | − | Trehalose | + | 5-keto-gluconate | − |
| Dulcitol | − | Inuline | − | ||
| Inositol | + | Melizitose | + |
| Biogenic Amine (mM) | Result |
|---|---|
| Tyramine | ND a |
| Histamine | ND |
| Putrescine | ND |
| 2-Phenethylamine | ND |
| Cadaverine | ND |
| Tryptamine | ND |
| D-/L- lactate proportion | Result |
| L-lactate (g/l) | ND |
| D-lactate (g/L) | ND |
| L-form (%) | ND |
| D-form (%) | ND |
| Group | Dose (g/kg BW 1) | Day after Administration | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | ||
| 9 weeks old | 300 | 216.2 ± 9.0 | 240.3 ± 8.9 | 251.3 ± 13.7 | 257.1 ± 16.2 | 269.7 ± 16.1 |
| 2000 | 205.5 ± 4.2 | 232.6 ± 2.7 | 237.3 ± 2.6 | 246.5 ± 4.3 | 254.2 ± 7.1 | |
| 10 weeks old | 300 | 231.8 ± 9.6 | 257.5 ± 13.8 | 254.6 ± 14.3 | 261.2 ± 16.8 | 266.6 ± 12.1 |
| 2000 | 220.4 ± 3.9 | 246.0 ± 4.2 | 256.2 ± 6.4 | 263.7 ± 4.3 | 266.2 ± 6.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Yehuala, G.A.; Bang, W.Y.; Yang, J.; Jung, Y.H.; Park, M.-K. Safety Evaluation of Bacillus subtilis IDCC1101, Newly Isolated from Cheonggukjang, for Industrial Applications. Microorganisms 2022, 10, 2494. https://doi.org/10.3390/microorganisms10122494
Kim S-H, Yehuala GA, Bang WY, Yang J, Jung YH, Park M-K. Safety Evaluation of Bacillus subtilis IDCC1101, Newly Isolated from Cheonggukjang, for Industrial Applications. Microorganisms. 2022; 10(12):2494. https://doi.org/10.3390/microorganisms10122494
Chicago/Turabian StyleKim, Su-Hyeon, Gashaw Assefa Yehuala, Won Yeong Bang, Jungwoo Yang, Young Hoon Jung, and Mi-Kyung Park. 2022. "Safety Evaluation of Bacillus subtilis IDCC1101, Newly Isolated from Cheonggukjang, for Industrial Applications" Microorganisms 10, no. 12: 2494. https://doi.org/10.3390/microorganisms10122494
APA StyleKim, S.-H., Yehuala, G. A., Bang, W. Y., Yang, J., Jung, Y. H., & Park, M.-K. (2022). Safety Evaluation of Bacillus subtilis IDCC1101, Newly Isolated from Cheonggukjang, for Industrial Applications. Microorganisms, 10(12), 2494. https://doi.org/10.3390/microorganisms10122494






