Comparison of Reference-Based Assembly and De Novo Assembly for Bacterial Plasmid Reconstruction and AMR Gene Localization in Salmonella enterica Serovar Schwarzengrund Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and DNA Preparation
2.2. Whole-Genome Sequencing
2.3. De Novo Assembly of Both Short and Long Reads
2.4. Reference-Based Assembly of Illumina Short Reads Mapped onto the Long Reads Assembly
2.5. Plasmid Annotation
2.6. S1-PFGE
2.7. Antimicrobial Resistance Gene Identification and Location
3. Results
3.1. Quality Control of Short and Long Reads
3.2. Comparison of De Novo and Reference-Based Assembly
3.3. Plasmid Annotation by PlasmidFinder and Confirmation by S1-PFGE Analysis
3.4. Antimicrobial Resistance Gene Identification and Location
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 31 October 2021).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef] [Green Version]
- Trusts, T.P.C. Could Efforts to Fight the Coronavirus Lead to Overuse of Antibiotics? Available online: https://www.pewtrusts.org/-/media/assets/2021/03/could_efforts_to_fight_coronavirus_lead_to_overuse_of_antibiotics_final.pdf (accessed on 31 October 2021).
- Metin, N.; Turan, Ç.; Utlu, Z. Changes in dermatological complaints among healthcare professionals during the COVID-19 outbreak in Turkey. Acta Dermatovenerol. Alp. Pannon. Adriat. 2020, 29, 115–122. [Google Scholar] [CrossRef]
- CDC. 2019 AR Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 31 October 2021).
- San Millan, A. Evolution of Plasmid-Mediated Antibiotic Resistance in the Clinical Context. Trends Microbiol. 2018, 26, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Beltrán, J.; DelaFuente, J.; León-Sampedro, R.; MacLean, R.C.; San Millán, Á. Beyond horizontal gene transfer: The role of plasmids in bacterial evolution. Nat. Rev. Microbiol. 2021, 19, 347–359. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2021, 41, 25–43. [Google Scholar] [CrossRef]
- Croucher, N.J.; Didelot, X. The application of genomics to tracing bacterial pathogen transmission. Curr. Opin. Microbiol. 2015, 23, 62–67. [Google Scholar] [CrossRef]
- Arredondo-Alonso, S.; Willems, R.J.; van Schaik, W.; Schürch, A.C. On the (im)possibility of reconstructing plasmids from whole-genome short-read sequencing data. Microb. Genom. 2017, 3, e000128. [Google Scholar] [CrossRef]
- Tyson, J.R.; O’Neil, N.J.; Jain, M.; Olsen, H.E.; Hieter, P.; Snutch, T.P. MinION-based long-read sequencing and assembly extends the Caenorhabditis elegans reference genome. Genome Res. 2018, 28, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, S.; Beka, L.; Graf, J.; Klassen, J.L. Evaluation of strategies for the assembly of diverse bacterial genomes using MinION long-read sequencing. BMC Genom. 2019, 20, 23. [Google Scholar] [CrossRef] [Green Version]
- George, S.; Pankhurst, L.; Hubbard, A.; Votintseva, A.; Stoesser, N.; Sheppard, A.E.; Mathers, A.; Norris, R.; Navickaite, I.; Eaton, C.; et al. Resolving plasmid structures in Enterobacteriaceae using the MinION nanopore sequencer: Assessment of MinION and MinION/Illumina hybrid data assembly approaches. Microb. Genom. 2017, 3, e000118. [Google Scholar] [CrossRef] [Green Version]
- Berbers, B.; Saltykova, A.; Garcia-Graells, C.; Philipp, P.; Arella, F.; Marchal, K.; Winand, R.; Vanneste, K.; Roosens, N.H.C.; De Keersmaecker, S.C.J. Combining short and long read sequencing to characterize antimicrobial resistance genes on plasmids applied to an unauthorized genetically modified Bacillus. Sci. Rep. 2020, 10, 4310. [Google Scholar] [CrossRef]
- EFSA. Antimicrobial Resistance in the EU: Infections with Foodborne Bacteria Becoming Harder to Treat. Available online: https://www.efsa.europa.eu/en/news/antimicrobial-resistance-eu-infections-foodborne-bacteria-becoming-harder-treat (accessed on 3 November 2021).
- Li, I.C.; Wu, H.H.; Chen, Z.W.; Chou, C.H. Prevalence of IncFIB Plasmids Found among Salmonella enterica Serovar Schwarzengrund Isolates from Animal Sources in Taiwan Using Whole-Genome Sequencing. Pathogens 2021, 10, 1024. [Google Scholar] [CrossRef]
- Li, I.C.; Wu, R.; Hu, C.-W.; Wu, K.-M.; Chen, Z.-W.; Chou, C.-H. Comparison of Conventional Molecular and Whole-Genome Sequencing Methods for Differentiating Salmonella enterica Serovar Schwarzengrund Isolates Obtained from Food and Animal Sources. Microorganisms 2021, 9, 46. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Wick, R.R.; Judd, L.M.; Holt, K.E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 2019, 20, 129. [Google Scholar] [CrossRef] [Green Version]
- Wick, R. Porechop. Available online: https://github.com/rrwick/Porechop (accessed on 8 November 2021).
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Lander, E.S.; Waterman, M.S. Genomic mapping by fingerprinting random clones: A mathematical analysis. Genomics 1988, 2, 231–239. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 8 November 2021).
- Fukasawa, Y.; Ermini, L.; Wang, H.; Carty, K.; Cheung, M.-S. LongQC: A Quality Control Tool for Third Generation Sequencing Long Read Data. G3 Genes Genomes Genet. 2020, 10, 1193–1196. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H. SeqTk. Available online: https://github.com/lh3/seqtk (accessed on 8 November 2021).
- Torsten, S. ABRicate. Available online: https://github.com/tseemann/abricate (accessed on 8 November 2021).
- Carattoli, A.; Hasman, H. PlasmidFinder and In Silico pMLST: Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS). Methods Mol. Biol. 2020, 2075, 285–294. [Google Scholar] [CrossRef] [PubMed]
- FDA. Notice to US Food and Drug Administration of the Conclusion that the Intended Use of Lactobacillus plantarum ECGC 13110402 (LPLDL®) is Generally Recognized as Safe. Available online: https://www.fda.gov/media/134878/download (accessed on 8 November 2021).
- Barton, B.M.; Harding, G.P.; Zuccarelli, A.J. A general method for detecting and sizing large plasmids. Anal. Biochem. 1995, 226, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, U.H.; Cézard, T.; Bridgett, S.; Montazam, A.; Nichols, J.; Blaxter, M.; Gharbi, K. Quality control of next-generation sequencing data without a reference. Front. Genet. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, M.; Khan, A.S.; Adesiyun, A.A.; Georges, K.; Gonzalez-Escalona, N. Genomic comparison of eight closed genomes of multidrug resistant Salmonella enterica strains isolated from broiler farms and processing plants in Trinidad and Tobago. bioRxiv 2021. [Google Scholar] [CrossRef]
- Juraschek, K.; Borowiak, M.; Tausch, S.H.; Malorny, B.; Käsbohrer, A.; Otani, S.; Schwarz, S.; Meemken, D.; Deneke, C.; Hammerl, J.A. Outcome of Different Sequencing and Assembly Approaches on the Detection of Plasmids and Localization of Antimicrobial Resistance Genes in Commensal Escherichia coli. Microorganisms 2021, 9, 598. [Google Scholar] [CrossRef]
- Murigneux, V.; Rai, S.K.; Furtado, A.; Bruxner, T.J.C.; Tian, W.; Harliwong, I.; Wei, H.; Yang, B.; Ye, Q.; Anderson, E.; et al. Comparison of long-read methods for sequencing and assembly of a plant genome. GigaScience 2020, 9, giaa146. [Google Scholar] [CrossRef]
- Koren, S.; Phillippy, A.M. One chromosome, one contig: Complete microbial genomes from long-read sequencing and assembly. Curr. Opin. Microbiol. 2015, 23, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Nurk, S.; Walenz, B.P.; Rhie, A.; Vollger, M.R.; Logsdon, G.A.; Grothe, R.; Miga, K.H.; Eichler, E.E.; Phillippy, A.M.; Koren, S. HiCanu: Accurate assembly of segmental duplications, satellites, and allelic variants from high-fidelity long reads. Genome Res. 2020, 30, 1291–1305. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Gu, X.-X.; Zhang, J.; Yang, L.; Lu, Y.-W.; Fang, L.-X.; Jiang, H.-X. Characterization of a fosA3 Carrying IncC–IncN Plasmid From a Multidrug-Resistant ST17 Salmonella Indiana Isolate. Front. Microbiol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Marasini, D.; Fakhr, M.K. Exploring PFGE for Detecting Large Plasmids in Campylobacter jejuni and Campylobacter coli Isolated from Various Retail Meats. Pathogens 2014, 3, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Neyaz, L.; Rajagopal, N.; Wells, H.; Fakhr, M.K. Molecular Characterization of Staphylococcus aureus Plasmids Associated With Strains Isolated From Various Retail Meats. Front. Microbiol. 2020, 11, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2018, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Cummins, M.L.; Sanderson-Smith, M.; Newton, P.; Carlile, N.; Phalen, D.N.; Maute, K.; Monahan, L.G.; Hoye, B.J.; Djordjevic, S.P. Whole-Genome Sequence Analysis of an Extensively Drug-Resistant Salmonella enterica Serovar Agona Isolate from an Australian Silver Gull (Chroicocephalus novaehollandiae) Reveals the Acquisition of Multidrug Resistance Plasmids. mSphere 2020, 5, e00743-20. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, A.L.; Yushina, Y.K.; Zaiko, E.V.; Bataeva, D.S.; Kuznetsova, O.A.; Semenova, A.A.; Ermolaeva, S.A.; Beletskiy, A.V.; Kolganova, T.y.V.; Mardanov, A.V.; et al. Evaluation of Antibiotic Resistance of Salmonella Serotypes and Whole-Genome Sequencing of Multiresistant Strains Isolated from Food Products in Russia. Antibiotics 2022, 11, 1. [Google Scholar] [CrossRef]
- McMillan, E.A.; Gupta, S.K.; Williams, L.E.; Jové, T.; Hiott, L.M.; Woodley, T.A.; Barrett, J.B.; Jackson, C.R.; Wasilenko, J.L.; Simmons, M.; et al. Antimicrobial Resistance Genes, Cassettes, and Plasmids Present in Salmonella enterica Associated With United States Food Animals. Front. Microbiol. 2019, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Lindsay, J.A. The distribution of plasmids that carry virulence and resistance genes in Staphylococcus aureus is lineage associated. BMC Microbiol. 2012, 12, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.J.; Thorsness, J.L.; Anderson, C.P.; Lynne, A.M.; Foley, S.L.; Han, J.; Fricke, W.F.; McDermott, P.F.; White, D.G.; Khatri, M.; et al. Horizontal gene transfer of a ColV plasmid has resulted in a dominant avian clonal type of Salmonella enterica serovar Kentucky. PLoS ONE 2010, 5, e15524. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, N.H.; Khajanchi, B.K.; Weston, K.; Deck, J.; Cox, J.; Singh, R.; Gilbert, J.; Sanad, Y.M.; Han, J.; Nayak, R.; et al. Genotypic and Phenotypic Characterization of Incompatibility Group FIB Positive Salmonella enterica Serovar Typhimurium Isolates from Food Animal Sources. Genes 2020, 11, 1307. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine. Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 26 December 2021).

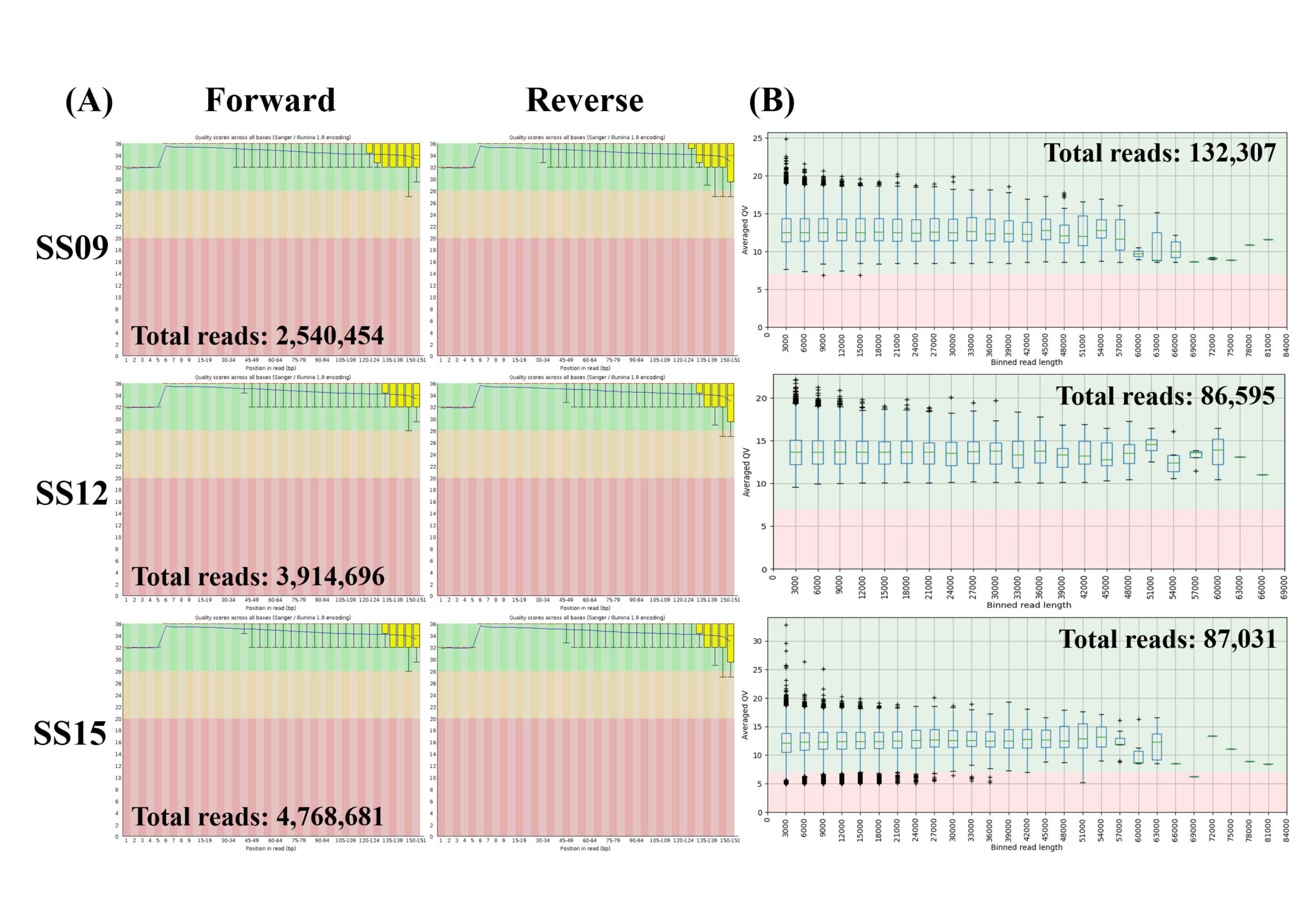

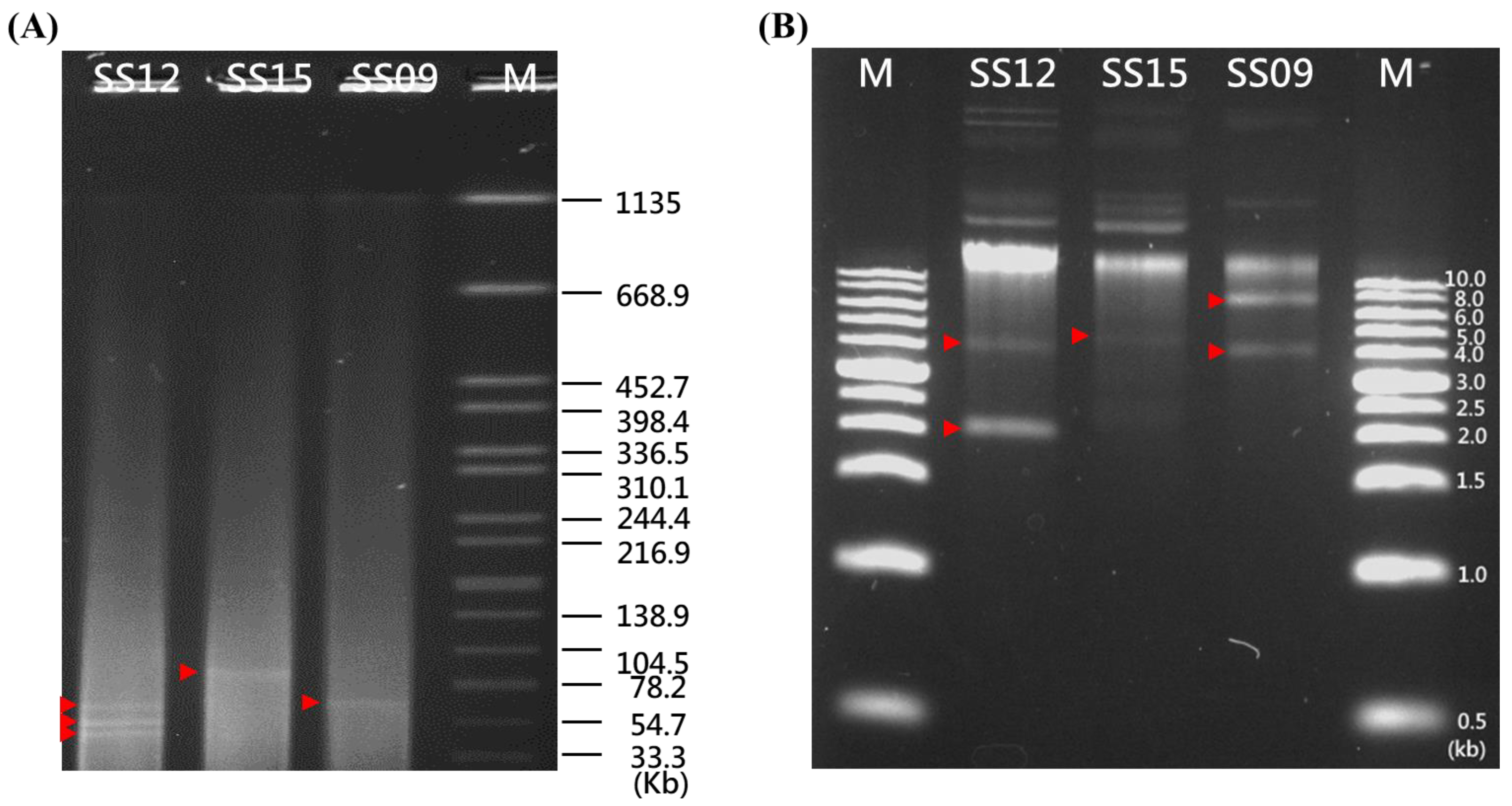
| De Novo Assembly | Reference-Based Assembly | |||||
|---|---|---|---|---|---|---|
| Strains | SS09 | SS12 | SS15 | SS09 | SS12 | SS15 |
| Total length (bp) | 4,769,113 | 4,859,186 | 4,829,437 | 4,767,778 | 4,857,568 | 4,826,252 |
| GC (%) | 52.19 | 52.13 | 52.21 | 51.19 | 52.14 | 52.21 |
| N50 | 4,684,845 | 4,671,981 | 4,713,162 | 4,686,238 | 4,672,103 | 4,712,860 |
| L50 | 1 | 1 | 1 | 1 | 1 | 1 |
| Contig | 5 | 8 | 6 | 3 | 7 | 5 |
| Size contig 1 (bp) | 4,684,845 | 4,671,981 | 4,713,162 | 4,686,238 | 4,672,198 | 4,712,860 |
| Size contig 2 (bp) | 70,463 | 67,491 | 92,935 | 70,788 | 67,818 | 93,257 |
| Size contig 3 (bp) | 6747 | 58,438 | 8131 | 6745 | 58,566 | 8128 |
| Size contig 4 (bp) | 4013 | 45,793 | 6079 | 4007 | 45,784 | 6106 |
| Size contig 5 (bp) | 3045 | 5686 | 5907 | 5682 | 5901 | |
| Size contig 6 (bp) | 4663 | 3223 | 4663 | |||
| Size contig 7 (bp) | 3045 | 3042 | ||||
| Size contig 8 (bp) | 2089 | |||||
| De Novo Assembly | Reference-Based Assembly | ||||||
|---|---|---|---|---|---|---|---|
| Replicon | Contig | Identity (%) | Coverage (%) | Replicon | Contig | Identity (%) | Coverage (%) |
| SS09 | SS09 | ||||||
| IncFIB(K) | 2 | 98.93 | 100 | IncFIB(K) | 2 | 98.93 | 100 |
| Col156 | 3 | 98.03 | 98.7 | Col156 | 3 | 98.03 | 98.7 |
| Col440II | 4 | 96.44 | 99.65 | Col440II | 4 | 96.44 | 99.65 |
| SS12 | SS12 | ||||||
| IncFIB(K) | 2 | 98.93 | 100 | IncFIB(K) | 2 | 98.75 | 99.82 |
| IncL/M | 3 | 94.63 | 89.46 | IncL/M | 3 | 94.46 | 89.31 |
| IncX1 | 4 | 98.93 | 100 | IncX1 | 4 | 98.93 | 100 |
| ColRNAI | 5 | 90.84 | 100 | ColRNAI | 5 | 90.08 | 99.23 |
| Col156 | 6 | 93.51 | 100 | Col156 | 6 | 93.51 | 100 |
| Col(BS512) | 8 | 100 | 100 | ||||
| SS15 | SS15 | ||||||
| IncFIB(K) | 2 | 98.93 | 100 | IncFIB(K) | 2 | 98.93 | 100 |
| IncQ1 | 3 | 100 | 81.28 | IncQ1 | 3 | 100 | 81.28 |
| ColRNAI | 4 | 87.79 | 100 | ColRNAI | 4 | 83.97 | 99.23 |
| De Novo Assembly | Reference-Based Assembly | ||||||
|---|---|---|---|---|---|---|---|
| Resistance Gene | Contig | Identity (%) | Coverage (%) | Resistance Gene | Contig | Identity (%) | Coverage (%) |
| SS09 | SS09 | ||||||
| AAC(3)-IV | 1 | 100 | 100 | AAC(3)-IV | 1 | 100 | 100 |
| AAC(6′)-Iy | 1 | 98.4 | 100 | AAC(6′)-Iy | 1 | 98.4 | 100 |
| aadA2 | 1 | 100 | 100 | aadA2 | 1 | 100 | 100 |
| aadA2 | 2 | 100 | 100 | aadA2 | 2 | 99.87 | 100 |
| APH(4)-Ia | 1 | 100 | 100 | APH(4)-Ia | 1 | 100 | 100 |
| cmlA1 | 1 | 99.92 | 100 | cmlA1 | 1 | 99.92 | 100 |
| dfrA12 | 2 | 100 | 100 | dfrA12 | 2 | 100 | 100 |
| golS | 1 | 99.36 | 100 | golS | 1 | 99.36 | 100 |
| mdsA | 1 | 98.78 | 100 | mdsA | 1 | 98.78 | 100 |
| mdsB | 1 | 99.02 | 100 | mdsB | 1 | 99.02 | 100 |
| mdsC | 1 | 98.28 | 100 | mdsC | 1 | 98.28 | 100 |
| mdtK | 1 | 98.88 | 100 | mdtK | 1 | 98.88 | 100 |
| qacH | 1 | 91.59 | 100 | qacH | 1 | 91.59 | 100 |
| sdiA | 1 | 98.75 | 100 | sdiA | 1 | 98.75 | 100 |
| sul1 | 2 | 100 | 100 | sul1 | 2 | 99.88 | 99.88 |
| sul3 | 1 | 100 | 100 | sul3 | 1 | 100 | 100 |
| TEM-1 | 1 | 99.88 | 100 | TEM-1 | 1 | 99.88 | 100 |
| tet(A) | 2 | 100 | 97.8 | tet(A) | 2 | 99.68 | 97.65 |
| SS12 | SS12 | ||||||
| AAC(3)-IV | 4 | 100 | 100 | AAC(3)-IV | 4 | 99.87 | 99.87 |
| AAC(6′)-Iy | 1 | 98.4 | 100 | AAC(6′)-Iy | 1 | 98.4 | 100 |
| aadA2 | 1 | 100 | 100 | aadA2 | 1 | 100 | 100 |
| aadA2 | 2 | 100 | 100 | aadA2 | 2 | 99.87 | 100 |
| APH(4)-Ia | 4 | 100 | 100 | APH(4)-Ia | 4 | 100 | 100 |
| cmlA1 | 1 | 99.92 | 100 | cmlA1 | 1 | 99.92 | 100 |
| dfrA12 | 2 | 100 | 100 | dfrA12 | 2 | 100 | 100 |
| floR | 4 | 99.75 | 100 | floR | 4 | 99.75 | 100 |
| golS | 1 | 99.36 | 100 | golS | 1 | 99.36 | 100 |
| mdsA | 1 | 98.78 | 100 | mdsA | 1 | 98.78 | 100 |
| mdsB | 1 | 99.02 | 100 | mdsB | 1 | 99.02 | 100 |
| mdsC | 1 | 98.28 | 100 | mdsC | 1 | 98.28 | 100 |
| mdtK | 1 | 98.88 | 100 | mdtK | 1 | 98.88 | 100 |
| qacH | 1 | 91.59 | 100 | qacH | 1 | 91.59 | 100 |
| sdiA | 1 | 98.75 | 100 | sdiA | 1 | 98.75 | 100 |
| sul2 | 1 | 100 | 100 | sul2 | 1 | 99.88 | 99.88 |
| TEM-1 | 1 | 99.88 | 100 | TEM-1 | 1 | 99.88 | 100 |
| TEM-1 | 4 | 99.88 | 100 | TEM-1 | 4 | 99.88 | 100 |
| tet(A) | 2 | 100 | 97.8 | tet(A) | 2 | 100 | 97.8 |
| SS15 | SS15 | ||||||
| AAC(3)-IV | 2 | 100 | 100 | AAC(3)-IV | 2 | 100 | 100 |
| AAC(6′)-Iy | 1 | 98.4 | 100 | AAC(6′)-Iy | 1 | 98.4 | 100 |
| aadA2 | 2 | 99.87 | 100 | aadA2 | 2 | 99.87 | 100 |
| APH(4)-Ia | 2 | 100 | 100 | APH(4)-Ia | 2 | 100 | 100 |
| cmlA1 | 2 | 99.92 | 100 | cmlA1 | 2 | 99.92 | 100 |
| dfrA12 | 2 | 100 | 100 | dfrA12 | 2 | 100 | 100 |
| floR | 2 | 99.67 | 100 | floR | 2 | 99.59 | 99.92 |
| golS | 1 | 99.36 | 100 | golS | 1 | 99.36 | 100 |
| mdsA | 1 | 98.78 | 100 | mdsA | 1 | 98.78 | 100 |
| mdsB | 1 | 99.02 | 100 | mdsB | 1 | 98.83 | 99.81 |
| mdsC | 1 | 98.21 | 100 | mdsC | 1 | 98.21 | 100 |
| mdtK | 1 | 98.88 | 100 | mdtK | 1 | 98.88 | 100 |
| qacH | 2 | 91.59 | 100 | qacH | 2 | 91.59 | 100 |
| sdiA | 1 | 98.75 | 100 | sdiA | 1 | 98.75 | 100 |
| sul1 | 2 | 100 | 100 | sul1 | 2 | 100 | 100 |
| sul3 | 2 | 100 | 100 | sul3 | 2 | 99.87 | 99.87 |
| tet(A) | 2 | 100 | 97.8 | tet(A) | 2 | 99.84 | 97.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, I.-C.; Yu, G.-Y.; Huang, J.-F.; Chen, Z.-W.; Chou, C.-H. Comparison of Reference-Based Assembly and De Novo Assembly for Bacterial Plasmid Reconstruction and AMR Gene Localization in Salmonella enterica Serovar Schwarzengrund Isolates. Microorganisms 2022, 10, 227. https://doi.org/10.3390/microorganisms10020227
Li I-C, Yu G-Y, Huang J-F, Chen Z-W, Chou C-H. Comparison of Reference-Based Assembly and De Novo Assembly for Bacterial Plasmid Reconstruction and AMR Gene Localization in Salmonella enterica Serovar Schwarzengrund Isolates. Microorganisms. 2022; 10(2):227. https://doi.org/10.3390/microorganisms10020227
Chicago/Turabian StyleLi, I-Chen, Gine-Ye Yu, Jing-Fang Huang, Zeng-Weng Chen, and Chung-Hsi Chou. 2022. "Comparison of Reference-Based Assembly and De Novo Assembly for Bacterial Plasmid Reconstruction and AMR Gene Localization in Salmonella enterica Serovar Schwarzengrund Isolates" Microorganisms 10, no. 2: 227. https://doi.org/10.3390/microorganisms10020227
APA StyleLi, I.-C., Yu, G.-Y., Huang, J.-F., Chen, Z.-W., & Chou, C.-H. (2022). Comparison of Reference-Based Assembly and De Novo Assembly for Bacterial Plasmid Reconstruction and AMR Gene Localization in Salmonella enterica Serovar Schwarzengrund Isolates. Microorganisms, 10(2), 227. https://doi.org/10.3390/microorganisms10020227






