Impact of Different Anthropogenic Environments on Ticks and Tick-Associated Pathogens in Alsace, a French Region Highly Endemic for Tick-Borne Diseases
Abstract
:1. Introduction
2. Materials and Methods
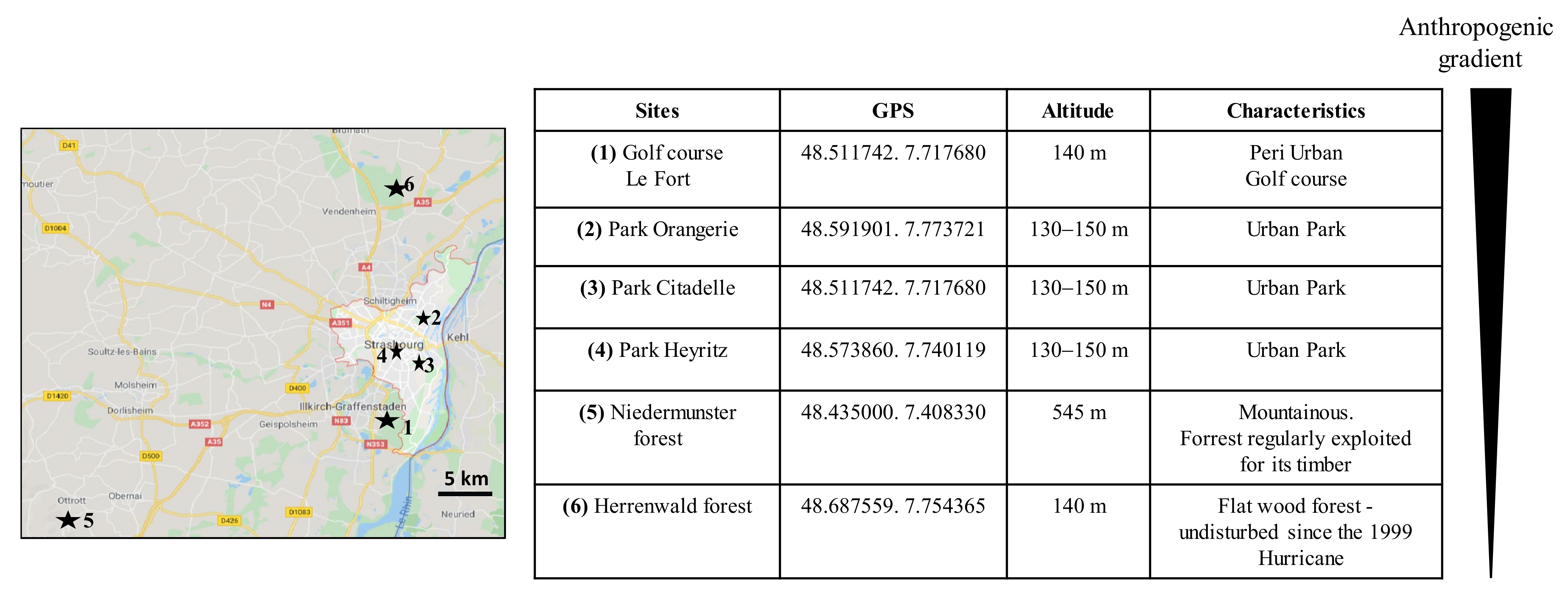
2.1. Site Description
2.2. Field Sampling of Questing Ticks
2.3. Tick Identification
2.4. DNA Extraction
2.5. PCR Detection of Tick-Borne Microorganisms
2.6. Statistical Analyses
3. Results
3.1. Spatiotemporal Variation of Ixodes Ricinus Nymph Density
3.2. Risk of Infection
3.3. Repartition of the Ixodes-Borne Microorganisms According to the Site
3.4. Dermacentor Reticulatus and Rickettsia Raoulti
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilpatrick, A.; Randolph, S. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- McMahon, B.J.; Morand, S.; Gray, J.S. Ecosystem change and zoonoses in the Anthropocene. Zoonoses Public Health 2018, 65, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of Land Use Changes and Habitat Fragmentation on the Eco-epidemiology of Tick-Borne Diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef] [PubMed]
- Fish, D. Range expansion of Ixodes scapularis in the United States. In Climate, Ticks and Disease; Nuttall, P., Ed.; CAB International: Wallingford, UK, 2022. [Google Scholar]
- Sprong, H.; Azagi, T.; Hoornstra, D.; Nijhof, A.; Knorr, S.; Baarsma, M.; Hovius, J. Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasites Vectors 2018, 11, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef]
- Eisen, R.J.; Paddock, C.D. Tick and Tickborne Pathogen Surveillance as a Public Health Tool in the United States. J. Med. Entomol. 2021, 58, 1490–1502. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.-C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.-K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Sonenshine, D.E. Range expansion of tick disease vectors in north america: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [Green Version]
- Eisen, R.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef]
- Wikel, S. Ticks and Tick-Borne Infections: Complex Ecology, Agents, and Host Interactions. Vet. Sci. 2018, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, A.; Dobson, A.; Levi, T.; Salkeld, D.; Swei, A.; Ginsberg, H.; Kjemtrup, A.; Padgett, K.; Jensen, P.; Fish, D.; et al. Lyme disease ecology in a changing world: Consensus, uncertainty and critical gaps for improving control. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372, 1722. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Farkas, R.; Estrada-Peña, A.; Jaenson, T.; Pascucci, I.; Madder, M. Basic biology and geographical distribution of tick species involved in the transmission of animal pathogens, including zoonoses. In Ticks and Tick-Borne Diseases: Geographical Distribution and Control Strategies in the Euro-Asia Region; Salman, M., Tarrés-Call, J.J., Eds.; CAB International: Wallingford, UK, 2013; pp. 6–26. [Google Scholar]
- Karbowiak, G. The occurrence of the Dermacentor reticulatus tick-Its expansion to new areas and possible causes. Ann. Parasitol. 2014, 60, 37–47. [Google Scholar] [PubMed]
- Humair, P.; Gern, L. The wild hidden face of Lyme borreliosis in Europe. Microbes Infect. 2000, 2, 915–922. [Google Scholar] [CrossRef]
- Levi, T.; Kilpatrick, A.M.; Mangel, M.; Wilmers, C.C. Deer, predators, and the emergence of Lyme disease. Proc. Natl. Acad. Sci. USA 2012, 109, 10942–10947. [Google Scholar] [CrossRef] [Green Version]
- Barbour, A.; Fish, D. The biological and social phenomenon of Lyme disease. Science 1993, 260, 1610–1616. [Google Scholar] [CrossRef] [Green Version]
- Rubel, F.; Brugger, K.; Walter, M.; Vogelgesang, J.R.; Didyk, Y.M.; Fu, S.; Kahl, O. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick-Borne Dis. 2018, 9, 1080–1089. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G. The Hard Ticks of the World: (Acari: Ixodida: Ixodidae); Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7496-4. [Google Scholar]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The Spatial Distribution of Dermacentor Ticks (Ixodidae) in Germany—Evidence of a Continuing Spread of Dermacentor reticulatus. Front. Vet. Sci. 2020, 7, 661. [Google Scholar] [CrossRef]
- Estrada-Pena, A.; Mihalca, A.D.; Petney, T. Ticks of Europe and Northern Africa: A Guide to Species Identification; COST; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick-Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.S.; Estrada-Peña, A.; Zintl, A. Vectors of Babesiosis. Annu. Rev. Entomol. 2019, 64, 149–165. [Google Scholar] [CrossRef]
- Dwużnik-Szarek, D.; Mierzejewska, E.J.; Rodo, A.; Goździk, K.; Behnke-Borowczyk, J.; Kiewra, D.; Kartawik, N.; Bajer, A. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasites Vectors 2021, 14, 267. [Google Scholar] [CrossRef]
- Gern, L.; Humair, P. Ecology of Borrelia burgdorferi sensu lato in Europe. In Lyme borreliosis: Biology, Epidemiology and Control; Gray, J., Kahl, O., Lane, R.S., Stanek, G., Eds.; CABI Publishing: Wallingford, UK, 2002; p. 347. [Google Scholar]
- Rizzoli, A.; Hauffe, H.; Carpi, G.; Vourc, H.G.; Neteler, M.; Rosa, R. Lyme borreliosis in Europe. Eurosurveilliance 2011, 16, 19906. [Google Scholar] [CrossRef]
- Humair, P.; Postic, D.; Wallich, R.; Gern, L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zent. Bakteriol. 1998, 287, 521–538. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalská, E.; et al. Ixodes ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Kazimírová, M.; Hamšíková, Z.; Špitalská, E.; Minichová, L.; Mahríková, L.; Caban, R.; Sprong, H.; Fonville, M.; Schnittger, L.; Kocianová, E. Diverse tick-borne microorganisms identified in free-living ungulates in Slovakia. Parasite Vectors 2018, 11, 1–18. [Google Scholar] [CrossRef]
- Mysterud, A.; Easterday, W.; Stigum, V.; Aas, A.; Meisingset, E.; Viljugrein, H. Contrasting emergence of Lyme disease across ecosystems. Nat. Commun. 2016, 7, 11882. [Google Scholar] [CrossRef]
- Randolph, S.; Gern, L.; Nuttall, P. Co-feeding ticks: Epidemiological significance for tick-borne pathogen transmission. Parasitol. Today 1996, 12, 472–479. [Google Scholar] [CrossRef]
- Fabri, N.D.; Sprong, H.; Hofmeester, T.R.; Heesterbeek, H.; Donnars, B.F.; Widemo, F.; Ecke, F.; Cromsigt, J.P.G.M. Wild ungulate species differ in their contribution to the transmission of Ixodes ricinus-borne pathogens. Parasites Vectors 2021, 14, 360. [Google Scholar] [CrossRef]
- Bordes, F.; Blasdell, K.; Morand, S. Transmission ecology of rodent-borne diseases: New frontiers. Integr. Zool. 2015, 10, 424–435. [Google Scholar] [CrossRef]
- Septfons, A.; Goronflot, T.; Jaulhac, B.; Roussel, V.; De Martino, S.; Guerreiro, S.; Launay, T.; Fournier, L.; De Valk, H.; Figoni, J.; et al. Epidemiology of Lyme borreliosis through two surveillance systems: The national Sentinelles GP network and the national hospital discharge database, France, 2005 to 2016. Eurosurveilliance 2019, 24, 1800134. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, V.; Boulanger, N.; Schwartz, D.; George, J.-C.; Ertlen, D.; Zilliox, L.; Schaeffer, M.; Jaulhac, B. Factors responsible for Ixodes ricinus nymph abundance: Are soil features indicators of tick abundance in a French region where Lyme borreliosis is endemic? Ticks Tick-Borne Dis. 2018, 9, 938–944. [Google Scholar] [CrossRef]
- Boyer, P.H.; Boulanger, N.; Nebbak, A.; Collin, E.; Jaulhac, B.; Almeras, L. Assessment of MALDI-TOF MS biotyping for Borrelia burgdorferi sl detection in Ixodes ricinus. PLoS ONE 2017, 12, e0185430. [Google Scholar] [CrossRef] [Green Version]
- Guy, E.C.; Stanek, G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J. Clin. Pathol. 1991, 44, 610–611. [Google Scholar] [CrossRef] [Green Version]
- Rijpkema, S.; Golubi6, D.; Molkenboer, M.; Verbeek-De Kruif, N.; Schellekens, J. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northem Croatia. Exp. Appl. Acarol. 1996, 20, 23–30. [Google Scholar]
- Koebel, C.; Kern, A.; Edouard, S.; Hoang, A.; Celestin, N.; Hansmann, Y.; Jaulhac, B.; Brouqui, P.; De Martino, S.J. Human granulocytic anaplasmosis in eastern France: Clinical presentation and laboratory diagnosis. Diagn. Microbiol. Infect. Dis. 2012, 72, 214–218. [Google Scholar] [CrossRef]
- Hovius, J.W.R.; de Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef] [Green Version]
- Labruna, M.B.; Whitworth, T.; Bouyer, D.H.; McBride, J.; Camargo, L.M.A.; Camargo, E.P.; Popov, V.; Walker, D.H. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondônia, Western Amazon, Brazil. J. Med. Entomol. 2004, 41, 1073–1081. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, S.H.; Park, K.H.; Koh, Y.S.; Lee, K.H.; Baik, H.S.; Choi, M.S.; Kim, I.S.; Jang, W.J. Evaluation of PCR-based assay for diagnosis of spotted fever group rickettsiosis in human serum samples. Clin. Diagn. Lab. Immunol. 2005, 12, 759–763. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, N.; Zilliox, L.; Goldstein, V.; Boyer, P.; Napolitano, D.; Jaulhac, B. Surveillance du vecteur de la borréliose de Lyme, Ixodes ricinus, en Alsace de 2013 à 2016. Bull. Epidemiol. Hebd. 2018, 19–20, 400–405. [Google Scholar]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Jaenson, D.G.E.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vail, S.G.; Smith, G. Air temperature and relative humidity effects on behavioral activity of blacklegged tick (Acari: Ixodidae) nymphs in New Jersey. J. Med. Entomol. 1998, 35, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.A.; Ginsberg, H.S.; Gonzalez, L.; Mather, T.N. Relative humidity and activity patterns of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 2014, 51, 769–776. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Hung, R.W. Suppression of subadult Ixodes scapularis (Acari: Ixodidae) following removal of leaf litter. J. Med. Entomol. 1995, 32, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Gassner, F.; Hansford, K.; Medlock, J. Greener cities, a wild card for ticks? In Ecology and Prevention of Lyme borreliosis; Braks, M.A.H., Van Wieren, S.E., Takken, W., Sprong, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 187–203. [Google Scholar]
- Heylen, D.; Lasters, R.; Adriaensen, F.; Fonville, M.; Sprong, H.; Matthysen, E. Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area. Sci. Total Environ. 2019, 670, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Guy, E.; Farquhar, R. Borrelia burgdorferi in urban parks. Lancet 1991, 338, 253. [Google Scholar] [CrossRef]
- Daniel, M.; Cerný, V. Occurrence of the tick Ixodes ricinus (L.) under the conditions of anthropopressure. Folia Parasitol. 1990, 37, 183–186. [Google Scholar]
- Kahl, O.; Radda, A. Occurrence of tick-borne encephalitis (TBE) virus in Berlin (West). Zent. Bakteriol. Mikrobiol. Hyg. A 1988, 268, 482–486. [Google Scholar] [CrossRef]
- Pichot, J.; Gilot, B.; Almire, N.; Polette, K.; Degeilh, B. Ixodes populations (Ixodes ricinus Linné, 1758; Ixodes hexagonus Leach, 1815) in the city of Lyon (France) and its outskirts: Preliminary results. Parasite 1997, 2, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Pangrácová, L.; Derdáková, M.; Pekárik, L.; Hviščová, I.; Víchová, B.; Stanko, M.; Hlavatá, H.; Peťko, B. Ixodes ricinus abundance and its infection with the tick-borne pathogens in urban and suburban areas of Eastern Slovakia. Parasites Vectors 2013, 6, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wodecka, B.; Rymaszewska, A.; Skotarczak, B. Host and pathogen DNA identification in blood meals of nymphal Ixodes ricinus ticks from forest parks and rural forests of Poland. Exp. Appl. Acarol. 2014, 62, 543–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahfari, S.; Ruyts, S.C.; Frazer-Mendelewska, E.; Jaarsma, R.; Verheyen, K.; Sprong, H. Melting pot of tick-borne zoonoses: The European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasites Vectors 2017, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millán, J.; Proboste, T.; Fernández de Mera, I.G.; Chirife, A.D.; de la Fuente, J.; Altet, L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human-wildlife interface. Ticks Tick-Borne Dis. 2016, 7, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hamer, S.A.; Hickling, G.J.; Keith, R.; Sidge, J.L.; Walker, E.D.; Tsao, J.I. Associations of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan, U.S.A. Parasites Vectors 2012, 5, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, K. Tick Management Handbook; The Connecticut Agricultural Experiment Station: New Haven, CT, USA, 2007. [Google Scholar]
- Hubálek, Z.; Halouzka, J.; Juricová, Z.; Sikutová, S.; Rudolf, I. Effect of forest clearing on the abundance of Ixodes ricinus ticks and the prevalence of Borrelia burgdorferi s.l. Med. Vet. Entomol. 2006, 20, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Dolan, M.C. Evidence for Personal Protective Measures to Reduce Human Contact with Blacklegged Ticks and for Environmentally Based Control Methods to Suppress Host-Seeking Blacklegged Ticks and Reduce Infection with Lyme Disease Spirochetes in Tick Vectors and Rodent Reservoirs. J. Med. Entomol. 2016, 53, 1063–1092. [Google Scholar]
- Ferquel, E.; Garnier, M.; Marie, J.; Baranton, G.; Pérezeid, C.; Postic, D. Prevalence of Borrelia burgdorferi Sensu Lato and Anaplasmataceae Members in Ixodes ricinus Ticks in Alsace, a Focus of Lyme Borreliosis Endemicity in France Prevalence of Borrelia burgdorferi Sensu Lato and Anaplasmataceae Members in Ixodes ricinus Tick. Appl. Env. Microbiol. 2006, 72, 3074–3078. [Google Scholar] [CrossRef] [Green Version]
- Norte, A.C.; Boyer, P.H.; Castillo-Ramirez, S.; Chvostáč, M.; Brahami, M.O.; Rollins, R.E.; Woudenberg, T.; Didyk, Y.M.; Derdakova, M.; Núncio, M.S.; et al. The Population Structure of Borrelia lusitaniae Is Reflected by a Population Division of Its Ixodes Vector. Microorganisms 2021, 9, 933. [Google Scholar] [CrossRef]
- Norte, A.C.; Alves da Silva, A.; Alves, J.; da Silva, L.P.; Núncio, M.S.; Escudero, R.; Anda, P.; Ramos, J.A.; Lopes de Carvalho, I. The importance of lizards and small mammals as reservoirs for Borrelia lusitaniae in Portugal. Environ. Microbiol. Rep. 2015, 7, 188–193. [Google Scholar] [CrossRef]
- Nebbak, A.; Dahmana, H.; Almeras, L.; Raoult, D.; Boulanger, N.; Jaulhac, B.; Mediannikov, O.; Parola, P. Co-infection of bacteria and protozoan parasites in Ixodes ricinus nymphs collected in the Alsace region, France. Ticks Tick-Borne Dis. 2019, 10, 101241. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, Y.; Jaulhac, B.; Kieffer, P.; Martinot, M.; Wurtz, E.; Dukic, R.; Boess, G.; Michel, A.; Strady, C.; Sagez, J.F.; et al. Value of PCR, Serology, and Blood Smears for Human Granulocytic Anaplasmosis Diagnosis, France. Emerg. Infect. Dis. 2019, 25, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.H.; Koetsveld, J.; Zilliox, L.; Sprong, H.; Talagrand-Reboul, É.; Hansmann, Y.; de Martino, S.J.; Boulanger, N.; Hovius, J.W.; Jaulhac, B. Assessment of Borrelia miyamotoi in febrile patients and ticks in Alsace, an endemic area for Lyme borreliosis in France. Parasites Vectors 2020, 13, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, P.H.; Baldinger, L.; Degeilh, B.; Wirth, X.; Kamdem, C.M.; Hansmann, Y.; Zilliox, L.; Boulanger, N.; Jaulhac, B. The emerging tick-borne pathogen Neoehrlichia mikurensis: First French case series and vector epidemiology. Emerg. Microbes Infect. 2021, 10, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Hrazdilová, K.; Lesiczka, P.M.; Bardoň, J.; Vyroubalová, Š.; Šimek, B.; Zurek, L.; Modrý, D. Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick-Borne Dis. 2021, 12, 101558. [Google Scholar] [CrossRef]
- Lejal, E.; Marsot, M.; Chalvet-Monfray, K.; Cosson, J.-F.; Moutailler, S.; Vayssier-Taussat, M.; Pollet, T. A three-years assessment of Ixodes ricinus-borne pathogens in a French peri-urban forest. Parasites Vectors 2019, 12, 551. [Google Scholar] [CrossRef] [Green Version]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; van Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; van Maanen, C.; Butler, C.M.; Földvári, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vectors 2014, 7, 365. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.; Cote, M.; Paul, R.; Bonnet, S. Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis. 2011, 11, 907–916. [Google Scholar] [CrossRef]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasites Vectors 2016, 9, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, S.; de la Fuente, J.; Nicollet, P.; Liu, X.; Madani, N.; Blanchard, B.; Maingourd, C.; Alongi, A.; Torina, A.; Fernandez de Mera, I.G.; et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector Borne Zoonotic Dis. 2013, 13, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Perez-Eid, C. Les Tiques: Identification, Biologie, Importance Médicale et Vétérinaire; Larpent, J.-P., Ed.; Editions TEC & DOC; Lavoisier: Cachan, France, 2007. [Google Scholar]
- Dautel, H.; Dippel, C.; Oehme, R.; Hartelt, K.; Schettler, E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int. J. Med. Microbiol. 2006, 296, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bajer, A.; Rodo, A.; Alsarraf, M.; Dwużnik, D.; Behnke, J.M.; Mierzejewska, E.J. Abundance of the tick Dermacentor reticulatus in an ecosystem of abandoned meadows: Experimental intervention and the critical importance of mowing. Vet. Parasitol. 2017, 246, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewska, E.J.; Alsarraf, M.; Behnke, J.M.; Bajer, A. The effect of changes in agricultural practices on the density of Dermacentor reticulatus ticks. Vet. Parasitol. 2015, 211, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Rigó, K.; Lakos, A. Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn. Microbiol. Infect. Dis. 2013, 76, 387–389. [Google Scholar] [CrossRef]
- Jongejan, F.; Ringenier, M.; Putting, M.; Berger, L.; Burgers, S.; Kortekaas, R.; Lenssen, J.; van Roessel, M.; Wijnveld, M.; Madder, M. Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasites Vectors 2015, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Parola, P.; Rovery, C.; Rolain, J.M.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in tick-borne Rickettsioses. Emerg. Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef]
- Gray, J.; Kahl, O.; Robertson, J.; Daniel, M.; Estrada-Peña, A.; Gettinby, G.; Jaenson, T.; Jensen, P.; Jongejan, F.; Korenberg, E.; et al. Lyme borreliosis habitat assessment. Zent. Bakteriol. 1998, 287, 211–228. [Google Scholar] [CrossRef]
- Roome, A.; Spathis, R.; Hill, L.; Darcy, J.; Garruto, R. Lyme Disease Transmission Risk: Seasonal Variation in the Built Environment. Healthcare 2018, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Hauser, G.; Rais, O.; Morán Cadenas, F.; Gonseth, Y.; Bouzelboudjen, M.; Gern, L. Influence of climatic factors on Ixodes ricinus nymph abundance and phenology over a long-term monthly observation in Switzerland (2000–2014). Parasites Vectors. 2018, 11, 289. [Google Scholar] [CrossRef]
- Coipan, E.C.; Jahfari, S.; Fonville, M.; Maassen, C.B.; van der Giessen, J.; Takken, W.; Takumi, K.; Sprong, H. Spatiotemporal dynamics of emerging pathogens in questing Ixodes ricinus. Front. Cell. Infect. Microbiol. 2013, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| DON: Density of Nymphs | NIP (All Microorganisms) | NIP (Borrelia) | DIN (All Microorganisms) | DIN (Borrelia) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Relative Risk | p-Value | OR | p-Value | OR | p-Value | Relative Risk | p-Value | Relative Risk | p-Value | |
| By Site | ||||||||||
| Niedermunster | Reference | Reference | Reference | Reference | Reference | |||||
| Golf | 0.0515 | <0.001 | 2.227 | 0.152 | 1.872 | 0.3569 | 0.106 | 0.00595 | 0.097 | 0.02556 |
| Herrenwald | 1.438 | <0.001 | 1.454 | 0.225 | 2.002 | 0.0473 | 1.978 | 0.02780 | 2.719 | <0.001 |
| Three urban parks | 0.001 | 0.007 | 0.000 | 0.982 | 0.000 | 0.9886 | 0.000 | 0.99693 | 0.000 | 0.99699 |
| By month | ||||||||||
| April | Reference | Reference | Reference | Reference | Reference | |||||
| May | 1.761 | <0.001 | 1.177 | 0.478 | 0.665 | 0.322 | 1.273 | 0.471 | 1.127 | 0.741 |
| June | 0.827 | 0.202 | 0.770 | 0.636 | 1.044 | 0.909 | 0.768 | 0.487 | 0.648 | 0.304 |
| Dermacentor Adult | Ixodes Adult | Ixodes Nymph | ||||
|---|---|---|---|---|---|---|
| Total Number | Density/100 m2 | Total Number | Density/100 m2 | Total Number | Density/100 m2 | |
| March 2019 | 114 | 38 | 6 | 2 | 58 | 19 |
| April 2019 | 49 | 16 | 4 | 1 | 197 | 65 |
| May 2019 2020 | 55 | 18 | 4 | 1 | 335 | 111 |
| 42 | 14 | 21 | 7 | 100 | 33 | |
| June 2019 2020 | 8 | 2 | 10 | 3 | 91 | 30 |
| 7 | 2 | 17 | 6 | 84 | 28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyer, P.H.; Barthel, C.; Mohseni-Zadeh, M.; Talagrand-Reboul, E.; Frickert, M.; Jaulhac, B.; Boulanger, N. Impact of Different Anthropogenic Environments on Ticks and Tick-Associated Pathogens in Alsace, a French Region Highly Endemic for Tick-Borne Diseases. Microorganisms 2022, 10, 245. https://doi.org/10.3390/microorganisms10020245
Boyer PH, Barthel C, Mohseni-Zadeh M, Talagrand-Reboul E, Frickert M, Jaulhac B, Boulanger N. Impact of Different Anthropogenic Environments on Ticks and Tick-Associated Pathogens in Alsace, a French Region Highly Endemic for Tick-Borne Diseases. Microorganisms. 2022; 10(2):245. https://doi.org/10.3390/microorganisms10020245
Chicago/Turabian StyleBoyer, Pierre H., Cathy Barthel, Mahsa Mohseni-Zadeh, Emilie Talagrand-Reboul, Mathieu Frickert, Benoit Jaulhac, and Nathalie Boulanger. 2022. "Impact of Different Anthropogenic Environments on Ticks and Tick-Associated Pathogens in Alsace, a French Region Highly Endemic for Tick-Borne Diseases" Microorganisms 10, no. 2: 245. https://doi.org/10.3390/microorganisms10020245
APA StyleBoyer, P. H., Barthel, C., Mohseni-Zadeh, M., Talagrand-Reboul, E., Frickert, M., Jaulhac, B., & Boulanger, N. (2022). Impact of Different Anthropogenic Environments on Ticks and Tick-Associated Pathogens in Alsace, a French Region Highly Endemic for Tick-Borne Diseases. Microorganisms, 10(2), 245. https://doi.org/10.3390/microorganisms10020245





