Ocular Surface Infection by SARS-CoV-2 in COVID-19 Pneumonia Patients Admitted to Sub-Intensive Unit: Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. RT-PCR
2.3. Cell and Virus
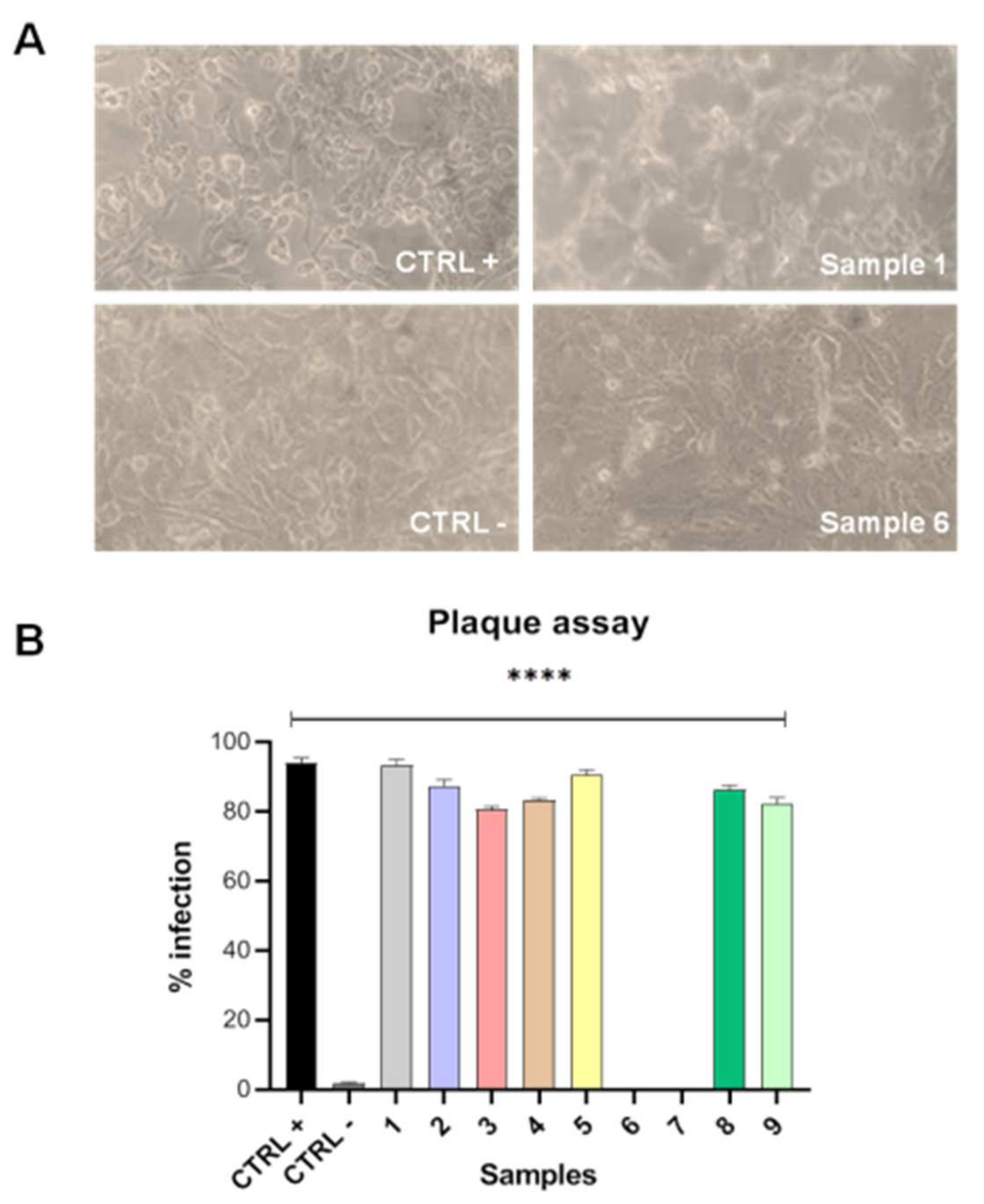
2.4. Plaque Assay
2.5. Statistical Analysis
3. Results
3.1. Patients Clinical Data
3.2. RT-PCR
3.3. Plaque Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lipsitch, M.; Swerdlow, D.L.; Finelli, L. Defining the Epidemiology of Covid-19—Studies Needed. N. Engl. J. Med. 2020, 382, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Dell’Omo, R.; Filippelli, M.; Semeraro, F.; Avitabile, T.; Giansanti, F.; Parmeggiani, F.; Romano, M.R.; Strianese, D.; Romano, V.; Virgili, G.; et al. Effects of the first month of lockdown for COVID-19 in Italy: A preliminary analysis on the eyecare system from six centers. Eur. J. Ophthalmol. 2020, 31, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Liu, X.-F.; Jia, Z.-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet 2020, 395, e39. [Google Scholar] [CrossRef] [Green Version]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.; Xia, J.; Shen, Y.; Tong, J. SARS-CoV-2 may be related to conjunctivitis but not necessarily spread through the conjunctiva SARS-CoV-2 and conjunctiva. J. Med. Virol. 2020, 92, 1757–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efron, N.; Morgan, P.B.; Katsara, S.S. Validation of grading scales for contact lens complications. Ophthalmic Physiol. Opt. 2001, 21, 17–29. [Google Scholar] [PubMed]
- Guan, W.-j.; Ni, Z.-y.; Hu, Y.; Liang, W.-h.; Ou, C.-q.; He, J.-x.; Liu, L.; Shan, H.; Lei, C.-l.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colavita, F.; Lapa, D.; Carletti, F.; Lalle, E.; Bordi, L.; Marsella, P.; Nicastri, E.; Bevilacqua, N.; Giancola, M.L.; Corpolongo, A.; et al. SARS-CoV-2 Isolation From Ocular Secretions of a Patient With COVID-19 in Italy With Prolonged Viral RNA Detection. Ann. Intern. Med. 2020, 173, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, M.; Zhang, Z.; Qiao, K.; Huang, T.; Chen, M.; Xin, N.; Huang, Z.; Liu, L.; Zhang, G.; et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br. J. Ophthalmol. 2020, 104, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Chen, L.; Deng, C.; Zou, X.; Liu, W.; Yu, H.; Chen, B.; Sun, X. The evidence of SARS-CoV-2 infection on ocular surface. Ocul. Surf. 2020, 18, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Raboud, J.; Shigayeva, A.; McGeer, A.; Bontovics, E.; Chapman, M.; Gravel, D.; Henry, B.; Lapinsky, S.; Loeb, M.; McDonald, L.C.; et al. Risk Factors for SARS Transmission from Patients Requiring Intubation: A Multicentre Investigation in Toronto, Canada. PLoS ONE 2010, 5, e10717. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Gambini, G.; Savastano, A.; Falsini, B.; De Vico, U.; Sanguinetti, M.; Cattani, P.; Marchetti, S.; Larici, A.R.; Franceschi, F.; et al. Evidence-based of conjunctival COVID-19 positivity: An Italian experience: Gemelli Against COVID Group. Eur. J. Ophthalmol. 2021, 6, 2886–2893. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; West, W.B.; Marx, D.P. Face Mask-Associated Ocular Irritation and Dryness. Ophthalmol. Ther. 2020, 9, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Ghionni, N.; Ensor, W.; Olubiyi, O.; Mann, R.K.; Simcox, B.; Gulati, S.; Nyugen, D.; Saad, K.; Chowdhury, J.; Valentino, D. 1190: Exposure keratopathy in the intensive care unit is often iatrogenic. Crit. Care Med. 2018, 46, 579. [Google Scholar] [CrossRef]
- Petronio, G.P.; Di Marco, R.; Costagliola, C. Do Ocular Fluids Represent a Transmission Route of SARS-CoV-2 Infection? Front. Med. 2021, 7, 620412. [Google Scholar] [CrossRef] [PubMed]
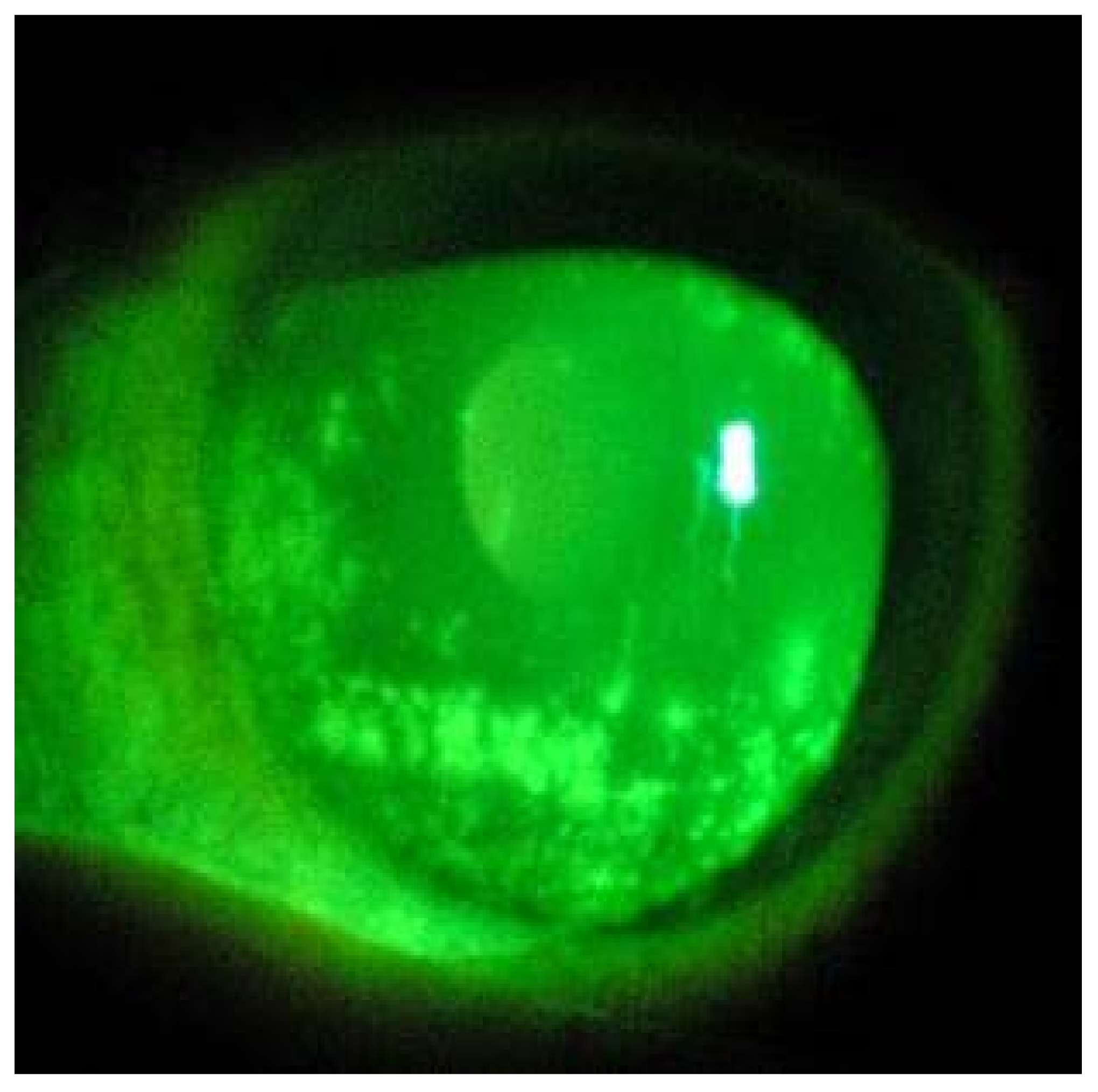
| Patient | Gender | Age | Clinical History | Days Elapsed from the First Swab Positive for SARS-CoV-2 | Systemic Therapy | Oxygen Supplement Mode |
|---|---|---|---|---|---|---|
| 1 | Male | 64 | T2DM, Hypertension | 25 days | Piperacillin/Tazobactam Enoxaparin, Dexamethasone, Omeprazole, Amlodipine, Insulin | Helmet CPAP |
| 2 | Female | 93 | Hypertension, LBBB | 14 days | Clarithromycin, Dexamethasone, Omeprazole, ARBs | Venturi mask |
| 3 | Female | 64 | Ovarian neoplasia Colostomy | 9 days | Clarithromycin, Betametasone, Pantoprazole, Albumin, Furosemide | Face mask CPAP |
| 4 | Male | 65 | T2DM, Hypertension | 3 days | Clarithromycin, Enoxaparin, Dexamethasone, Pantoprazole, Amlodipine, Metformin | Venturi mask |
| 5 | Male | 63 | Heart failure | 12 days | Enoxaparin, Azytromicin, Amlodipine, Dexamethasone, Pantoprazole | Face maskCPAP |
| 6 | Male | 79 | Hypertension, T1DM, CVD | 11 days | Bisoprolol, Amlodipine, Insulin, Enoxaparin, Pantoprazole | Nasal Cannula |
| 7 | Male | 68 | Hypertension, T2DM, Psoriasic arthritis | 9 days | Azytromicin, Enoxaparin, Insulin, Dexamethasone, Pantoprazole, ACE-inhibitor/tiazide | Nasal Cannula |
| 8 | Male | 82 | Hypertension, COPD, Prostatic neoplasia | 17 days | Azytromicin, Enoxaparin, Amlodipine, Dexamethasone, Pantoprazole, Silodosin | Venturi mask |
| 9 | Male | 83 | Hypertension, T2DM, Kidney neoplasia | 16 days | Piperacillin/Tazobactam Enoxaparin, Dexamethasone, Pantoprazole, Nebivolol, Rapid insulin, Albumin, Amlodipine | Helmet CPAP |
| Patient | Conjunctival Hyperemia (Efron Scale) | Corneal Fluorescein Test Result | RT-PCR-SARS-CoV-2 Conjunctival Swab | Ct | |
|---|---|---|---|---|---|
| 1 | Right eye: Left eye: | Grade 4 Grade 3 | Positive positive | positive positive | 18.83 18 |
| 2 | Right eye: Left eye: | Grade 2 Grade 3 | Negative positive | positive positive | 22.76 21.9 |
| 3 | Right eye: Left eye: | Grade 2 Grade 3 | Negative positive | positive positive | 27.66 27.05 |
| 4 | Right eye: Left eye: | Grade 4 Grade 3 | Positive negative | positive positive | 25.16 24.07 |
| 5 | Right eye: Left eye: | Grade 3 Grade 3 | Positive positive | positive positive | 20.18 19.12 |
| 6 | Right eye: Left eye: | Grade 0 Grade 1 | Negative negative | negative negative | N/A N/A |
| 7 | Right eye: Left eye: | Grade 2 Grade 0 | Negative negative | negative negative | N/A N/A |
| 8 | Right eye: Left eye: | Grade 3 Grade 3 | Positive negative | positive negative | 24.88 25.12 |
| 9 | Right eye: Left eye: | Grade 3 Grade 4 | Negative positive | positive positive | 28.02 27.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troisi, M.; Zannella, C.; Troisi, S.; De Bernardo, M.; Galdiero, M.; Franci, G.; Rosa, N. Ocular Surface Infection by SARS-CoV-2 in COVID-19 Pneumonia Patients Admitted to Sub-Intensive Unit: Preliminary Results. Microorganisms 2022, 10, 347. https://doi.org/10.3390/microorganisms10020347
Troisi M, Zannella C, Troisi S, De Bernardo M, Galdiero M, Franci G, Rosa N. Ocular Surface Infection by SARS-CoV-2 in COVID-19 Pneumonia Patients Admitted to Sub-Intensive Unit: Preliminary Results. Microorganisms. 2022; 10(2):347. https://doi.org/10.3390/microorganisms10020347
Chicago/Turabian StyleTroisi, Mario, Carla Zannella, Salvatore Troisi, Maddalena De Bernardo, Massimiliano Galdiero, Gianluigi Franci, and Nicola Rosa. 2022. "Ocular Surface Infection by SARS-CoV-2 in COVID-19 Pneumonia Patients Admitted to Sub-Intensive Unit: Preliminary Results" Microorganisms 10, no. 2: 347. https://doi.org/10.3390/microorganisms10020347
APA StyleTroisi, M., Zannella, C., Troisi, S., De Bernardo, M., Galdiero, M., Franci, G., & Rosa, N. (2022). Ocular Surface Infection by SARS-CoV-2 in COVID-19 Pneumonia Patients Admitted to Sub-Intensive Unit: Preliminary Results. Microorganisms, 10(2), 347. https://doi.org/10.3390/microorganisms10020347








