Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms
Abstract
:1. Introduction
1.1. Chemical Constituents
1.1.1. Proteins and Peptides
1.1.2. Polysaccharides
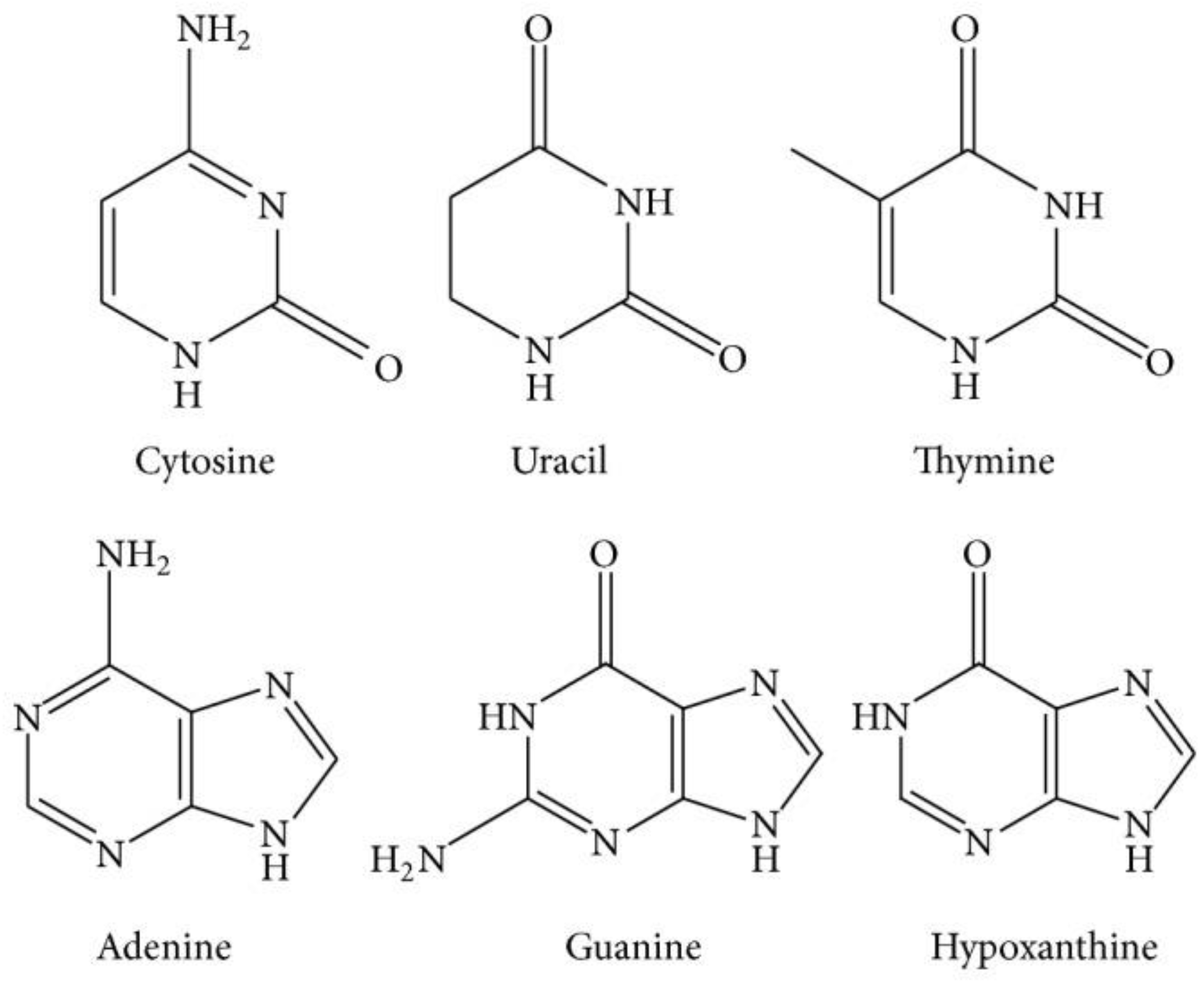
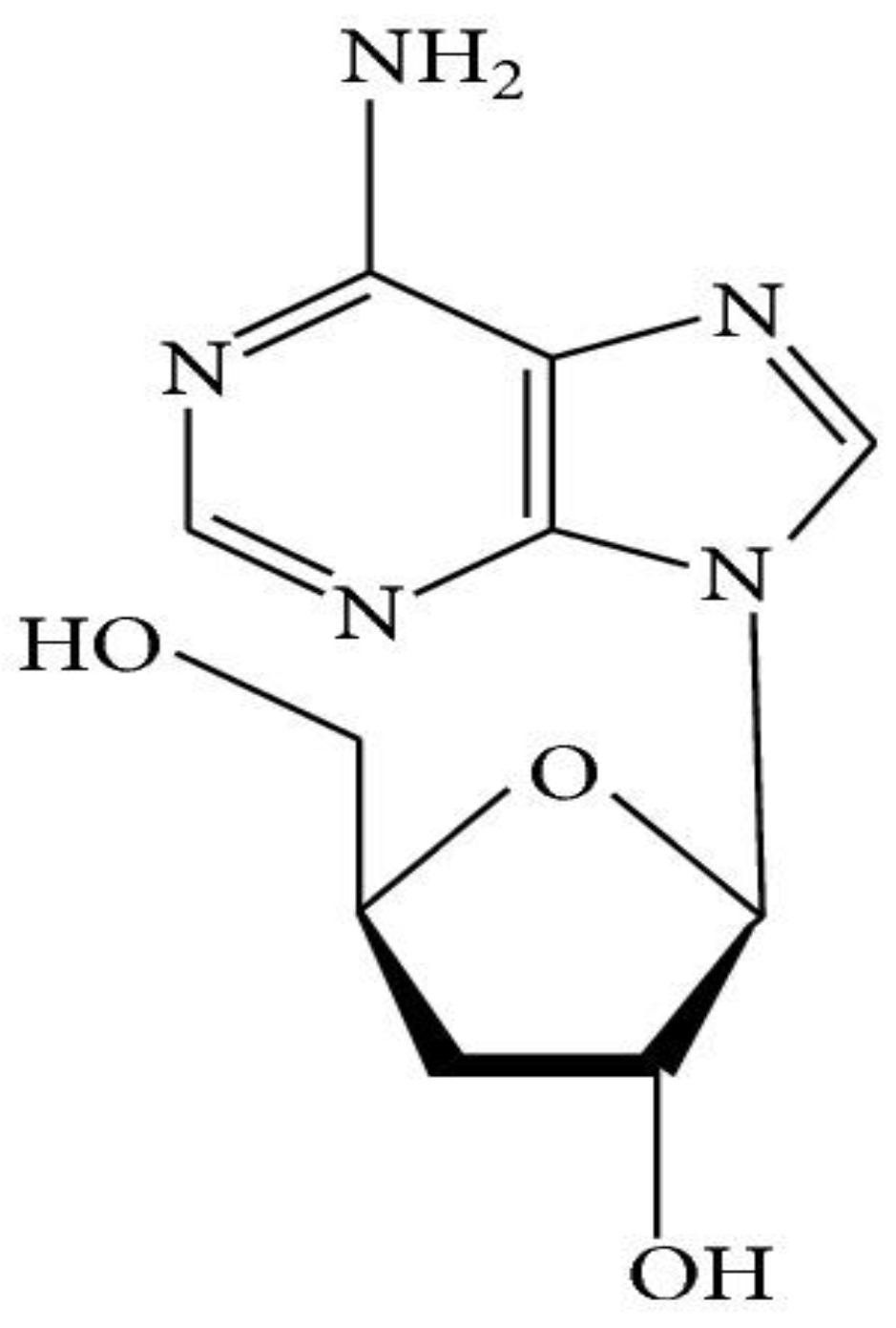
1.1.3. Nucleosides
1.1.4. Phenolic Compounds
1.1.5. Others
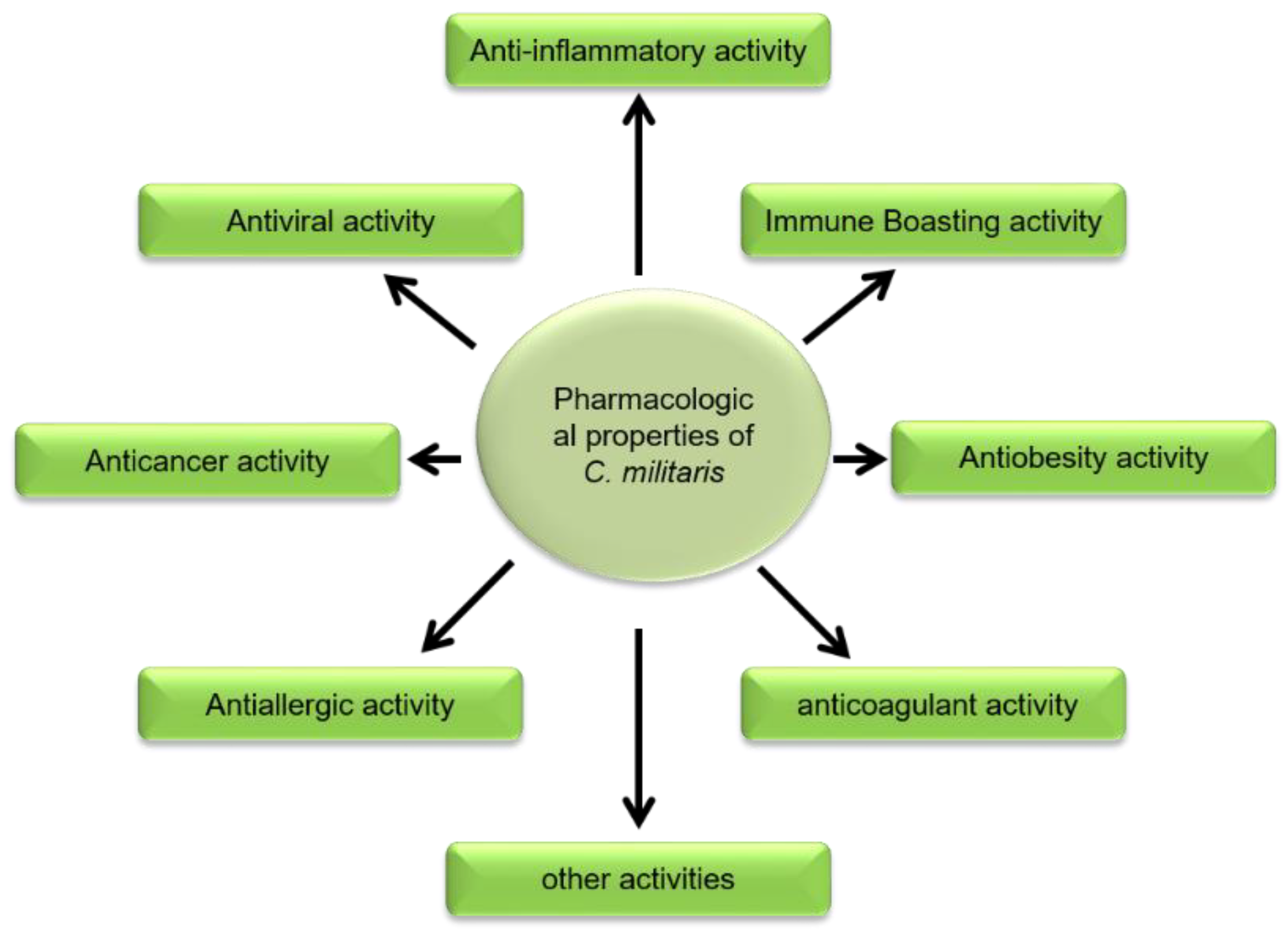
2. Pharmacological Actions of Cordyceps militaris
2.1. Immune Boosting Activity
2.2. Antiviral Potential
2.3. Anticoagulant Activity
2.4. Anticancer Activity
2.5. Anti-Obesity Activity
2.6. Anti-Allergic Activity
2.7. Other
3. Inflammation
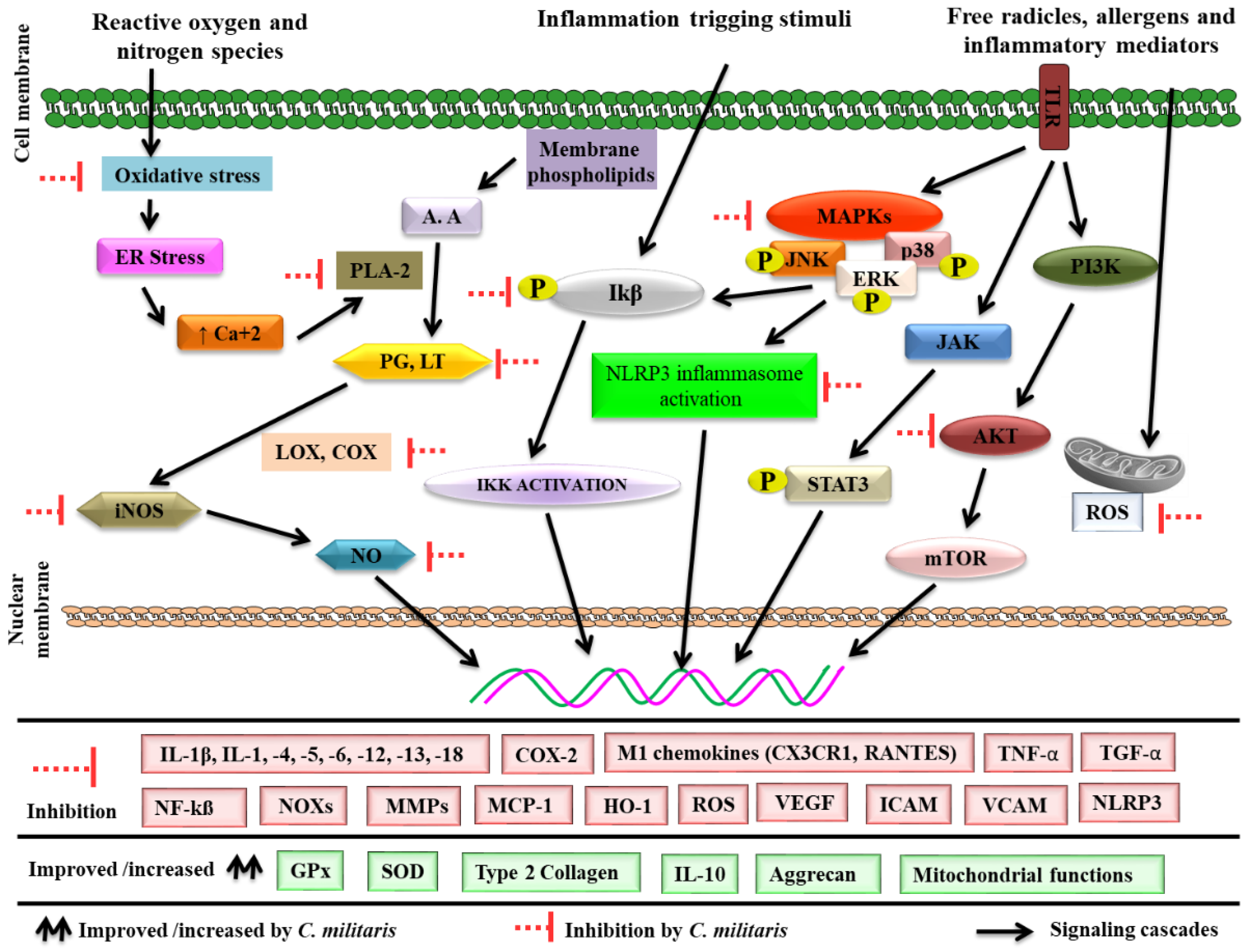
4. Cordyceps militaris and Inflammation
4.1. Antioxidant Potential
4.2. Effects on Proinflammatory Enzymes
4.3. Effects on Inflammation-Associated Gene Expression
4.4. Effects on Transcription Factors
4.5. Effects on Adhesion Molecules
4.6. Effects on Matrix Metalloproteinase
| Bioactive Component | Dose/Disease Model | Study Type/Experimental Model | Results/Mechanism | References |
|---|---|---|---|---|
| Cordycepin | 2.5–10 mg per kg of rat/Parkinson’s disease | In vivo/ Male Sprague-Dawley rats | Reduced neuro-inflammation, dynamin-related protein 1 (Drp1), IL-1β, IL-18 and tyrosine hydroxylase. Amplified NLRP3 inflammasome activation, ATP production, AMP-activated protein kinase and mitochondrial functions | [164] |
| Cordycepin | 0.0005–0.008 nM/L | In vitro/ PC12 rat pheochromocytoma cell line | Improved mitochondrial functioning by increased ATP content, maintaining membrane potential, inhibiting fission protein 1(Fis1) and mitochondrial ROS levels. | [164] |
| Cordycepin | 0–40 µg per mL/ TNF-α-induced inhibition of osteogenic differentiation in ADMSCs | In vitro/ ADMSCs | Restoration of cell proliferation and osteogenic differentiation by regulating Runx2 and Osx mRNA expressions, and NF-κB signaling via inhibition of IκBα phosphorylation. | [165] |
| Cordycepin | 0–40 µg per mL/LPS-stimulated RAW264.7 cells | In vitro/ RAW264.7 cells | Reduced proinflammatory chemicals such as IL-1β, IL-6, TNF-α, iNOS, COX-2 and NO synthesis | [64] |
| C. militaris extract (WIB801C) | 20, 50, 100 mg per kg of rat/Focal cerebral ischemia | In vivo/ Male Sprague-Dawley rat | Neuroprotection, inhibited MCP-1-induced microglial migration, oedema and the infiltration of ED-1-and MPO-positive inflammatory cells. | [158] |
| Asterina pectinifera fermented C. militaris extract (FACM) | 0–40 µg per mL/LPS-induced RAW264.7 macrophages | In vitro/ RAW264.7 macrophages | Amelioration of LPS-stimulated phosphorylation levels of MAPKs (p38, JNK1/2, and ERK1/2), NO synthase expression, IL-6 and TNF-α. | [140] |
| Cordycepin | 10, 20, 400 mg per kg of rat/ Acute lung injury, asthma. | In vivo/ Male BALB/c mice | Inhibited OVA-specific immunoglobulin (Ig) E, mucus hypersecretion, eotaxin, IL-4, -5, -13 and ICAM-1, NF-kB activation and p38-MAPK signaling cascades, recruitment of inflammatory cells in an experimental model. | [143] |
| Militarin Derivatives | 0–100 µM/ LPS-treated RAW264.7 | In vitro/ RAW264.7 cells, peritoneal macrophages | Inhibited NO production and PGE2 by downregulating p38/AP-1, IKKe/IRF-3, and Syk/NF-kB pathways | [122] |
| Militarin Derivatives | 5–20 mg per kg in DSS-induced colitis, 5–30 mg per kg in gastritis model and ear oedema model | In vivo/ male C57BL/6 and ICR mice | Anti-inflammatory effects by reducing gastric damage (gastritis), inhibited colon size and up-regulated phospho-p38 (colitis), and inhibited ear oedema. | [122] |
| Cordycepin and adenosine | 0, 1, 10 and 100 µg per mL/ LPS induced inflammatory response | In vitro/ Murine macrophage | Inhibition of inflammation by reducing expression of M1 chemokines (CX3CR1, RANTES) and cytokines (IL-1β, TNF-α). | [166] |
| Extract of C. militaris grown on soybean | 5–20 mg per kg of mice/ DSS-induced colitis | In vivo/ C57BL/6 mice | Inhibited TNF-𝛼, iNOS, MMP-3, MMP-9 mRNA Expressions in colonic tissue of a colitis model. | [167] |
| Extract of C. militaris grown on soybean | 10 and 100 µg per mL/ LPS-induce RAW264.7 Cells. | In vitro/ RAW264.7 cells | Suppressed TNF-𝛼 and iNOS in a cell model | [167] |
| Extract (Mulberry leaves fermented with C. Militaris) | High fat diet-induce -obese mice | In vivo/ C57BL/6N male mice | Inhibited mast cell infiltration, COX-2, iNOS, IL-6, -1β, TNF-α, NF-κB. Anti-inflammatory response via the PI3K/AKT/mTOR signaling pathway. | [148] |
| Cordycepin | 0, 10, 50 or 100 µM/ Nucleus pulposus cell and intervertebral disc organ culture inflammatory models | In vitro/ rats | Increased type-II collagen, aggrecan synthesis. Inhibited PGE2, NO, and matrix damaging enzymes (MMP-3, -13; ADAMTS-4, and -5). | [146] |
| C. militaris extract, fractions, ergosterol | 0.1, 1, 10 and 100 µg per mL/ LPS-stimulated BV2 microglia cells | In vitro/ BV2 microglia cells | Significantly reduction in LPS induced nitric oxide. | [168] |
| C. militaris-fermented product extract | 0.603–1.809 g per kg per day/ liver fibrosis BALB/c mice | In vivo/ Male BALB/c mice | Suppressed proinflammatory cytokines, such as TNF-α, IL-6, and NF-κB. | [151] |
| Cordycepin, C. militaris butanol extract | 0–30 µg of Cordycepin per mL/ or 0–75 µg of extract per mL/ LPS-triggered RAW264.7 cells | In vitro/ RAW264.7 cells | Anti-inflammatory effect by inhibiting NO synthesis, NF-κB activation, iNOS, COX-2 expressions and phosphorylation of p38 and Akt. | [136] |
| Ergosterol palmitate; palmitic acid; ergosterol; ergosterol peroxide; 3,4-O-isopropylidene-d-mannitol; Cordycepin; d-mannitol; d-glucose | LPS/IFN-α stimulated murine peritoneal macrophage cells | In vitro/ macrophage cells | Suppressed synthesis of cytokines including IL-12 and TNF-α and NO production | [40] |
| Soya-cerebroside, C. militaris extract | 0, 1, 5, and 10 µM/ IL-1β-induced monocytes | In vitro/ Monocyte | Reduced monocytes migration and MCP-1 expressions. Downregulated SP1 expression by activating miR-432 and inducing phosphorylation of AKT and AMPK. | [159] |
| Soya-cerebroside, C. militaris extract | 3 and 10 mg per kg per day/ IL-1β-induced inflammatory rat model | In vivo/ Severe combined immunodeficiency | Inhibited edema and cartilage damage. Induction in CD68 and MCP-1 (a marker for monocyte/macrophages) positive cells, | [159] |
| Soya-cerebroside | 0, 1, 5, and 10 µM/ Osteoarthritis synovial fibroblasts (OASFs) | In vitro/ humans | Decreased monocyte migration, activated AKT and AMPK signaling pathways, MCP-1 and microRNA (miR)-432 expression in OASFs. | [159] |
| C. militaris extract | 1, 10, 100 and 1000 µg per mL/ In LPS-stimulated RAW264.7 and antigen-induced RBL-2H3 cells | In vitro/ RAW264.7 and RBL-2H3 cells | Inhibited nitrite production, iNOS, and TNF-α. | [169] |
| C. militaris extract | 500 mg per kg of animal per day/ DSS induced acute colitis | In vivo/ BALB/c mice | Alleviated the severity of the disease in a colitis mouse model by decreasing mRNA expression of TNF- α and iNOS. | [169] |
| GRC, GRC-ON89A | 250, 500 µg per mL/ LPS-induced Macrophages | In vitro/ RAW264.7 cells | Reduced NO production, iNOS, COX-2, and TNF-α mRNA expression, and that of MAPKs (ERK, JNK, and P38), NF-κB. | [120] |
| GRC, GRC-ON89A | 25 mg per kg of animal/ DNFB induced allergic contact dermatitis | In vivo/ BALB/c, C57BL/6N mice models | Decreased inflammatory response such as ear swelling in an experimental model | [120] |
| Cordycepin | 12.5, 25, 50, 100 µg per mL/ Cholecystokinin-stimulated pancreatic acinar cancer cell | In vitro/ pancreatic acinar cancer cell | Anti-inflammatory effect by down regulating NLRP3 inflammasome activation and NF-κB via AMPK. | [152] |
| Cordycepin | 100 mg per kg of animal/ Caerulein induced acute pancreatitis | In vivo/ Male ICR mice | Augmented neutrophil infiltration and reduced edema, acinar cell vacuolization, serum amylase, lipase levels. Inhibited TNF-α, IL-1β, IL-6 by suppressing the activation of NLRP3 inflammasome and NF-κB. | [152] |
| C. militaris aqueous extract | 1 and 2 g per kg of animal/ Cationic bovine serum albumin-induced membranous glomerulonephritis rat model | In vivo/ Wistar male rats | Amplification of total protein, serum albumin, MDA, SOD, and glutathione peroxidase. Attenuated IL-1, TNF-α, 6-keto-PGF1α, NF-κB p65. Reduced serum levels of VCAM-1, ICAM-1, and MCP-1 and urine protein serum creatinine, triglyceride, blood urea nitrogen and total cholesterol. | [144] |
| Extract of fruiting bodies C. militaris | 500 µg per mL/ LPS-induced inflammatory response in macrophages | In vitro/ RAW264.7 Macrophages | Reduced Synthesis of IL-6, NO, and TNF-α. | [170] |
| Cordycepin | 50, 100, and 200 g per kg / LPS-induced acute lung injury mice model | In vivo/ Male BALB/c mice | Inhibition of Nrf2 and HO-1 expressions, MDA content, IL-1β, TNF-α and NF-κB activation. | [133] |
| C. militaris and Rumex crispus Mixture | 50 and 100 µg per mL/ LPS-induced splenocytes | Ex vivo/ splenocytes | Suppressed COX-2, iNOS, IL-1β, IL-6, TNF-α, IFN-γ) and NO synthesis | [135] |
| C. militaris-based nanoemulsion | 25 and 50 µg per mL/ LPS-induced Macrophages | In vitro/ RAW264.7 Macrophages | Reduced expression of proinflammatory cytokines (TNF-α, IL-1β, IKKa, iNOS, IL-6, NF-kß) and NO production. | [171] |
| Mulberry leaves fermented with C. militaris | 100, 200 and 400 μg per mL/ LPS-induced Macrophages | In vitro/ RAW264.7 Macrophages | Anti-inflammatory activity by iNOS-mediated COX-2, expression of inflammatory cytokines (IL-1β, IL-6 and TNF-α), and MAPK signaling pathway | [148] |
| Cordycepin | 10, 50 and 100 μM/ IL-1β-stimulated human osteoarthritic chondrocytes | Ex vivo/ osteoarthritic chondrocytes | Suppressed IL-1β, PGE2, MMP-13, IL-6, iNOS, COX-2 and NO synthesis. | [145] |
| Cordycepin | PBMCs (Kawasaki disease patients), LPS-induced Macrophages | In vitro and Ex-Vivo/ PBMCs, macrophages | Inhibition of LPS-stimulated TNFα production in mouse macrophages and in PBMCs | [172] |
| Cordycepin | 1, 5, 10 and 20 mg per kg/ Traumatic brain injury | In vivo/ Sprague-Dawley rats | Increased arginase 1 and IL-10. Inhibition of IL-1β, iNOS, MPO and MMP-9, and NADPH oxidase expression. | [173] |
| C. militaris fruiting bodies extract | 4 g per kg/ OVA sensitized airway inflammatory mice model | In vivo/ BALB/c mice | Inhibited asthmatic airway inflammation and blocked bronchoconstriction mediators-leukotrienes | [97] |
| C. militaris, C. militaris fermented Haliotis discus hannai (HFCM-5) | 50, 100 and 200 µg per mL/ LPS-induced Macrophages | In vitro/ RAW264.7 Macrophages | Decreased proinflammatory cytokines, TNF-α and IL-6 in a concentration-dependent manner. In addition, showed nitric oxide inhibitory activity. | [174] |
| Cordycepin | 50 and 100 μM/ Palmitic acid and oleic acid in inflammation in Hepatocytes | In vitro/ hepatocytes | Attenuated the increased expression of inflammatory genes (TNF-α, IL-1β, Cxcl10, Ccl2 and Ccl5) | [121] |
| Cordycepin | 100 and 200 mg per kg/ Lipotoxic model), nonalcoholic steatohepatitis | In vivo/ Mice | Suppressed inflammatory genes (IL-1β, Cxcl2, Cxcl10, Ccl2, and Ccl5), activation of NF-κB signaling, and inflammatory cell infiltration. Anti-inflammatory effects through AMPK pathways | [121] |
| Spent mushroom (C. militaris) | 0.5, 1 and 1.5 g per kg/ | In vitro/ pigs | Improved health conditions. Inhibition of IL-1β and TNF-α. | [175] |
| Fermented cultured C. militaris (GRC-SC11) | 0–300 µg per mL/ allergic model (RBL-2H3 cells) | In vitro/ RBL-2H3 | IL-4 and TNF-α inhibition | [176] |
| Cordycepin | 2 g per liter in drinking water/ LPS stimulated animals | In vivo/ Male broilers (Ross 308) | Inhibition of COX-2 and iNOS | [123] |
| C. militaris powder | 3, 1.5, and 0.5 g powder per individual/ | In vivo/ Humans | Suppressed inflammatory cytokines including EGF, eotaxin, fractalkine, IP-10, IL-1α, -6, -8, IFN-α2, -γ, MIP-1α, -1β, GRO, G-CSF, GM-CSF, MCP-1, sCD40L, TGF-α, VEGF | [137] |
5. Functional Resemblance of Cordyceps militaris and Nonsteroidal Inflammation Preventing Drugs
- ➢
- Production of ROS
- ➢
- Triggering and augmented production of pro-inflammatory cytokines
- ➢
- Inflammation-associated markers, pro-inflammatory cytokine mediated regulation of CAMs
- ➢
- NF-κB activation
- ➢
- Enhancing the production of arachidonic acid metabolites
6. Limitations and Future Prospects
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ma, X.H.; Qin, C.; Tao, L.; Liu, X.; Shi, Z.; Zhang, C.L.; Tan, C.Y.; Chen, Y.Z.; Jiang, Y.Y. Drug discovery prospect from untapped species: Indications from approved natural product drugs. PLoS ONE 2012, 7, e39782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galm, U.; Shen, B. Natural product drug discovery: The times have never been better. Chem. Biol. 2007, 14, 1098–1104. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Song, H.; Yoon, D.H.; Song, B.W.; Park, S.M.; Sung, G.H.; Cho, J.Y.; Park, H.I.; Choi, S.; Song, W.O.; et al. Cordyceps pruinosa extracts induce apoptosis of HeLa cells by a caspase dependent pathway. J. Ethnopharmacol. 2010, 128, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Shrestha, B.; Han, S.K.; Sung, J.M. Cultural Characteristics of Ophiocordyceps heteropoda Collected from Korea. Mycobiology 2011, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Cleaver, P.D.; Loomis-Powers, M.; Patel, D. Analysis of quality and techniques for hybridization of medicinal fungus Cordyceps sinensis (Berk.) Sacc. (Ascomycetes). Int. J. Med. Mushrooms 2004, 6, 151–164. [Google Scholar]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Rivera, G.; Bocanegra-García, V.; Monge, A. Traditional plants as source of functional foods: A review Plantas tradicionales como fuente de alimentos funcionales: Una revisión. CyTA J. Food 2010, 8, 159–167. [Google Scholar] [CrossRef]
- Maroyi, A. Utilization of Bridelia mollis as herbal medicine, nutraceutical and functional food in southern Africa: A review. Trop. J. Pharm. Res. 2019, 18, 203–209. [Google Scholar] [CrossRef]
- Ukeyima, M.T.; Enujiugha, V.N.; Sanni, T.A. Current applications of probiotic foods in Africa. Afr. J. Biotechnol. 2010, 9, 394–401. [Google Scholar]
- Arvanitoyannis, I.S.; Van Houwelingen-Koukaliaroglou, M. Functional foods: A survey of health claims, pros and cons, and current legislation. Crit. Rev. Food Sci. Nutr. 2005, 45, 385–404. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Alves, R.C.; Oliveira, M.B.P.; Costa, H.S. Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods? Trends Food Sci. Technol. 2020, 98, 181–189. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Ziemer, C.J.; Gibson, G.R. An overview of probiotics, prebiotics and synbiotics in the functional food concept: Perspectives and future strategies. Int. Dairy J. 1998, 8, 473–479. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Cordyceps Medicinal Fungus and Its Nutraceutical and Therapeutic Potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef]
- Holliday, J.; Cleaver, M.P. Medicinal value of the caterpillar fungi species of the genus Cordyceps (Fr.) Link (Ascomycetes). A review. Int. J. Med. Mushrooms 2008, 10, 219–234. [Google Scholar] [CrossRef]
- Shrestha, B.; Zhang, W.; Zhang, Y.; Liu, X. The medicinal fungus Cordyceps militaris: Research and development. Mycol. Prog. 2012, 11, 599–614. [Google Scholar] [CrossRef]
- Dong, C.; Guo, S.; Wang, W.; Liu, X. Cordyceps industry in China. Mycology 2015, 6, 121–129. [Google Scholar] [CrossRef]
- Wong, K.L.; Wong, R.N.; Zhang, L.; Liu, W.K.; Ng, T.B.; Shaw, P.C.; Kwok, P.C.; Lai, Y.M.; Zhang, Z.J.; Zhang, Y.; et al. Bioactive proteins and peptides isolated from Chinese medicines with pharmaceutical potential. Chin. Med. 2014, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, Y.; Yan, Y.B.; Lei, W.; Wu, Z.X.; Liu, N.; Liu, S.; Shi, L.; Fan, Y. The Protective Effect of Cordymin, a Peptide Purified from the Medicinal Mushroom Cordyceps sinensis, on Diabetic Osteopenia in Alloxan-Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2013, 2013, 985636. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.C.; Kim, K.D.; Bae, C.H.; Kim, J.C.; Kim, D.K.; Kim, H.H. A mushroom lectin from ascomycete Cordyceps militaris. Biochim. Biophys. Acta 2007, 1770, 833–838. [Google Scholar] [CrossRef]
- Park, B.T.; Na, K.H.; Jung, E.C.; Park, J.W.; Kim, H.H. Antifungal and Anticancer Activities of a Protein from the Mushroom Cordyceps militaris. Korean J. Physiol. Pharm. 2009, 13, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.H.; Yen, Y.H.; Yang, J.P. Finding of polysaccharide-peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J. Food Drug Anal. 2015, 23, 63–70. [Google Scholar] [CrossRef]
- Liu, X.; Kopparapu, N.K.; Li, Y.; Deng, Y.; Zheng, X. Biochemical characterization of a novel fibrinolytic enzyme from Cordyceps militaris. Int. J. Biol. Macromol. 2017, 94 Pt B, 793–801. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, Y.; Yu, G.; Jin, Y.; Ye, X.; Shrestha, A.; Liu, W.; Yu, W.; Sun, H. A novel protein with anti-metastasis activity on 4T1 carcinoma from medicinal fungus Cordyceps militaris. Int. J. Biol. Macromol. 2015, 80, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kwon, D.S.; Lee, K.R.; Park, J.M.; Ha, S.J.; Hong, E.K. Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydr. Polym. 2015, 120, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, X.Y.; Yang, R.L.; Qin, T.; Wang, X.Y.; Zhang, K.Y.; Fei, C.Z.; Li, Y.; Hu, Y.; Xue, F.Q. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr. Polym. 2012, 89, 461–466. [Google Scholar] [CrossRef]
- Luo, X.; Duan, Y.; Yang, W.; Zhang, H.; Li, C.; Zhang, J. Structural elucidation and immunostimulatory activity of polysaccharide isolated by subcritical water extraction from Cordyceps militaris. Carbohydr. Polym. 2017, 157, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Huang, Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013, 58, 18–22. [Google Scholar] [CrossRef]
- Yu, R.; Wang, L.; Zhang, H.; Zhou, C.; Zhao, Y. Isolation, purification and identification of polysaccharides from cultured Cordyceps militaris. Fitoterapia 2004, 75, 662–666. [Google Scholar] [CrossRef]
- Jing, Y.; Cui, X.; Chen, Z.; Huang, L.; Song, L.; Liu, T.; Lv, W.; Yu, R. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr. Polym. 2014, 102, 288–296. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.-L.; Jin, C.; Chen, H.-J.; Li, S.-H.; Li, S.-Y.; Dou, X.-F.; Jia, J.-Q.; Gui, Z.-Z. Cordyceps militaris polysaccharide triggers apoptosis and G0/G1 cell arrest in cancer cells. J. Asia-Pac. Entomol. 2015, 18, 433–438. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Wu, W.S.; Tzeng, Y.M. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator’s production and human cancer cell proliferation. J. Ethnopharmacol. 2010, 131, 363–367. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, J.; Lee, Y.W.; Yoo, H.S.; Cho, C.K. Antitumor activity of water extracts from Cordyceps militaris in NCI-H460 cell xenografted nude mice. J. Acupunct. Meridian Stud. 2009, 2, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.Y.; Kang, J.S.; Kim, H.M.; Kim, Y.O.; Hong, I.P.; Lee, M.K.; Hong, J.T.; Kim, Y.; Han, S.B. Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food Chem. Toxicol. 2010, 48, 1926–1933. [Google Scholar] [CrossRef]
- Lin, R.; Liu, H.; Wu, S.; Pang, L.; Jia, M.; Fan, K.; Jia, S.; Jia, L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int. J. Biol. Macromol. 2012, 51, 153–157. [Google Scholar] [CrossRef]
- Liu, Y.; E, Q.; Zuo, J.; Tao, Y.; Liu, W. Protective effect of Cordyceps polysaccharide on hydrogen peroxide-induced mitochondrial dysfunction in HL-7702 cells. Mol. Med. Rep. 2013, 7, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Meng, X.; Yang, R.; Qin, T.; Li, Y.; Zhang, L.; Fei, C.; Zhen, W.; Zhang, K.; Wang, X.; et al. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2013, 59, 178–183. [Google Scholar] [CrossRef]
- Yu, R.; Song, L.; Zhao, Y.; Bin, W.; Wang, L.; Zhang, H.; Wu, Y.; Ye, W.; Yao, X. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia 2004, 75, 465–472. [Google Scholar] [CrossRef]
- Cheung, J.K.; Li, J.; Cheung, A.W.; Zhu, Y.; Zheng, K.Y.; Bi, C.W.; Duan, R.; Choi, R.C.; Lau, D.T.; Dong, T.T.; et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: Signaling cascade and induction of cytokines. J. Ethnopharmacol. 2009, 124, 61–68. [Google Scholar] [CrossRef]
- Bi, S.; Huang, W.; Chen, S.; Huang, C.; Li, C.; Guo, Z.; Yang, J.; Zhu, J.; Song, L.; Yu, R. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int. J. Biol. Macromol. 2020, 150, 261–280. [Google Scholar] [CrossRef]
- Bi, S.; Jing, Y.; Zhou, Q.; Hu, X.; Zhu, J.; Guo, Z.; Song, L.; Yu, R. Structural elucidation and immunostimulatory activity of a new polysaccharide from Cordyceps militaris. Food Funct. 2018, 9, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.T.; Bittencourt, V.C.B.; Lopes, L.C.L.; Sassaki, G.; Barreto-Bergter, E. Toll-like receptors (TLR2 and TLR4) recognize polysaccharides of Pseudallescheria boydii cell wall. Carbohydr. Res. 2012, 356, 260–264. [Google Scholar] [CrossRef]
- Wu, D.-T.; Xie, J.; Wang, L.-Y.; Ju, Y.-J.; Lv, G.-P.; Leong, F.; Zhao, J.; Li, S.-P. Characterization of bioactive polysaccharides from Cordyceps militaris produced in China using saccharide mapping. J. Funct. Foods 2014, 9, 315–323. [Google Scholar] [CrossRef]
- Ribeiro, J.A. Purinergic inhibition of neurotransmitter release in the central nervous system. Pharm. Toxicol. 1995, 77, 299–305. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [Green Version]
- Tabrizchi, R.; Bedi, S. Pharmacology of adenosine receptors in the vasculature. Pharm. Ther. 2001, 91, 133–147. [Google Scholar] [CrossRef]
- Carlezon, W.A., Jr.; Mague, S.D.; Parow, A.M.; Stoll, A.L.; Cohen, B.M.; Renshaw, P.F. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol. Psychiatry 2005, 57, 343–350. [Google Scholar] [CrossRef]
- Vinadé, E.R.; Schmidt, A.P.; Frizzo, M.E.; Izquierdo, I.; Elisabetsky, E.; Souza, D.O. Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res. 2003, 977, 97–102. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Park, S.J.; Lee, S.G.; Shin, S.C.; Choi, D.H. Cordycepin: Selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. J. Agric. Food Chem. 2000, 48, 2744–2748. [Google Scholar] [CrossRef]
- Zhou, X.; Meyer, C.U.; Schmidtke, P.; Zepp, F. Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. Eur. J. Pharm. 2002, 453, 309–317. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech. 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Zhang, S.; Du, M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice. Nutr. Res. 2015, 35, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, S.; Kwon, J.; Moon, S.; Lee, S.; Lee, C.K.; Cho, K.; Ha, N.J.; Kim, K. Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages. Immune Netw. 2009, 9, 98–105. [Google Scholar] [CrossRef]
- Ji, D.B.; Ye, J.; Li, C.L.; Wang, Y.H.; Zhao, J.; Cai, S.Q. Antiaging effect of Cordyceps sinensis extract. Phytother. Res. 2009, 23, 116–122. [Google Scholar] [CrossRef]
- Patel, K.; Ingalhalli, R.S. Cordyceps militaris (L.: Fr.) Link-An Important Medicinal Mushroom. J. Pharmacogn. Phytochem. 2013, 2, 315–319. [Google Scholar]
- Thomson, D.M.; Winder, W.W. AMP-activated protein kinase control of fat metabolism in skeletal muscle. Acta Physiol. (Oxf.) 2009, 196, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, L.L.; Kozak, R.; Kelly, S.E.; Onay Besikci, A.; Russell, J.C.; Lopaschuk, G.D. Potential mechanisms and consequences of cardiac triacylglycerol accumulation in insulin-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E923–E930. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Luo, L.; Dressel, W.; Shadier, G.; Krumbiegel, D.; Schmidtke, P.; Zepp, F.; Meyer, C.U. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am. J. Chin. Med. 2008, 36, 967–980. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wang, Z.-L.; Qi, W.; Lei, W.; Zhao, G.-Y. Cordycepin (3′-deoxyadenosine) down-regulates the proinflammatory cytokines in inflammation-induced osteoporosis model. Inflammation 2014, 37, 1044–1049. [Google Scholar] [CrossRef]
- Trigg, P.I.; Gutteridge, W.E.; Williamson, J. The effects of cordycepin on malaria parasites. Trans. R. Soc. Trop. Med. Hyg. 1971, 65, 514–520. [Google Scholar] [CrossRef]
- Rodrigo, R.; Bosco, C. Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp. Biochem. Physiol. C Toxicol. Pharm. 2006, 142, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.M.; Wang, B.S.; Huang, S.C.; Duh, P.D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006, 54, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Sagar, A.; Kanwar, S.S.; Singh, S. Anticancer, antibacterial and antioxidant activities of Cordyceps militaris. Indian J. Exp. Biol. 2019, 57, 15–20. [Google Scholar]
- Cui, J.D. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Li-Tong, C.; Hong-Feng, C.; Wen-Fang, H.J. Chemical composition, pharmic effect and application of Cordyceps militaris. Guangzhou Food Sci. Technol. 2005, 21, 192–197. [Google Scholar]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Cirić, A.; van Griensven, L.J.; Soković, M.; Ferreira, I.C. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.Z.; Wang, S.H.; Ai, X.R.; Yao, L.; Sun, Z.W.; Lei, C.; Wang, Y.; Wang, Q.J. Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J. Funct. Foods 2013, 5, 1450–1455. [Google Scholar] [CrossRef]
- Chiu, C.P.; Liu, S.C.; Tang, C.H.; Chan, Y.; El-Shazly, M.; Lee, C.L.; Du, Y.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Anti-inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef]
- Kim, C.S.; Lee, S.Y.; Cho, S.H.; Ko, Y.M.; Kim, B.H.; Kim, H.J.; Park, J.C.; Kim, D.K.; Ahn, H.; Kim, B.O.; et al. Cordyceps militaris induces the IL-18 expression via its promoter activation for IFN-gamma production. J. Ethnopharmacol. 2008, 120, 366–371. [Google Scholar] [CrossRef]
- Zhu, S.J.; Pan, J.; Zhao, B.; Liang, J.; Ze-Yu, W.; Yang, J.J. Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. J. Ethnopharmacol. 2013, 149, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Baik, H.W.; Kim, S.J.; Lee, S.G.; Ahn, H.Y.; Park, J.S.; Park, S.J.; Jang, E.J.; Park, S.W.; Choi, J.Y.; et al. Cordyceps militaris Enhances Cell-Mediated Immunity in Healthy Korean Men. J. Med. Food 2015, 18, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Park, H.; Sung, G.H.; Lee, K.; Lee, T.; Lee, I.; Park, M.S.; Jung, Y.W.; Shin, Y.S.; Kang, H.; et al. Anti-influenza effect of Cordyceps militaris through immunomodulation in a DBA/2 mouse model. J. Microbiol. 2014, 52, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Mori, K.; Satoh, S.; Dansako, H.; Ikeda, M.; Kato, N. Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 2014, 447, 341–345. [Google Scholar] [CrossRef]
- Cui, L.; Dong, M.S.; Chen, X.H.; Jiang, M.; Lv, X.; Yan, G. A novel fibrinolytic enzyme from Cordyceps militaris, a Chinese traditional medicinal mushroom. World J. Microbiol. Biotechnol. 2008, 24, 483–489. [Google Scholar] [CrossRef]
- Liu, X.; Kopparapu, N.K.; Shi, X.; Deng, Y.; Zheng, X.; Wu, J. Purification and biochemical characterization of a novel fibrinolytic enzyme from culture supernatant of Cordyceps militaris. J. Agric. Food Chem. 2015, 63, 2215–2224. [Google Scholar] [CrossRef]
- Park, J.G.; Son, Y.J.; Lee, T.H.; Baek, N.J.; Yoon, D.H.; Kim, T.W.; Aravinthan, A.; Hong, S.; Kim, J.H.; Sung, G.H.; et al. Anticancer Efficacy of Cordyceps militaris Ethanol Extract in a Xenografted Leukemia Model. Evid. Based Complement. Altern. Med. 2017, 2017, 8474703. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, J.; Zhang, H.; Xiao, L.; Lei, Z.; Kaul, S.C.; Wadhwa, R.; Zhang, Z. Low Dose of Fluoride in the Culture Medium of Cordyceps militaris Promotes Its Growth and Enhances Bioactives with Antioxidant and Anticancer Properties. J. Fungi 2021, 7, 342. [Google Scholar] [CrossRef]
- Jin, C.Y.; Kim, G.Y.; Choi, Y.H. Induction of apoptosis by aqueous extract of Cordyceps militaris through activation of caspases and inactivation of Akt in human breast cancer MDA-MB-231 Cells. J. Microbiol. Biotechnol. 2008, 18, 1997–2003. [Google Scholar]
- Lee, H.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. Daru 2015, 23, 35. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.M.; Lai, W.J.; Hong, T.; Tsai, S.H.; Chen, Y.H.; Kao, C.H.; Chu, R.; Shen, T.L.; Li, T.K. Involvement of p38 MAPK in the Anticancer Activity of Cultivated Cordyceps militaris. Am. J. Chin. Med. 2015, 43, 1043–1057. [Google Scholar] [CrossRef]
- Jo, E.; Jang, H.J.; Shen, L.; Yang, K.E.; Jang, M.S.; Huh, Y.H.; Yoo, H.S.; Park, J.; Jang, I.S.; Park, S.J. Cordyceps militaris Exerts Anticancer Effect on Non-Small Cell Lung Cancer by Inhibiting Hedgehog Signaling via Suppression of TCTN3. Integr. Cancer 2020, 19, 1534735420923756. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Choi, Y.W.; Kim, K.M.; Song, H.; Hwang, D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019, 17, 2185–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Hong, I.P.; Ahn, M.J.; Yoo, H.S.; Han, S.B.; Hwang, B.Y.; Lee, M.K. Anti-adipogenic activity of Cordyceps militaris in 3T3-L1 cells. Nat. Prod. Commun. 2011, 6, 1839–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Li, K.; Kang, J.S.; Kang, N.J.; Son, B.G.; Choi, Y.W. Strawberry fermentation with Cordyceps militaris has anti-adipogenesis activity. Food Biosci. 2020, 35, 100576. [Google Scholar] [CrossRef]
- Shimada, T.; Hiramatsu, N.; Kasai, A.; Mukai, M.; Okamura, M.; Yao, J.; Huang, T.; Tamai, M.; Takahashi, S.; Nakamura, T.; et al. Suppression of adipocyte differentiation by Cordyceps militaris through activation of the aryl hydrocarbon receptor. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E859–E867. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.H.; Sun, H.L.; Sheu, J.N.; Ku, M.S.; Hu, C.M.; Chan, Y.; Lue, K.H. Effects of the immunomodulatory agent Cordyceps militaris on airway inflammation in a mouse asthma model. Pediatr. Neonatol. 2008, 49, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Choi, W.S.; Lee, C.H.; Park, H.J. The ethyl acetate extract of Cordyceps militaris inhibits IgE-mediated allergic responses in mast cells and passive cutaneous anaphylaxis reaction in mice. J. Ethnopharmacol. 2011, 135, 422–429. [Google Scholar] [CrossRef]
- Park, D.K.; Choi, W.S.; Park, H.J. Antiallergic activity of novel isoflavone methyl-glycosides from Cordyceps militaris grown on germinated soybeans in antigen-stimulated mast cells. J. Agric. Food Chem. 2012, 60, 2309–2315. [Google Scholar] [CrossRef]
- Wu, T.F.; Chan, Y.Y.; Shi, W.Y.; Jhong, M.T. Uncovering the Molecular Mechanism of Anti-Allergic Activity of Silkworm Pupa-Grown Cordyceps militaris Fruit Body. Am. J. Chin. Med. 2017, 45, 497–513. [Google Scholar] [CrossRef]
- Dong, Y.; Jing, T.; Meng, Q.; Liu, C.; Hu, S.; Ma, Y.; Liu, Y.; Lu, J.; Cheng, Y.; Wang, D.; et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. BioMed Res. Int. 2014, 2014, 160980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012, 56, 1–29. [Google Scholar] [CrossRef]
- Mehra, A.; Zaidi, K.U.; Mani, A.; Thawani, V.J.K. The health benefits of Cordyceps militaris—A review. Kavaka 2017, 48, 27–32. [Google Scholar]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Segal, J.P.; Tresidder, K.A.; Bhatt, C.; Gilron, I.; Ghasemlou, N.J. Circadian control of pain and neuroinflammation. J. Neurosci. Res. 2018, 96, 1002–1020. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vega, M.T. The inflammatory process underlying atherosclerosis. Crit. Rev. Immunol. 2012, 32, 373–462. [Google Scholar] [CrossRef]
- Kornstädt, L.; Pierre, S.; Weigert, A.; Ebersberger, S.; Schäufele, T.J.; Kolbinger, A.; Schmid, T.; Cohnen, J.; Thomas, D.; Ferreirós, N. Bacterial and Fungal Toll-Like Receptor Activation Elicits Type I IFN Responses in Mast Cells. Front. Immunol. 2021, 11, 3872. [Google Scholar] [CrossRef] [PubMed]
- Galvão, I.; Sugimoto, M.A.; Vago, J.P.; Machado, M.G.; Sousa, L.P. Mediators of inflammation. In Immunopharmacology and Inflammation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–32. [Google Scholar]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A. Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 2014, 54, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Sommer, C.; Lindenlaub, T.; Teuteberg, P.; Schäfers, M.; Hartung, T.; Toyka, K.V. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001, 913, 86–89. [Google Scholar] [CrossRef]
- Phull, A.R.; Nasir, B.; Haq, I.U.; Kim, S.J. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S., Jr. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Tissot, B.; Montdargent, B.; Chevolot, L.; Varenne, A.; Descroix, S.; Gareil, P.; Daniel, R. Interaction of fucoidan with the proteins of the complement classical pathway. Biochim. Biophys. Acta 2003, 1651, 5–16. [Google Scholar] [CrossRef]
- Maity, N.; Nema, N.K.; Sarkar, B.K.; Mukherjee, P.K. Standardized Clitoria ternatea leaf extract as hyaluronidase, elastase and matrix-metalloproteinase-1 inhibitor. Indian J. Pharm. 2012, 44, 584–587. [Google Scholar]
- Terzi, M.; Altun, G.; Şen, S.; Kocaman, A.; Kaplan, A.A.; Yurt, K.K.; Kaplan, S. The use of non-steroidal anti-inflammatory drugs in neurological diseases. J. Chem. Neuroanat. 2018, 87, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Majid, M.; Haq, I.-U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Kwon, H.K.; Song, M.J.; Lee, H.J.; Park, T.S.; Kim, M.I.; Park, H.J. Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. Int. J. Mol. Sci. 2018, 19, 3504. [Google Scholar] [CrossRef] [Green Version]
- Lan, T.; Yu, Y.; Zhang, J.; Li, H.; Weng, Q.; Jiang, S.; Tian, S.; Xu, T.; Hu, S.; Yang, G.; et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 686–703. [Google Scholar] [CrossRef]
- Yu, T.; Shim, J.; Yang, Y.; Byeon, S.E.; Kim, J.H.; Rho, H.S.; Park, H.; Sung, G.H.; Kim, T.W.; Rhee, M.H.; et al. 3-(4-(tert-Octyl)phenoxy)propane-1,2-diol suppresses inflammatory responses via inhibition of multiple kinases. Biochem. Pharm. 2012, 83, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Hsieh, Y.C.; Yu, Y.H. Effect of Cordyceps militaris Hot Water Extract on Immunomodulation-associated Gene Expression in Broilers, Gallus gallus. J. Poult. Sci. 2019, 56, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.F.; Shi, W.Y.; Chiu, Y.C.; Chan, Y.Y. Investigation of the molecular mechanism underlying the inhibitory activities of ethanol extract of Bombyx mori pupa-incubated Cordyceps militaris fruiting bodies toward allergic rhinitis. Biomed. Pharm. 2021, 135, 111248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Joe, H.I.; Zhang, Z.; Woo Lee, S.; Lee, K.Y.; Kook, Y.B.; An, H.J. Anti-inflammatory effect of Acalypha australis L. via suppression of NF-κB signaling in LPS-stimulated RAW264.7 macrophages and LPS-induced septic mice. Mol. Immunol. 2020, 119, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharm. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Gu, S.; Pan, L.; Sun, H.; Gong, E.; Zhu, Z.; Wen, T.; Daba, G.M.; Elkhateeb, W.A. Structure analysis and antioxidant activity of polysaccharide-iron (III) from Cordyceps militaris mycelia. Int. J. Biol Macromol. 2021, 178, 170–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Cui, Y.; Liu, H.; Dong, C.; Sun, Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydr. Polym. 2020, 235, 115969. [Google Scholar] [CrossRef]
- Wu, H.C.; Chen, S.T.; Chang, J.C.; Hsu, T.Y.; Cheng, C.C.; Chang, H.S.; Liu, C.S.; Wu, Y.C.; Liang, Z.C. Radical Scavenging and Antiproliferative Effects of Cordycepin-Rich Ethanol Extract from Brown Rice-Cultivated Cordyceps militaris (Ascomycetes) Mycelium on Breast Cancer Cell Lines. Int. J. Med. Mushrooms 2019, 21, 657–669. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, Z. Using Cordyceps militaris extracellular polysaccharides to prevent Pb(2+)-induced liver and kidney toxicity by activating Nrf2 signals and modulating gut microbiota. Food Funct. 2020, 11, 9226–9239. [Google Scholar] [CrossRef]
- He, M.T.; Lee, A.Y.; Park, C.H.; Cho, E.J. Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro. Nutr. Res. Pract. 2019, 13, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wei, Y.; Song, P.; Li, Y.; Zhang, T.; Feng, Q.; Xu, G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharm. 2018, 818, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Wilhelms, D.B.; Mirrasekhian, E.; Jaarola, M.; Blomqvist, A.; Engblom, D. Inflammation-induced anorexia and fever are elicited by distinct prostaglandin dependent mechanisms, whereas conditioned taste aversion is prostaglandin independent. Brain Behav. Immun. 2017, 61, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Song, G.H.; Kim, S.H.; Lee, S.M.; Kim, Y.G.; Lim, Y.L.; Kang, S.A.; Park, K.Y. Rumex crispus and Cordyceps militaris Mixture Ameliorates Production of Pro-Inflammatory Cytokines Induced by Lipopolysaccharide in C57BL/6 Mice Splenocytes. Prev. Nutr. Food Sci. 2018, 23, 374–381. [Google Scholar] [CrossRef]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW264.7 macrophage cells. Eur. J. Pharm. 2006, 545, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shao, Y.; Zhang, Z.; Wang, L.; Mariga, A.M.; Pang, G.; Geng, C.; Ho, C.T.; Hu, Q.; Zhao, L. Regulation of human cytokines by Cordyceps militaris. J. Food Drug Anal. 2014, 22, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Phull, A.R.; Kim, S.J. Fucoidan from Undaria pinnatifida regulates type II collagen and COX-2 expression via MAPK and PI3K pathways in rabbit articular chondrocytes. Biologia 2017, 72, 1362–1369. [Google Scholar] [CrossRef]
- Sharma, J.; Mohammed, L.A. The role of leukotrienes in the pathophysiology of inflammatory disorders: Is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology 2006, 14, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Shin, W.B.; Dong, X.; Kim, E.K.; Nawarathna, W.; Kim, H.; Park, P.J. Anti-inflammatory effect of the extract from fermented Asterina pectinifera with Cordyceps militaris mycelia in LPS-induced RAW264.7 macrophages. Food Sci. Biotechnol. 2017, 26, 1633–1640. [Google Scholar] [CrossRef]
- Sheng, W.Y.; Chen, Y.R.; Wang, T.C. A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 2006, 580, 6819–6824. [Google Scholar] [CrossRef] [Green Version]
- Phull, A.; Eo, S.; Kim, S.J.C.; Biology, M. Oleanolic acid (OA) regulates inflammation and cellular dedifferentiation of chondrocytes via MAPK signaling pathways. Cell. Mol. Biol. (Noisy-le-Grand) 2017, 63, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; He, Y.; Li, T.; Wang, W.; Zhang, J.; Wei, J.; Deng, Y.; Lin, R. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int. Immunopharmacol. 2015, 26, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Liu, C.; Huang, Y.; He, L.; Cai, X.; Lu, J.; Liu, Y.; Wang, D. Cordyceps militaris fruit body extract ameliorates membranous glomerulonephritis by attenuating oxidative stress and renal inflammation via the NF-κB pathway. Food Funct. 2016, 7, 2006–2015. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Peng, L.; Chen, H.; Shen, Y.; Yu, K.; Cheng, S. Cordycepin prevented IL-β-induced expression of inflammatory mediators in human osteoarthritis chondrocytes. Int. Orthop. 2014, 38, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, K.; Mao, L.; Han, X.; Zhang, K.; Zhao, C.; Zhao, J. Cordycepin inhibits LPS-induced inflammatory and matrix degradation in the intervertebral disc. PeerJ 2016, 4, e1992. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Yamori, T. Phosphatidylinositol 3-kinase inhibitors: Promising drug candidates for cancer therapy. Cancer Sci. 2008, 99, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Kim, J.E.; Park, J.J.; Choi, J.Y.; Song, B.R.; Choi, Y.W.; Kim, D.S.; Kim, K.M.; Song, H.K.; Hwang, D.Y. Protective role of fermented mulberry leave extract in LPS-induced inflammation and autophagy of RAW264.7 macrophage cells. Mol. Med. Rep. 2020, 22, 4685–4695. [Google Scholar] [CrossRef]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar] [CrossRef]
- Ju, S.M.; Song, H.Y.; Lee, S.J.; Seo, W.Y.; Sin, D.H.; Goh, A.R.; Kang, Y.-H.; Kang, I.-J.; Won, M.-H.; Yi, J.-S.; et al. Suppression of thymus-and activation-regulated chemokine (TARC/CCL17) production by 1, 2, 3, 4, 6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009, 387, 115–120. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lee, C.L. Higher Anti-Liver Fibrosis Effect of Cordyceps militaris-Fermented Product Cultured with Deep Ocean Water via Inhibiting Proinflammatory Factors and Fibrosis-Related Factors Expressions. Mar. Drugs 2017, 15, 168. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Shi, J. Cordycepin protects against acute pancreatitis by modulating NF-κB and NLRP3 inflammasome activation via AMPK. Life Sci. 2020, 251, 117645. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Kang, K.S.; Chun, K.H.; Hwang, G.S. Cordyceps militaris mushroom and cordycepin inhibit RANKL-induced osteoclast differentiation. J. Med. Food 2015, 18, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Wulfert, F.M.; van Meurs, M.; Kurniati, N.F.; Jongman, R.M.; Houwertjes, M.C.; Heeringa, P.; Struys, M.M.; Zijlstra, J.G.; Molema, G. Age-dependent role of microvascular endothelial and polymorphonuclear cells in lipopolysaccharide-induced acute kidney injury. J. Am. Soc. Anesthesiol. 2012, 117, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etter, H.; Althaus, R.; Eugster, H.P.; Santamaria-Babi, L.F.; Weber, L.; Moser, R. IL-4 and IL-13 downregulate rolling adhesion of leukocytes to IL-1 or TNF-alpha-activated endothelial cells by limiting the interval of E-selectin expression. Cytokine 1998, 10, 395–403. [Google Scholar] [CrossRef]
- Ley, K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996, 32, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, B.; Müller, C.; Brigelius-Flohé, R. Inhibition of tumor necrosis factor-alpha- and interleukin-1-induced endothelial E-selectin expression by thiol-modifying agents. Arter. Thromb. Vasc. Biol. 1998, 18, 1829–1837. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Cho, G.S.; Ryu, S.; Kim, H.J.; Song, H.Y.; Yune, T.Y.; Ju, C.; Kim, W.K. Post-ischemic treatment of WIB801C, standardized Cordyceps extract, reduces cerebral ischemic injury via inhibition of inflammatory cell migration. J. Ethnopharmacol. 2016, 186, 169–180. [Google Scholar] [CrossRef]
- Liu, S.C.; Chiu, C.P.; Tsai, C.H.; Hung, C.Y.; Li, T.M.; Wu, Y.C.; Tang, C.H. Soya-cerebroside, an extract of Cordyceps militaris, suppresses monocyte migration and prevents cartilage degradation in inflammatory animal models. Sci. Rep. 2017, 7, 43205. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar]
- Alameddine, H.S.; Morgan, J.E. Matrix Metalloproteinases and Tissue Inhibitor of Metalloproteinases in Inflammation and Fibrosis of Skeletal Muscles. J. Neuromuscul. Dis. 2016, 3, 455–473. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.-C.; Choi, W.H. Mithramycin A alleviates osteoarthritic cartilage destruction by inhibiting HIF-2α expression. Int. J. Mol. Sci. 2018, 19, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, E.M.; Kim, J.S.; Hur, H.; Park, B.H.; Song, E.K.; Han, M.K.; Kwon, K.B.; Yoo, W.H.; Shim, I.K.; Lee, S.J.; et al. Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxf.) 2009, 48, 45–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.L.; Huang, W.M.; Tang, P.C.; Sun, Y.; Zhang, X.; Qiu, L.; Yu, B.C.; Zhang, X.Y.; Hong, Y.X.; He, Y.; et al. Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission. Neurotoxicology 2021, 84, 1–13. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Y.; Lv, Z.; Jiang, T.; Wang, L.; Li, Z. Cordycepin protected against the TNF-α-induced inhibition of osteogenic differentiation of human adipose-derived mesenchymal stem cells. Int. J. Immunopathol. Pharm. 2015, 28, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Moon, S.; Park, Y.; Kwon, J.; Lee, S.; Lee, C.K.; Cho, K.; Ha, N.J.; Kim, K. Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines. Immune Netw. 2009, 9, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Park, D.K.; Park, H.J. Ethanol extract of Cordyceps militaris grown on germinated soybeans attenuates dextran-sodium-sulfate-(DSS-) induced colitis by suppressing the expression of matrix metalloproteinases and inflammatory mediators. Biomed. Res. Int. 2013, 2013, 102918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nallathamby, N.; Guan-Serm, L.; Vidyadaran, S.; Abd Malek, S.N.; Raman, J.; Sabaratnam, V. Ergosterol of Cordyceps militaris Attenuates LPS Induced Inflammation in BV2 Microglia Cells. Nat. Prod. Commun. 2015, 10, 885–886. [Google Scholar] [CrossRef] [Green Version]
- Han, E.S.; Oh, J.Y.; Park, H.J. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. J. Ethnopharmacol. 2011, 134, 703–710. [Google Scholar] [CrossRef]
- Chien, R.C.; Lin, L.M.; Chang, Y.H.; Lin, Y.C.; Wu, P.H.; Asatiani, M.D.; Wasser, S.G.; Krakhmalnyi, M.; Agbarya, A.; Wasser, S.P.; et al. Anti-Inflammation Properties of Fruiting Bodies and Submerged Cultured Mycelia of Culinary-Medicinal Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms 2016, 18, 999–1009. [Google Scholar] [CrossRef]
- Rupa, E.J.; Li, J.F.; Arif, M.H.; Yaxi, H.; Puja, A.M.; Chan, A.J.; Hoang, V.A.; Kaliraj, L.; Yang, D.C.; Kang, S.C. Cordyceps militaris Fungus Extracts-Mediated Nanoemulsion for Improvement Antioxidant, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2020, 25, 5733. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xu, Y.; Shen, J. Cordycepin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production via activating amp-activated protein kinase (AMPK) signaling. Int. J. Mol. Sci. 2014, 15, 12119–12134. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Wang, A.; He, Y.; Si, Z.; Xu, S.; Zhang, S.; Wang, K.; Wang, D.; Liu, Y. Cordycepin attenuates traumatic brain injury-induced impairments of blood-brain barrier integrity in rats. Brain Res. Bull. 2016, 127, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.J.; Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Jeong, J.H.; Lee, J.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Anti-inflammatory effects of extract from Haliotis discus hannai fermented with Cordyceps militaris mycelia in RAW264.7 macrophages through TRIF-dependent signaling pathway. Fish. Shellfish Immunol. 2014, 38, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Boontiam, W.; Wachirapakorn, C.; Phaengphairee, P.; Wattanachai, S. Effect of Spent Mushroom (Cordyceps militaris) on Growth Performance, Immunity, and Intestinal Microflora in Weaning Pigs. Animals 2020, 10, 2360. [Google Scholar] [CrossRef]
- Phull, A.-R.; Dhong, K.-R.; Park, H.-J. Lactic Acid Bacteria Fermented Cordyceps militaris (GRC-SC11) Suppresses IgE Mediated Mast Cell Activation and Type I Hypersensitive Allergic Murine Model. Nutrients 2021, 13, 3849. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phull, A.-R.; Ahmed, M.; Park, H.-J. Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms. Microorganisms 2022, 10, 405. https://doi.org/10.3390/microorganisms10020405
Phull A-R, Ahmed M, Park H-J. Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms. Microorganisms. 2022; 10(2):405. https://doi.org/10.3390/microorganisms10020405
Chicago/Turabian StylePhull, Abdul-Rehman, Madiha Ahmed, and Hye-Jin Park. 2022. "Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms" Microorganisms 10, no. 2: 405. https://doi.org/10.3390/microorganisms10020405
APA StylePhull, A.-R., Ahmed, M., & Park, H.-J. (2022). Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms. Microorganisms, 10(2), 405. https://doi.org/10.3390/microorganisms10020405







