Probabilistic Analysis of a French Legionellosis Outbreak Shows Potential Role of Wastewater Basin
Abstract
1. Introduction
2. Material and Methods
2.1. Source of Data: Legionellosis Outbreak
2.2. Back-Calculation of Date of Exposure
2.3. Probability of Exposure to the Potential Sources of Contamination
2.4. Risk Assessment of Each Source of Contamination
3. Results
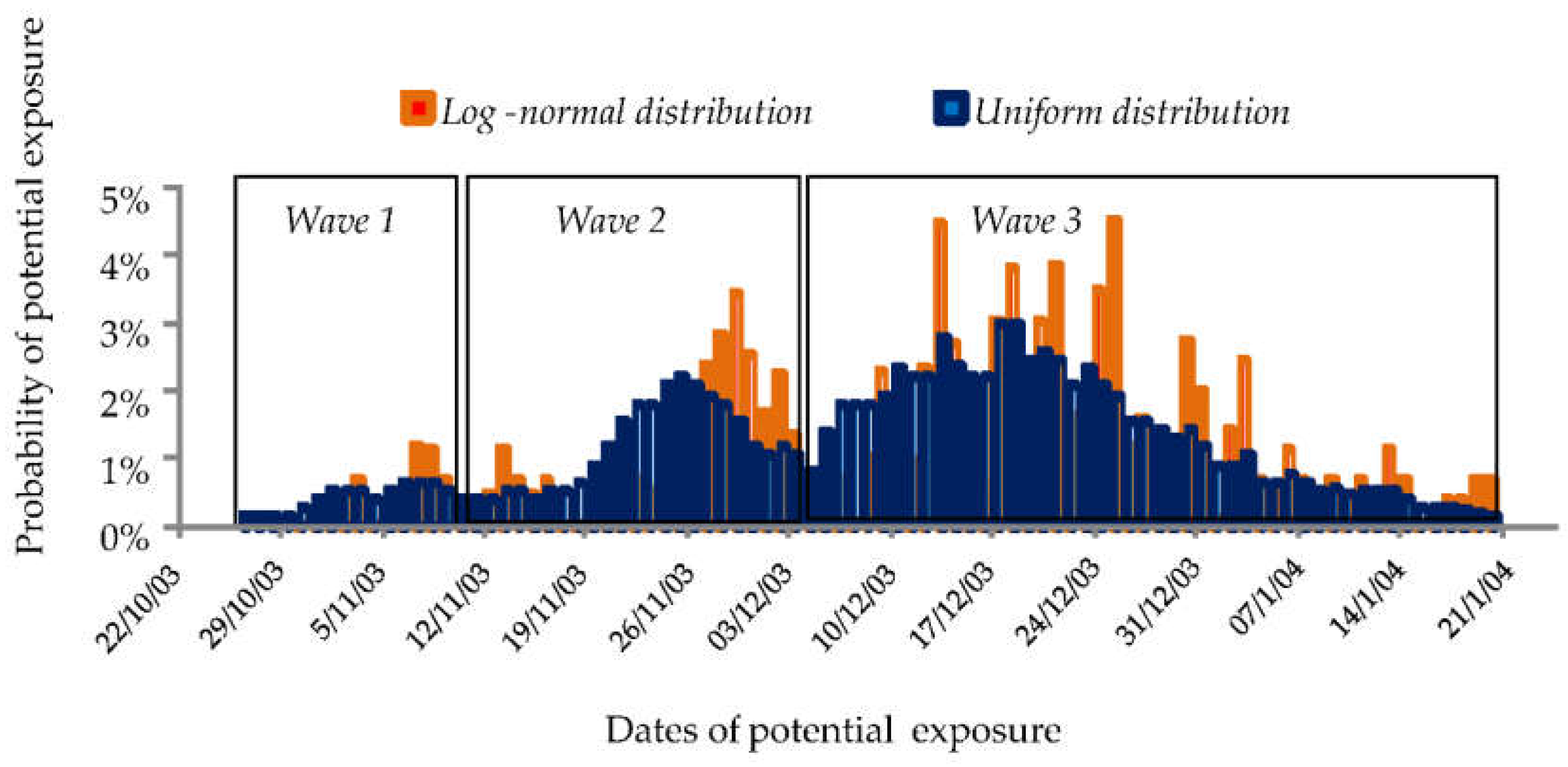
3.1. Outbreak in Three Waves
3.2. Probability of Exposure to the Potential Sources of Contamination
3.3. Risk Assessment of Each Source of Contamination
3.3.1. First Wave of the Outbreak
3.3.2. Second Wave of the Outbreak
3.3.3. Third Wave of the Outbreak
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Herwaldt, L.A.; Marra, A.R. Legionella: A reemerging pathogen. Curr. Opin. Infect. Dis. 2018, 31, 325–333. [Google Scholar] [CrossRef]
- Dominguez, A.; Alvarez, J.; Sabria, M.; Carmona, G.; Torner, N.; Oviedo, M.; Cayla, J.; Minguell, S.; Barrabeig, I.; Sala, M.; et al. Factors influencing the case-fatality rate of legionnaires’ disease. Int. J. Tuberc Lung Dis. 2009, 13, 407–412. [Google Scholar]
- Joseph, C.A.; Harrison, T.G.; Ilijic-Car, D.; Bartlett, C.L. Legionnaires’ disease in residents of england and wales: 1998. Commun. Dis. Public Health 1999, 2, 280–284. [Google Scholar]
- Walser, S.M.; Gerstner, D.G.; Brenner, B.; Holler, C.; Liebl, B.; Herr, C.E. Assessing the environmental health relevance of cooling towers—a systematic review of legionellosis outbreaks. Int. J. Hyg. Environ. Health 2014, 217, 145–154. [Google Scholar] [CrossRef]
- Marston, B.J.; Lipman, H.B.; Breiman, R.F. Surveillance for legionnaires’ disease. Risk factors for morbidity and mortality. Arch. Intern. Med. 1994, 154, 2417–2422. [Google Scholar] [CrossRef]
- Tkatch, L.S.; Kusne, S.; Irish, W.D.; Krystofiak, S.; Wing, E. Epidemiology of legionella pneumonia and factors associated with legionella-related mortality at a tertiary care center. Clin. Infect. Dis. 1998, 27, 1479–1486. [Google Scholar] [CrossRef]
- Beaute, J.; Zucs, P.; de Jong, B. Legionnaires disease in europe, 2009–2010. Eurosurveilliance 2013, 18, 20417. [Google Scholar] [CrossRef]
- Carratala, J.; Garcia-Vidal, C. An update on legionella. Curr. Opin. Infect. Dis. 2010, 23, 152–157. [Google Scholar] [CrossRef]
- Hruba, L. The colonization of hot water systems by legionella. Ann. Agric. Environ. Med. 2009, 16, 115–119. [Google Scholar]
- Kao, P.M.; Tung, M.C.; Hsu, B.M.; Hsu, S.Y.; Huang, J.T.; Liu, J.H.; Huang, Y.L. Differential legionella spp. Survival between intracellular and extracellular forms in thermal spring environments. Environ. Sci. Pollut. Res. Int. 2013, 20, 3098–3106. [Google Scholar] [CrossRef]
- WHO. Legionella and the Prevention of Legionellosis; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Thomas, R.J. Particle size and pathogenicity in the respiratory tract. Virulence 2013, 4, 847–858. [Google Scholar] [CrossRef]
- Parr, A.; Whitney, E.A.; Berkelman, R.L. Legionellosis on the rise: A review of guidelines for prevention in the united states. J. Public Health Manag. Pract. 2015, 21, E17–E26. [Google Scholar] [CrossRef]
- Cristino, S.; Legnani, P.P.; Leoni, E. Plan for the control of legionella infections in long-term care facilities: Role of environmental monitoring. Int. J. Hyg. Environ. Health 2012, 215, 279–285. [Google Scholar] [CrossRef]
- Wallet, F.; Fontenay, L.; Cabanes, P. Probabilistic analysis of legionellosis outbreak data and its potential contribution to microbial risk assessment. In Annual Meeting Abstract, Proceedings of the 2012 Annual Meeting of the Society for Risk Analysis, San Francisco, CA, USA, 9–12 December 2012; Society for Risk Analysis: Herndon, VA, USA, 2012. [Google Scholar]
- Bretin, P.; Capek, I.; Cabanes, P.; Marcel, F.; Merchat, M. Epidémie de Légionellose Dans le Pas-de-Calais—Rapport de la Mission D’appui; Ineris, Institut National de Veille Sanitaire: Verneuil-en-Halatte, Saint-Maurice, 2004. [Google Scholar]
- Centre National de Référence des Légionelles; Préfécture du Pas-de-Calais; DRIRE du Nord Pas-de-Calais; DDASS du Pas-de-Calais; Institut National de Veille Sanitaire. Epidémie Communautaire de Légionellose pas-de-Calais France Novembre 2003–Janvier 2004; Centre National de Référence des Légionelles; Préfecture du Pas-de-Calais; DRIRE du Nord Pas-de-Calais; DDASS du Pas-de-Calais; Institut National de Veille Sanitaire: Lyon, Arras, Saint-Maurice, 2004. [Google Scholar]
- Nguyen, T.M.; Ilef, D.; Jarraud, S.; Rouil, L.; Campese, C.; Che, D.; Haeghebaert, S.; Ganiayre, F.; Marcel, F.; Etienne, J.; et al. A community-wide outbreak of legionnaires disease linked to industrial cooling towers—how far can contaminated aerosols spread? J. Infect. Dis. 2006, 193, 102–111. [Google Scholar] [CrossRef]
- Egan, J.R.; Hall, I.M. A review of back-calculation techniques and their potential to inform mitigation strategies with application to non-transmissible acute infectious diseases. J. R. Soc. Interface 2015, 12, 20150096. [Google Scholar] [CrossRef]
- Merchat, M. Etude des Moyens Mis en Oeuvre Pour la Gestion du Risque Légionelles Chez Noroxo—Epidémie de Légionellose du Nord pas-de-Calais Mission D’appui; Climespace: Paris, France, 2004. [Google Scholar]
- Egan, J.R.; Hall, I.M.; Lemon, D.J.; Leach, S. Modeling legionnaires’ disease outbreaks: Estimating the timing of an aerosolized release using symptom-onset dates. Epidemiology 2011, 22, 188–198. [Google Scholar] [CrossRef]
- Gosselin, F.; Duval, J.F.; Simonet, J.; Ginevra, C.; Gaboriaud, F.; Jarraud, S.; Mathieu, L. Impact of the virulence-associated mab3/1 epitope on the physicochemical surface properties of legionella pneumophila sg1: An issue to explain infection potential? Colloids Surf. B Biointerfaces 2011, 82, 283–290. [Google Scholar] [CrossRef]
- Kondo, K. The lognormal distribution of the incubation time of exogenous diseases. Genetic interpretations and a computer simulation. Jinrui Idengaku Zasshi 1977, 21, 217–237. [Google Scholar]
- Nishiura, H. Early efforts in modeling the incubation period of infectious diseases with an acute course of illness. Emerg. Themes Epidemiol. 2007, 4, 2. [Google Scholar] [CrossRef]
- Sartwell, P.E. The incubation period and the dynamics of infectious disease. Am. J. Epidemiol. 1966, 83, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Valero, L.; Rusniok, C.; Rolando, M.; Neou, M.; Dervins-Ravault, D.; Demirtas, J.; Rouy, Z.; Moore, R.J.; Chen, H.; Petty, N.K.; et al. Comparative analyses of legionella species identifies genetic features of strains causing legionnaires’ disease. Genome Biol. 2014, 15, 505. [Google Scholar]
- Fykse, E.M.; Aarskaug, T.; Thrane, I.; Blatny, J.M. Legionella and non-legionella bacteria in a biological treatment plant. Can. J. Microbiol. 2013, 59, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kusnetsov, J.; Neuvonen, L.K.; Korpio, T.; Uldum, S.A.; Mentula, S.; Putus, T.; Tran Minh, N.N.; Martimo, K.P. Two legionnaires’ disease cases associated with industrial waste water treatment plants: A case report. BMC Infect. Dis. 2010, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.S.; Aarskaug, T.; Thrane, I.; Pourcel, C.; Ask, E.; Johansen, G.; Waagen, V.; Blatny, J.M. Alternative routes for dissemination of legionella pneumophila causing three outbreaks in norway. Environ. Sci. Technol. 2010, 44, 8712–8717. [Google Scholar] [CrossRef]
- Blatny, J.M.; Fossum, H.; Ho, J.; Tutkun, M.; Skogan, G.; Andreassen, O.; Fykse, E.M.; Waagen, V.; Reif, B.A. Dispersion of legionella-containing aerosols from a biological treatment plant, norway. Front. Biosci. 2011, 3, 1300–1309. [Google Scholar] [CrossRef][Green Version]
- Blatny, J.M.; Reif, B.A.; Skogan, G.; Andreassen, O.; Hoiby, E.A.; Ask, E.; Waagen, V.; Aanonsen, D.; Aaberge, I.S.; Caugant, D.A. Tracking airborne legionella and legionella pneumophila at a biological treatment plant. Environ. Sci. Technol. 2008, 42, 7360–7367. [Google Scholar] [CrossRef]
- Mathieu, L.; Robine, E.; Deloge-Abarkan, M.; Ritoux, S.; Pauly, D.; Hartemann, P.; Zmirou-Navier, D. Legionella bacteria in aerosols: Sampling and analytical approaches used during the legionnaires disease outbreak in pas-de-calais. J. Infect. Dis. 2006, 193, 1333–1335. [Google Scholar] [CrossRef]
- Caicedo, C.; Rosenwinkel, K.H.; Exner, M.; Verstraete, W.; Suchenwirth, R.; Hartemann, P.; Nogueira, R. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: Review. Water Res. 2019, 149, 21–34. [Google Scholar] [CrossRef]
- Loenenbach, A.D.; Beulens, C.; Euser, S.M.; van Leuken, J.P.G.; Bom, B.; van der Hoek, W.; Husman, A.M.R.; Ruijs, W.L.M.; Bartels, A.A.; Rietveld, A.; et al. Two community clusters of legionnaires’ disease directly linked to a biologic wastewater treatment plant, the netherlands. Emerg. Infect. Dis. 2018, 24, 1914–1918. [Google Scholar] [CrossRef]
- Vermeulen, L.C.; Brandsema, P.S.; van de Kassteele, J.; Bom, B.C.J.; Sterk, H.A.M.; Sauter, F.J.; van den Berg, H.; de Roda Husman, A.M. Atmospheric dispersion and transmission of legionella from wastewater treatment plants: A 6-year case-control study. Int. J. Hyg. Environ. Health 2021, 237, 113811. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, L.; Pietronave, S.; Rinaldi, M.; Giovanna Martinotti, M. Site-related airborne biological hazard and seasonal variations in two wastewater treatment plants. Water Res. 2006, 40, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, P.; Li, F.; Castelluccio, V.; Matsumoto, M.; Asa-Awuku, A. Real-time ultrafine aerosol measurements from wastewater treatment facilities. Environ. Sci. Technol. 2016, 50, 11137–11144. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, J.; Keywood, M.; Sinclair, M.; Leder, K. Risk in the mist? Deriving data to quantify microbial health risks associated with aerosol generation by water-efficient devices during typical domestic water-using activities. Water Sci. Technol. 2009, 60, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.; O’Toole, J.; Sinclair, M.; Keywood, M.; Leder, K. Endotoxin heath risk associated with high pressure cleaning using reclaimed water. Microb. Risk Anal. 2017, 5, 65–70. [Google Scholar] [CrossRef]
- Sinclair, M.; Roddick, F.; Nguyen, T.; O’Toole, J.; Leder, K. Measuring water ingestion from spray exposures. Water Res. 2016, 99, 1–6. [Google Scholar] [CrossRef]
- Ruiz, J.; Kaiser, A.S.; Lucas, M. Experimental determination of drift and pm10 cooling tower emissions: Influence of components and operating conditions. Environ. Pollut. 2017, 230, 422–431. [Google Scholar] [CrossRef]
- Madsen, A.M.; Matthiesen, C.B. Exposure to aerosols during high-pressure cleaning and relationship with health effects. Ann. Agric. Environ. Med. 2013, 20, 420–425. [Google Scholar]
- Roubaty, J.; Pradelle, F.; Roux, P.; Jarlier, V. Evaluation des risques de dissémination bactériennes liés au couplage d’une source concentrée de légionelles, d’un système générateur d’aérosols et d’un système dispersif. Environ. Risque St. 2015, 14, 151–162. [Google Scholar]
- Blatny, J.M.; Ho, J.; Skogan, G.; Fykse, E.M.; Aarskaug, T.; Waagen, V. Airborne legionella bacteria from pulp waste treatment plant: Aerosol particles characterized as aggregates and their potential hazard. Aerobiologia 2011, 27, 147–162. [Google Scholar] [CrossRef]
- Caicedo, C.; Beutel, S.; Scheper, T.; Rosenwinkel, K.H.; Nogueira, R. Occurrence of legionella in wastewater treatment plants linked to wastewater characteristics. Environ. Sci. Pollut. Res. Int. 2016, 23, 16873–16881. [Google Scholar] [CrossRef] [PubMed]
- Wallet, F. Evaluation du risque légionelles: De l’environnement au risque sanitaire, synthèse des connaissances. Environ. Risques St. 2018, 17, 572–582. [Google Scholar]
- Whiley, H.; Keegan, A.; Fallowfield, H.; Ross, K. Uncertainties associated with assessing the public health risk from legionella. Front. Microbiol. 2014, 5, 501. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nour, M.; Duncan, C.; Low, D.E.; Guyard, C. Biofilms: The stronghold of legionella pneumophila. Int. J. Mol. Sci. 2013, 14, 21660–21675. [Google Scholar] [CrossRef] [PubMed]
- Declerck, P. Biofilms: The environmental playground of legionella pneumophila. Environ. Microbiol. 2010, 12, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wallet, F.; Le-Brun, M.; Charton-Bissetta, J.; Musson-Genon, L.; Bickert, H.; Cabanes, P. Analysis of different quantitative microbial risk assessment models for legionella infection. In Annual Meeting Abstract, Proceedings of the 2010 Annual Meeting of the Society for Risk Analysis, Salt Lake City, UT, USA, 5–8 December 2010; Society for Risk Analysis: Herndon, VA, USA, 2010. [Google Scholar]
| Facility | Characteristics (L/h) | Contamination (CFU/L) | Legionella Emission (CFU/s) | Assumptions |
|---|---|---|---|---|
| Car wash | 500 | 1600 | 200 | The entire flow was aerosolized |
| High-pressure water cleaner | 500 | 100,000 | 700 | 5% of the flow aerosolized biofilm |
| Cooling tower | 90 | 100,000 | 5000 | Aerosol emission calculated for the two cooling towers |
| Wastewater treatment plant | 1.8 | 200,000,000 | 100,000 | Inverse dispersion modeling using single source Gaussian plume model based on air concentrations measurement |
| Probability Distribution Function | Wave of Outbreak | Duration (Days) | Start Date–End Date of the Wave | Number of Cases | Cases with a Nonzero Probability of Exposure to the Source (Number of Cases—% of Cases Per Wave) | |||
|---|---|---|---|---|---|---|---|---|
| Cooling Tower | High-Pressure Water Cleaner | Wastewater Basin | Car Wash Station | |||||
| Uniform distribution | Wave 1 | 16 | 26 October 2003 to 10 November 2003 | 7 | 7 (100%) | 7 (100%) | ||
| Wave 2 | 23 | 11 November 2003 to 3 December 2003 | 26 | 23 (88%) | 26 (100%) | |||
| Wave 3 | 50 | 4 December 2003 to 22 January 2004 | 53 | 26 (49%) | 32 (60%) | 53 (100%) | 33 (62%) | |
| Total duration of outbreak | 89 | 26 October 2003 to 22 January 2004 | 86 | 56 (65%) | 32 (37%) | 86 (100%) | 33 (38%) | |
| Lognormal distribution | Wave 1 | 9 | 1 November 2003 to 9 November 2003 | 4 | 4 (100%) | 4 (100%) | ||
| Wave 2 | 23 | 11 November 2003 to 3 December 2003 | 23 | 23 (100%) | 23 (100%) | |||
| Wave 3 | 50 | 4 December 2003 to 22 January 2004 | 59 | 23 (39%) | 16 (27%) | 59 (100%) | 24 (41%) | |
| Total duration of outbreak | 82 | 01 November 2003 to 22 January 2004 | 86 | 50 (58%) | 16 (19%) | 86 (100%) | 24 (28%) | |
| Sources | Wave 1 | Wave 2 | Wave 3 |
|---|---|---|---|
| Wastewater basin | 50% | 0.1% | 96.98% |
| Cooling tower | 50% | 99.9% | 2.91% |
| High-pressure water cleaner | 0.1% | ||
| Car wash station | 0.01% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallet, F.; Fontenay, L.; Cabanes, P.-A. Probabilistic Analysis of a French Legionellosis Outbreak Shows Potential Role of Wastewater Basin. Microorganisms 2022, 10, 422. https://doi.org/10.3390/microorganisms10020422
Wallet F, Fontenay L, Cabanes P-A. Probabilistic Analysis of a French Legionellosis Outbreak Shows Potential Role of Wastewater Basin. Microorganisms. 2022; 10(2):422. https://doi.org/10.3390/microorganisms10020422
Chicago/Turabian StyleWallet, France, Leila Fontenay, and Pierre-André Cabanes. 2022. "Probabilistic Analysis of a French Legionellosis Outbreak Shows Potential Role of Wastewater Basin" Microorganisms 10, no. 2: 422. https://doi.org/10.3390/microorganisms10020422
APA StyleWallet, F., Fontenay, L., & Cabanes, P.-A. (2022). Probabilistic Analysis of a French Legionellosis Outbreak Shows Potential Role of Wastewater Basin. Microorganisms, 10(2), 422. https://doi.org/10.3390/microorganisms10020422





