Picornavirus May Be Linked to Parkinson’s Disease through Viral Antigen in Dopamine-Containing Neurons of Substantia Nigra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Specimens
2.3. Immunohistochemistry
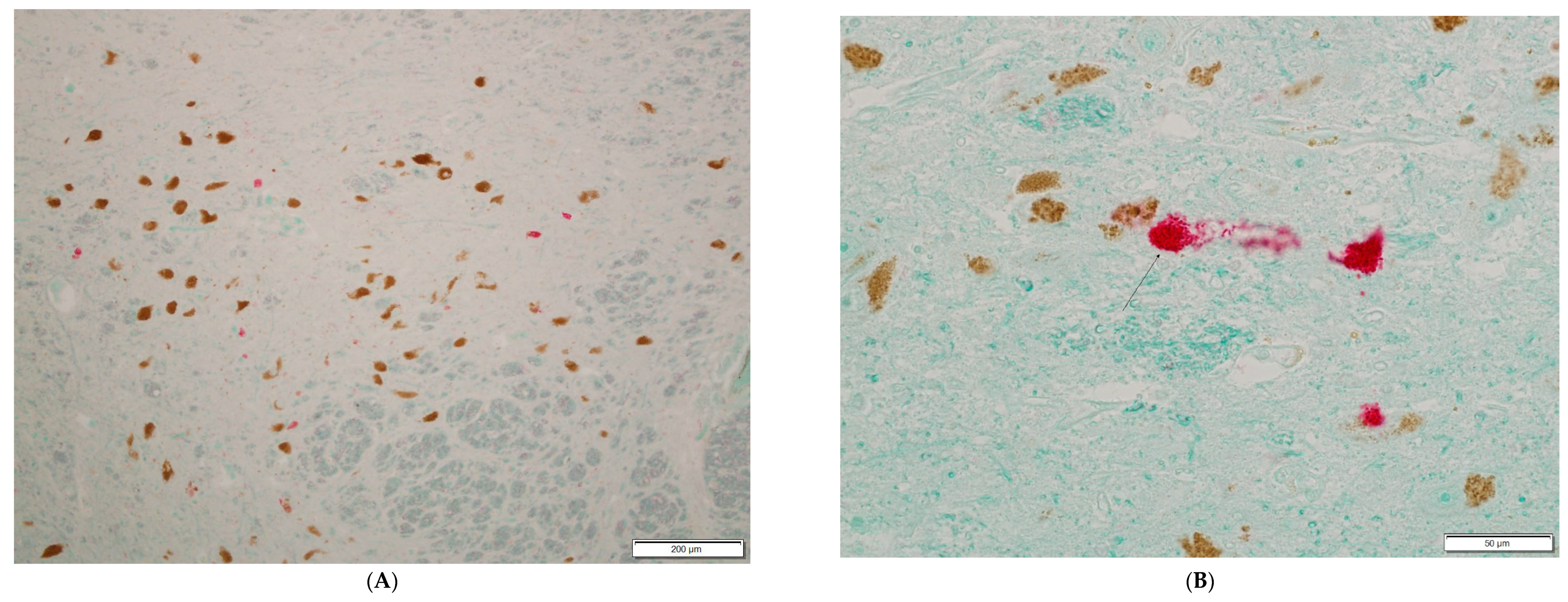
3. Results
4. Discussion
4.1. Evidence Suggesting a Chronic Persistent Picornavirus Infection in Parkinson’s Disease and Several Other Diseases of Unknown Etiology
4.2. Limitations of the Diagnostic Assay Used in the Present Study
4.3. Evidence of Persistent Picornavirus Infection
4.4. The Potential Role of Antiviral Treatment in Parkinson’s Disease and Several Other Chronic Diseases of Unknown Etiology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.; Trojanowski, J.Q. Spreading of pathology in neurodegenera-tive diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Koo, E.H.; Lansbury, P.T., Jr.; Kelly, J.W. Amyloid diseases: Abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. USA 1999, 96, 9989–9990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checkoway, H.; Lundin, J.I.; Kelada, S.N. Neurodegenerative diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- Bertram, L.; Tanzi, R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Miranda-Saksena, M.; Saksena, N.K. Viruses and neurodegeneration. Virol. J. 2013, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Kumar, A.; Calne, S.M.; Schulzer, M.; Mak, E.; Wszolek, Z.; Van Netten, C.; Tsui, J.K.; Stoessl, A.J.; Calne, D.B. Clustering of Parkinson disease: Shared cause or coincidence? Arch. Neurol. 2004, 61, 1057–1060. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.; Smeyne, R.J.; Jang, H.; Miller, B.; Okun, M.S. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat. Disord. 2010, 16, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.; Boltz, D.A.; Webster, R.G.; Smeyne, R.J. Viral parkinsonism. Biochim. Biophys. Acta 2009, 1792, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Casals, J.; Elizan, T.S.; Yahr, M.D. Postencephalitic parkinsonism—A review. J. Neural Transm. 1998, 105, 645–676. [Google Scholar] [CrossRef]
- Rebai, I.; Ben Rhouma, H.; Kraoua, I.; Klaa, H.; Rouissi, A.; Ben Youssef-Turki, I.; Gouider-Khouja, N. Postencephalitic parkinsonism and selective involvement of substantia nigra in childhood. Brain Dev. 2015, 37, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Smeyne, R.J.; Noyce, A.J.; Byrne, M.; Savica, R.; Marras, C. Infection and Risk of Parkinson’s Disease. J. Parkinsons Dis. 2021, 11, 31–43. [Google Scholar] [CrossRef]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. The role of viruses in the pathogenesis of Parkinson’s disease. Neural Regen. Res. 2021, 16, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Bantle, C.M.; Phillips, A.T.; Smeyne, R.J.; Rocha, S.M.; Olson, K.E.; Tjalkens, R.B. Infection with mosquito-borne alphavirus induces selective loss of dopaminergic neurons, neuroinflammation and widespread protein aggregation. Npj Parkinsons Dis. 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef]
- Niklasson, B.; Lindquist, L.; Klitz, W.; Netherlands Brain Bank; Englund, E. Picornavirus Identified in Alzheimer’s Disease Brains: A Pathogenic Path? J. Alzheimers Dis. Rep. 2020, 4, 141–146. [Google Scholar] [CrossRef]
- Klioueva, N.M.; Rademaker, M.C.; Dexter, D.T.; Al-Sarraj, S.; Seilhean, D.; Streichenberger, N.; Schmitz, P.; Bell, J.E.; Ironside, J.W.; Arzberger, T.; et al. BrainNet Europe’s Code of Conduct for brain banking. J. Neural Transm. 2015, 122, 937–940. [Google Scholar] [CrossRef] [Green Version]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Langston, J.W. The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann. Neurol. 2006, 59, 591–596. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Shi, S.R.; Cote, R.J.; Taylor, C.R. Antigen retrieval immunohistochemistry: Past, present, and future. J. Histochem. Cytochem. 1997, 45, 327–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolf, C.; Ekstrom, J.O.; Gullberg, M.; Arbrandt, G.; Niklasson, B.; Frisk, G.; Liljeqvist, J.A.; Edman, K.; Lindberg, A.M. Characterization of polyclonal antibodies against the capsid proteins of Ljungan virus. J. Virol. Methods 2008, 150, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K. The origin and virulence of the 1918 “Spanish” influenza virus. Proc. Am. Philos. Soc. 2006, 150, 86–112. [Google Scholar] [PubMed]
- Dourmashkin, R.R.; McCall, S.A.; Dourmashkin, N.; Hannah, M.J. Virus-like particles and enterovirus antigen found in the brainstem neurons of Parkinson’s disease. F1000Research 2018, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Poser, C.M.; Huntley, C.J.; Poland, J.D. Para-encephalitic parkinsonism. Report of an acute case due to coxsackie virus type B 2 and re-examination of the etiologic concepts of postencephalitic parkinsonism. Acta Neurol. Scand. 1969, 45, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Wesslen, L.; Pahlson, C.; Friman, G.; Fohlman, J.; Lindquist, O.; Johansson, C. Myocarditis caused by Chlamydia pneumoniae (TWAR) and sudden unexpected death in a Swedish elite orienteer. Lancet 1992, 340, 427–428. [Google Scholar] [CrossRef]
- Niklasson, B.; Kinnunen, L.; Hornfeldt, B.; Horling, J.; Benemar, C.; Hedlund, K.O.; Matskova, L.; Hyypia, T.; Winberg, G. A new picornavirus isolated from bank voles (Clethrionomys glareolus). Virology 1999, 255, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Niklasson, B.; Hornfeldt, B.; Lundman, B. Could myocarditis, insulin-dependent diabetes mellitus, and Guillain-Barre syndrome be caused by one or more infectious agents carried by rodents? Emerg. Infect. Dis. 1998, 4, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Jungeblut, C.W.; Dalldorf, G. Epidemiological and Experimental Observations on the Possible Significance of Rodents in a Suburban Epidemic of Poliomyelitis. Am. J. Public Health Nations Health 1943, 33, 169–172. [Google Scholar] [CrossRef]
- Buenz, E.J.; Rodriguez, M.; Howe, C.L. Disrupted spatial memory is a consequence of picornavirus infection. Neurobiol. Dis. 2006, 24, 266–273. [Google Scholar] [CrossRef]
- Carocci, M.; Bakkali-Kassimi, L. The encephalomyocarditis virus. Virulence 2012, 3, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsioe, A.; Feinstein, R.; Saade, G.; Sjoholm, A.; Hornfeldt, B.; Fundele, R.; Klitz, W.; Niklasson, B. Intrauterine death, fetal malformation, and delayed pregnancy in Ljungan virus-infected mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2006, 77, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, B.; Heller, K.E.; Schonecker, B.; Bildsoe, M.; Daniels, T.; Hampe, C.S.; Widlund, P.; Simonson, W.T.; Schaefer, J.B.; Rutledge, E.; et al. Development of type 1 diabetes in wild bank voles associated with islet autoantibodies and the novel ljungan virus. Int. J. Exp. Diabesity Res. 2003, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, B.; Samsioe, A.; Blixt, M.; Sandler, S.; Sjoholm, A.; Lagerquist, E.; Lernmark, A.; Klitz, W. Prenatal viral exposure followed by adult stress produces glucose intolerance in a mouse model. Diabetologia 2006, 49, 2192–2199. [Google Scholar] [CrossRef]
- Niklasson, B.; Almqvist, P.R.; Hornfeldt, B.; Klitz, W. Sudden infant death syndrome and Ljungan virus. Forensic. Sci. Med. Pathol. 2009, 5, 274–279. [Google Scholar] [CrossRef]
- Niklasson, B.; Samsioe, A.; Papadogiannakis, N.; Gustafsson, S.; Klitz, W. Zoonotic Ljungan virus associated with central nervous system malformations in terminated pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 542–545. [Google Scholar] [CrossRef]
- Niklasson, B.; Samsioe, A.; Papadogiannakis, N.; Kawecki, A.; Hornfeldt, B.; Saade, G.R.; Klitz, W. Association of zoonotic Ljungan virus with intrauterine fetal deaths. Birth Defects Res. A Clin. Mol. Teratol. 2007, 79, 488–493. [Google Scholar] [CrossRef]
- Rossi, C.; Zadra, N.; Fevola, C.; Ecke, F.; Hornfeldt, B.; Kallies, R.; Kazimirova, M.; Magnusson, M.; Olsson, G.E.; Ulrich, R.G.; et al. Evolutionary Relationships of Ljungan Virus Variants Circulating in Multi-Host Systems across Europe. Viruses 2021, 13, 1317. [Google Scholar] [CrossRef]
- Tolf, C.; Gullberg, M.; Johansson, E.S.; Tesh, R.B.; Andersson, B.; Lindberg, A.M. Molecular characterization of a novel Ljungan virus (Parechovirus; Picornaviridae) reveals a fourth genotype and indicates ancestral recombination. J. Gen. Virol. 2009, 90, 843–853. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Nix, W.A.; Maher, K.; Johansson, E.S.; Niklasson, B.; Lindberg, A.M.; Pallansch, M.A.; Oberste, M.S. Detection of all known parechoviruses by real-time PCR. J. Clin. Microbiol. 2008, 46, 2519–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoso Mantke, O.; Kallies, R.; Niklasson, B.; Nitsche, A.; Niedrig, M. A new quantitative real-time reverse transcriptase PCR assay and melting curve analysis for detection and genotyping of Ljungan virus strains. J. Virol. Methods 2007, 141, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Archard, L.C.; Richardson, P.J.; Olsen, E.G.; Dubowitz, V.; Sewry, C.; Bowles, N.E. The role of Coxsackie B viruses in the pathogenesis of myocarditis, dilated cardiomyopathy and inflammatory muscle disease. Biochem. Soc. Symp. 1987, 53, 51–62. [Google Scholar]
- Archard, L.C.; Bowles, N.E.; Behan, P.O.; Bell, E.J.; Doyle, D. Postviral fatigue syndrome: Persistence of enterovirus RNA in muscle and elevated creatine kinase. J. R. Soc. Med. 1988, 81, 326–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveque, N.; Renois, F.; Talmud, D.; Nguyen, Y.; Lesaffre, F.; Boulagnon, C.; Bruneval, P.; Fornes, P.; Andreoletti, L. Quantitative genomic and antigenomic enterovirus RNA detection in explanted heart tissue samples from patients with end-stage idiopathic dilated cardiomyopathy. J. Clin. Microbiol. 2012, 50, 3378–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, P.E.; Messner, R.P. Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: Viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J. Virol. 1999, 73, 10113–10121. [Google Scholar] [CrossRef] [Green Version]
- Oikarinen, M.; Tauriainen, S.; Oikarinen, S.; Honkanen, T.; Collin, P.; Rantala, I.; Maki, M.; Kaukinen, K.; Hyoty, H. Type 1 diabetes is associated with enterovirus infection in gut mucosa. Diabetes 2012, 61, 687–691. [Google Scholar] [CrossRef] [Green Version]
- Krogvold, L.; Edwin, B.; Buanes, T.; Frisk, G.; Skog, O.; Anagandula, M.; Korsgren, O.; Undlien, D.; Eike, M.C.; Richardson, S.J.; et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 2015, 64, 1682–1687. [Google Scholar] [CrossRef] [Green Version]
- Tracy, S.; Smithee, S.; Alhazmi, A.; Chapman, N. Coxsackievirus can persist in murine pancreas by deletion of 5’ terminal genomic sequences. J. Med. Virol. 2015, 87, 240–247. [Google Scholar] [CrossRef]
- Muir, P.; Nicholson, F.; Sharief, M.K.; Thompson, E.J.; Cairns, N.J.; Lantos, P.; Spencer, G.T.; Kaminski, H.J.; Banatvala, J.E. Evidence for persistent enterovirus infection of the central nervous system in patients with previous paralytic poliomyelitis. Ann. N. Y. Acad. Sci. 1995, 753, 219–232. [Google Scholar] [CrossRef]
- Julien, J.; Leparc-Goffart, I.; Lina, B.; Fuchs, F.; Foray, S.; Janatova, I.; Aymard, M.; Kopecka, H. Postpolio syndrome: Poliovirus persistence is involved in the pathogenesis. J. Neurol. 1999, 246, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Feuer, R.; Ruller, C.M.; An, N.; Tabor-Godwin, J.M.; Rhoades, R.E.; Maciejewski, S.; Pagarigan, R.R.; Cornell, C.T.; Crocker, S.J.; Kiosses, W.B.; et al. Viral persistence and chronic immunopathology in the adult central nervous system following Coxsackievirus infection during the neonatal period. J. Virol. 2009, 83, 9356–9369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, N.M.; Kim, K.S.; Drescher, K.M.; Oka, K.; Tracy, S. 5’ terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology 2008, 375, 480–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, K.; Witzenbichler, B.; Christmann, C.; Schultheiss, H.P. Disturbance of myocardial energy metabolism in experimental virus myocarditis by antibodies against the adenine nucleotide translocator. Cardiovasc. Res. 1999, 44, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, H.P.; Schulze, K.; Dorner, A. Significance of the adenine nucleotide translocator in the pathogenesis of viral heart disease. Mol. Cell Biochem. 1996, 163-164, 319–327. [Google Scholar] [CrossRef]
- Schulze, K.; Schultheiss, H.P. The role of the ADP/ATP carrier in the pathogenesis of viral heart disease. Eur. Heart J. 1995, 16 (Suppl. O), 64–67. [Google Scholar] [CrossRef]
- Schultheiss, H.P. Disturbance of the myocardial energy metabolism in dilated cardiomyopathy due to autoimmunological mechanisms. Circulation 1993, 87, IV43–IV48. [Google Scholar]
- Schulze, K.; Becker, B.F.; Schauer, R.; Schultheiss, H.P. Antibodies to ADP-ATP carrier--an autoantigen in myocarditis and dilated cardiomyopathy--impair cardiac function. Circulation 1990, 81, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Leveque, N.; Garcia, M.; Bouin, A.; Nguyen, J.H.C.; Tran, G.P.; Andreoletti, L.; Semler, B.L. Functional Consequences of RNA 5’-Terminal Deletions on Coxsackievirus B3 RNA Replication and Ribonucleoprotein Complex Formation. J. Virol. 2017, 91, e00423-17. [Google Scholar] [CrossRef] [Green Version]
- Gibson, J.P.; Righthand, V.F. Persistence of echovirus 6 in cloned human cells. J. Virol. 1985, 54, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Righthand, V.F.; Blackburn, R.V. Steady-state infection by echovirus 6 associated with nonlytic viral RNA and an unprocessed capsid polypeptide. J. Virol. 1989, 63, 5268–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Chapman, N.M.; Tracy, S. Replication of coxsackievirus B3 in primary cell cultures generates novel viral genome deletions. J. Virol. 2008, 82, 2033–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glenet, M.; N’Guyen, Y.; Mirand, A.; Henquell, C.; Lebreil, A.L.; Berri, F.; Bani-Sadr, F.; Lina, B.; Schuffenecker, I.; Andreoletti, L.; et al. Major 5’terminally deleted enterovirus populations modulate type I IFN response in acute myocarditis patients and in human cultured cardiomyocytes. Sci. Rep. 2020, 10, 11947. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Tracy, S.; Tapprich, W.; Bailey, J.; Lee, C.K.; Kim, K.; Barry, W.H.; Chapman, N.M. 5’-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J. Virol. 2005, 79, 7024–7041. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, L.; Bowles, N.E.; Lane, R.J.; Dubowitz, V.; Archard, L.C. Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J. Gen. Virol. 1990, 71 Pt 6, 1399–1402. [Google Scholar] [CrossRef]
- Archard, L.C.; Bowles, N.E.; Cunningham, L.; Freeke, C.A.; Olsen, E.G.; Rose, M.L.; Meany, B.; Why, H.J.; Richardson, P.J. Molecular probes for detection of persisting enterovirus infection of human heart and their prognostic value. Eur. Heart J. 1991, 12 (Suppl. D), 56–59. [Google Scholar] [CrossRef]
- Klingel, K.; Hohenadl, C.; Canu, A.; Albrecht, M.; Seemann, M.; Mall, G.; Kandolf, R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: Quantitative analysis of virus replication, tissue damage, and inflammation. Proc. Natl. Acad. Sci. USA 1992, 89, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Drescher, K.M.; Kono, K.; Bopegamage, S.; Carson, S.D.; Tracy, S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: Insulitis determines susceptibility of pancreatic islets to virus infection. Virology 2004, 329, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Beatman, E.L.; Massey, A.; Shives, K.D.; Burrack, K.S.; Chamanian, M.; Morrison, T.E.; Beckham, J.D. Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain. J. Virol. 2015, 90, 2767–2782. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Jin, U.; Park, S.M. Interaction between coxsackievirus B3 infection and alpha-synuclein in models of Parkinson’s disease. PLoS Pathog. 2021, 17, e1010018. [Google Scholar] [CrossRef]
- Mordes, J.P.; Bortell, R.; Blankenhorn, E.P.; Rossini, A.A.; Greiner, D.L. Rat models of type 1 diabetes: Genetics, environment, and autoimmunity. ILAR J. 2004, 45, 278–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niklasson, B.; Hultman, T.; Kallies, R.; Niedrig, M.; Nilsson, R.; Berggren, P.O.; Juntti-Berggren, L.; Efendic, S.; Lernmark, A.; Klitz, W. The BioBreeding rat diabetes model is infected with Ljungan virus. Diabetologia 2007, 50, 1559–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niklasson, B.; Arbrandt, G.; Kawecki, A.; Juntti-Berggren, L.; Berggren, P.O.; Al-Qahtani, S.M.; Gustafsson, A.L.; Bryzgalova, G.; Klitz, W. Diabetes Prevention Through Antiviral Treatment in Biobreeding Rats. Viral Immunol. 2016, 29, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, B.; Klitz, W.; Juntti-Berggren, L.; Berggren, P.O.; Lindquist, L. Effectiveness of Antivirals in a Type 1 Diabetes Model and the Move Toward Human Trials. Viral Immunol. 2020, 33, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Sheppard, L.; Fillenbaum, G.G.; Galasko, D.; Morris, J.C.; Koss, E.; Mohs, R.; Heyman, A. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: A clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s Disease. Arch. Neurol. 1999, 56, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, N.P.; Ringstrom, R.; Wiig, E.H.; Minthon, L. Associations between AQT processing speed and neuropsychological tests in neuropsychiatric patients. Am. J. Alzheimers Dis. Other Demen. 2007, 22, 202–210. [Google Scholar] [CrossRef]
- Lindblom, N.; Lindquist, L.; Westman, J.; Astrom, M.; Bullock, R.; Hendrix, S.; Wahlund, L.O. Potential Virus Involvement in Alzheimer’s Disease: Results from a Phase IIa Trial Evaluating Apovir, an Antiviral Drug Combination. J. Alzheimers Dis. Rep. 2021, 5, 413–431. [Google Scholar] [CrossRef]
- Schwab, R.S.; England, A.C., Jr.; Poskanzer, D.C.; Young, R.R. Amantadine in the treatment of Parkinson’s disease. JAMA 1969, 208, 1168–1170. [Google Scholar] [CrossRef]
- Benschop, K.S.; van der Avoort, H.G.; Duizer, E.; Koopmans, M.P. Antivirals against enteroviruses: A critical review from a public-health perspective. Antivir. Ther. 2015, 20, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Ianevski, A.; Yao, R.; Biza, S.; Zusinaite, E.; Mannik, A.; Kivi, G.; Planken, A.; Kurg, K.; Tombak, E.M.; Ustav, M., Jr.; et al. Identification and Tracking of Antiviral Drug Combinations. Viruses 2020, 12, 1178. [Google Scholar] [CrossRef]
- Maenza, J.; Flexner, C. Combination antiretroviral therapy for HIV infection. Am. Fam. Physician 1998, 57, 2789–2798. [Google Scholar] [PubMed]
- Bauer, L.; Manganaro, R.; Zonsics, B.; Strating, J.; El Kazzi, P.; Lorenzo Lopez, M.; Ulferts, R.; van Hoey, C.; Mate, M.J.; Langer, T.; et al. Fluoxetine Inhibits Enterovirus Replication by Targeting the Viral 2C Protein in a Stereospecific Manner. ACS Infect. Dis. 2019, 5, 1609–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pevear, D.C.; Tull, T.M.; Seipel, M.E.; Groarke, J.M. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 1999, 43, 2109–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niklasson, B.; Lindquist, L.; Klitz, W.; Fredrikson, S.; Morgell, R.; Mohammadi, R.; Netherlands Brain Bank; Karapetyan, Y.; Englund, E. Picornavirus May Be Linked to Parkinson’s Disease through Viral Antigen in Dopamine-Containing Neurons of Substantia Nigra. Microorganisms 2022, 10, 599. https://doi.org/10.3390/microorganisms10030599
Niklasson B, Lindquist L, Klitz W, Fredrikson S, Morgell R, Mohammadi R, Netherlands Brain Bank, Karapetyan Y, Englund E. Picornavirus May Be Linked to Parkinson’s Disease through Viral Antigen in Dopamine-Containing Neurons of Substantia Nigra. Microorganisms. 2022; 10(3):599. https://doi.org/10.3390/microorganisms10030599
Chicago/Turabian StyleNiklasson, Bo, Lars Lindquist, William Klitz, Sten Fredrikson, Roland Morgell, Reza Mohammadi, Netherlands Brain Bank, Yervand Karapetyan, and Elisabet Englund. 2022. "Picornavirus May Be Linked to Parkinson’s Disease through Viral Antigen in Dopamine-Containing Neurons of Substantia Nigra" Microorganisms 10, no. 3: 599. https://doi.org/10.3390/microorganisms10030599





