Phthalate Esters Metabolic Strain Gordonia sp. GZ-YC7, a Potential Soil Degrader for High Concentration Di-(2-ethylhexyl) Phthalate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
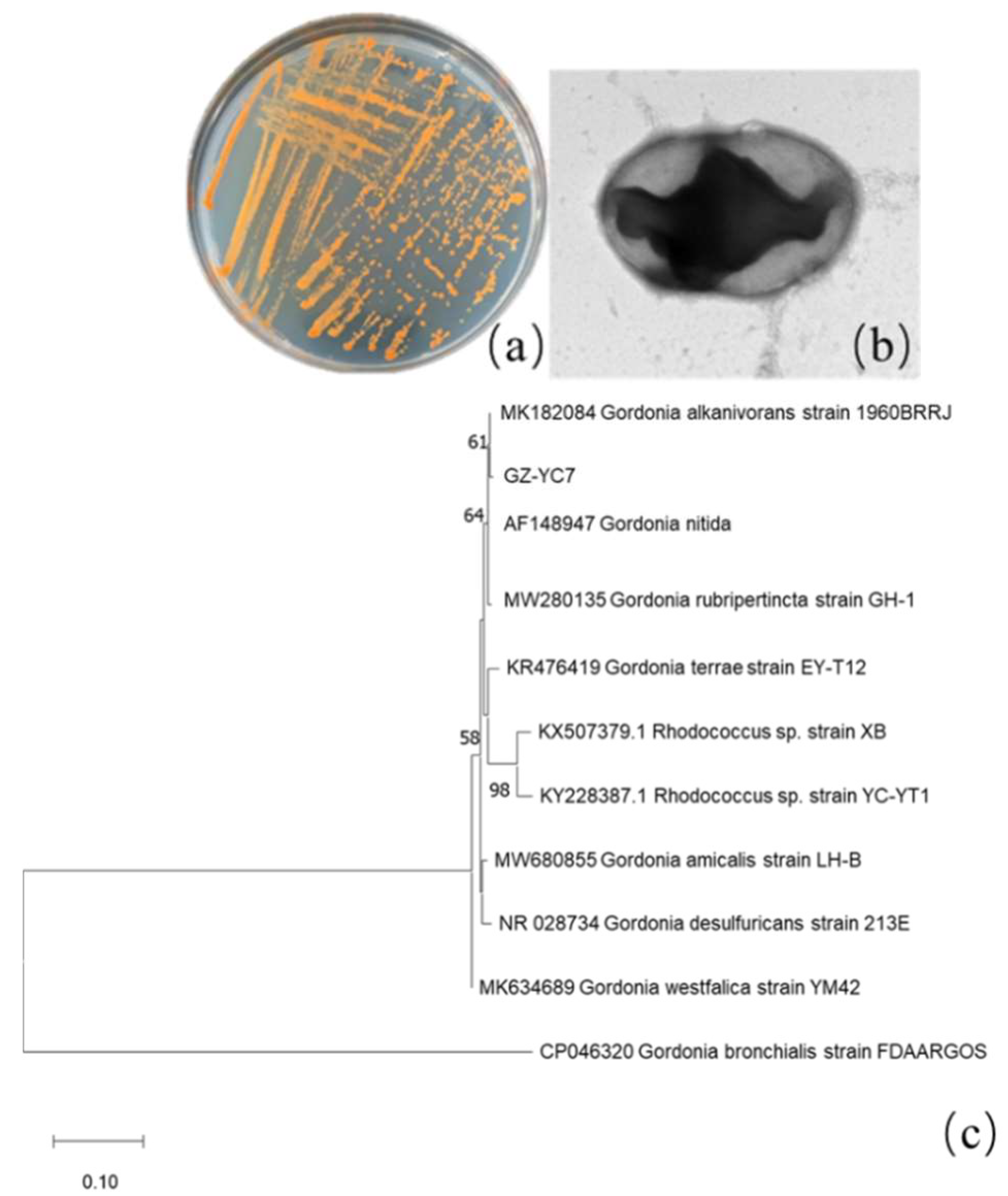
2.2. Isolation and Identification of Phthalate Esters Degrading Bacteria
2.3. Culture Conditions and Analytic Method for Phthalate Esters
2.4. Phthalate Esters Degradation Ability of Isolated Strains in BSM Medium
2.4.1. Degrading Substrates Spectrum
2.4.2. Tolerance to High Concentrations of Phthalate Esters Degrading Strains
2.4.3. Mixed Phthalate Esters Degradation
2.4.4. Effects of Environmental Factors on Di-(2-ethylhexyl) Phthalate Degradation
2.4.5. Degradation Kinetics of Di-(2-ethylhexyl) Phthalate
2.5. Degradation of Di-(2-ethylhexyl) Phthalate in Soil
2.6. Genome Sequence Analysis of Strain GZ-YC7
2.7. Gene Annotation and Protein Classification
3. Results and Discussion
3.1. Isolation and Characterization of Strains
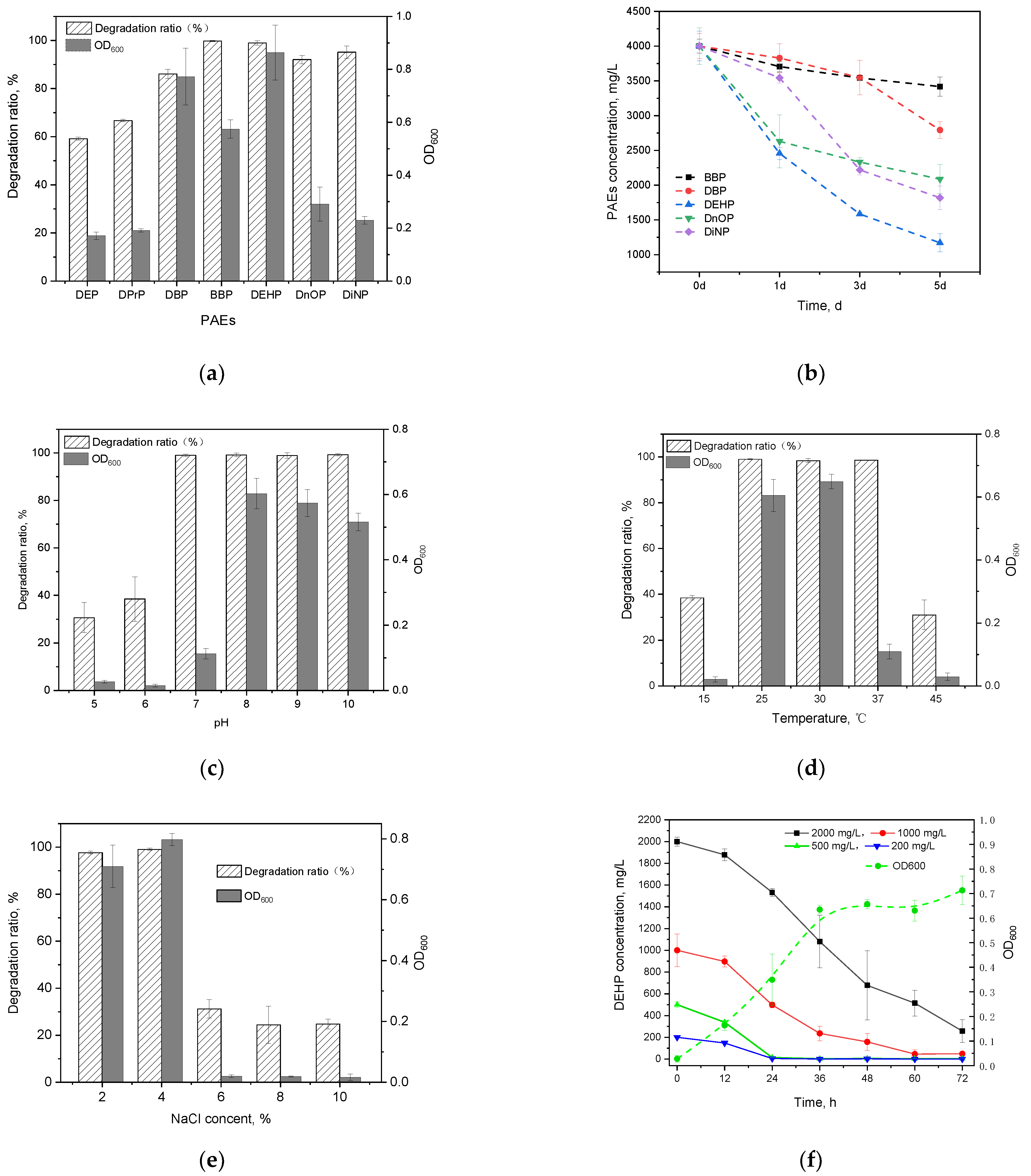
3.2. Phthalate Esters Degradation Ability of Isolated Strains in BSM Medium
3.2.1. Degrading Substrate Spectrum
3.2.2. Tolerance to High Concentrations of Phthalate Esters Degrading Strains
3.2.3. Mixed Phthalate Esters Degradation
3.2.4. Effects of Environmental Factors on Di-(2-ethylhexyl) Phthalate Degradation
3.2.5. Degradation Kinetics of Di-(2-ethylhexyl) Phthalate by Strain GZ-YC7
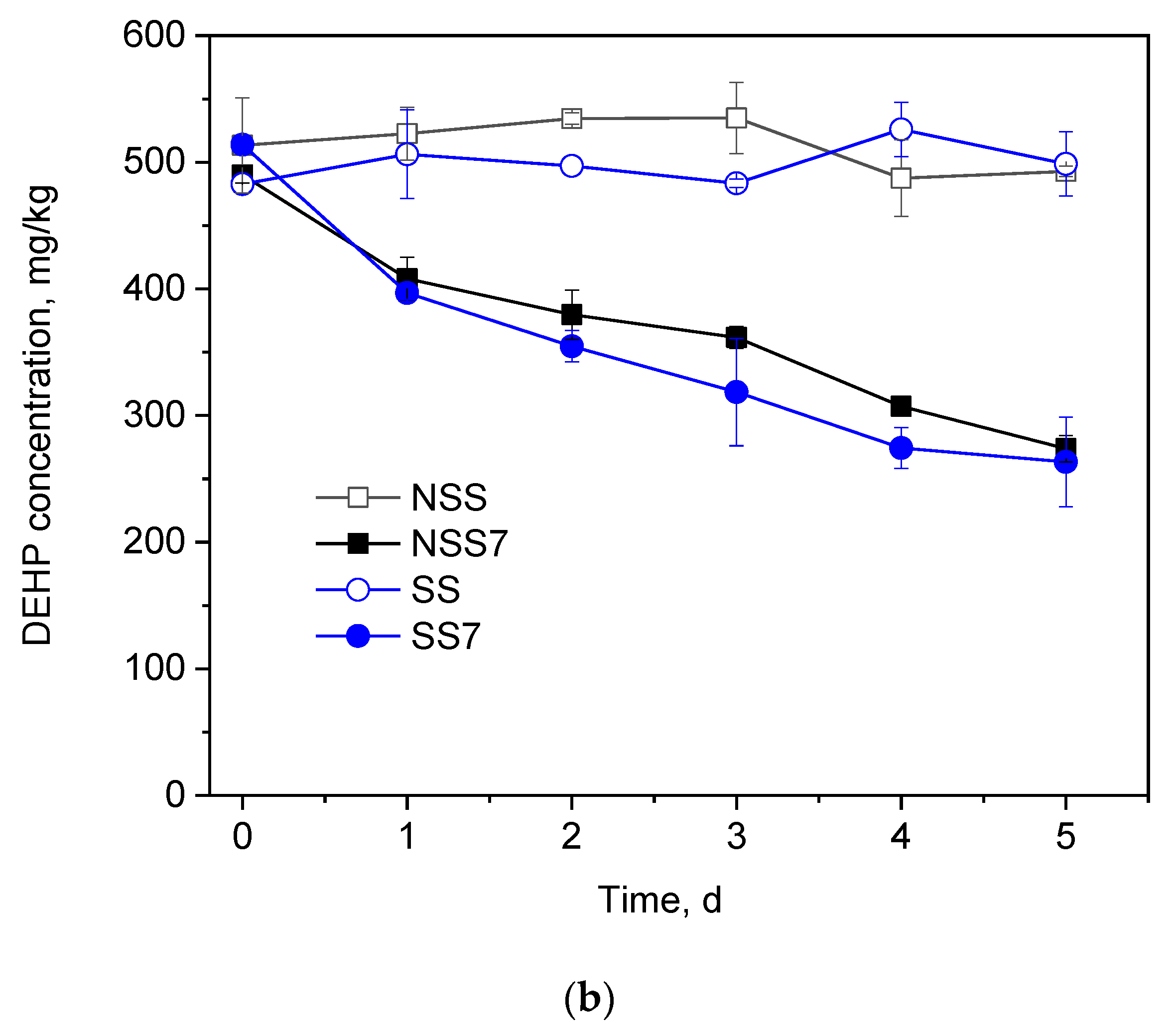
3.3. Degradation of di-(2-ethylhexyl) Phthalate in Soil
3.4. Genome Sequencing and Analysis
3.5. Comparison of Esterase Enzymes
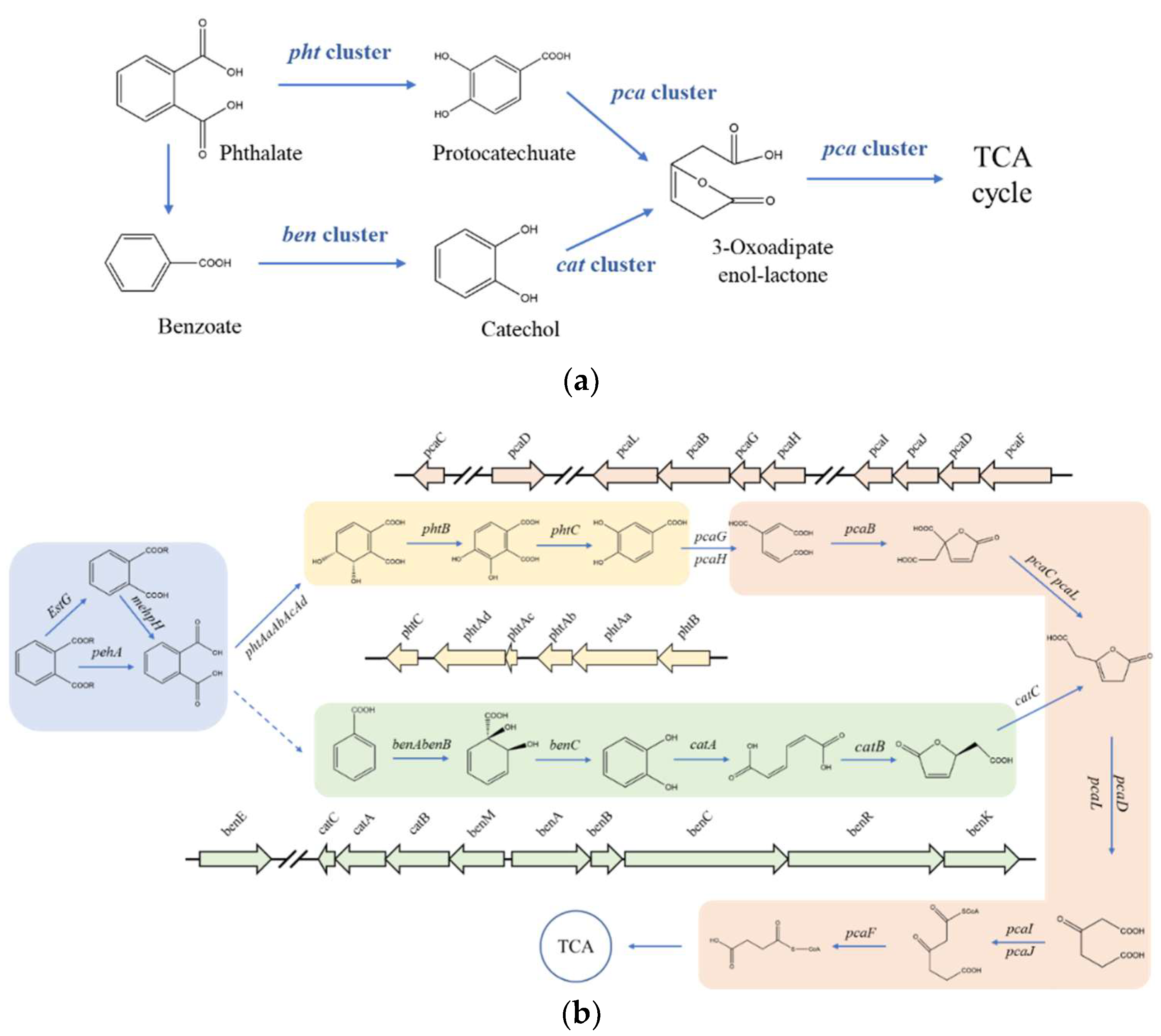
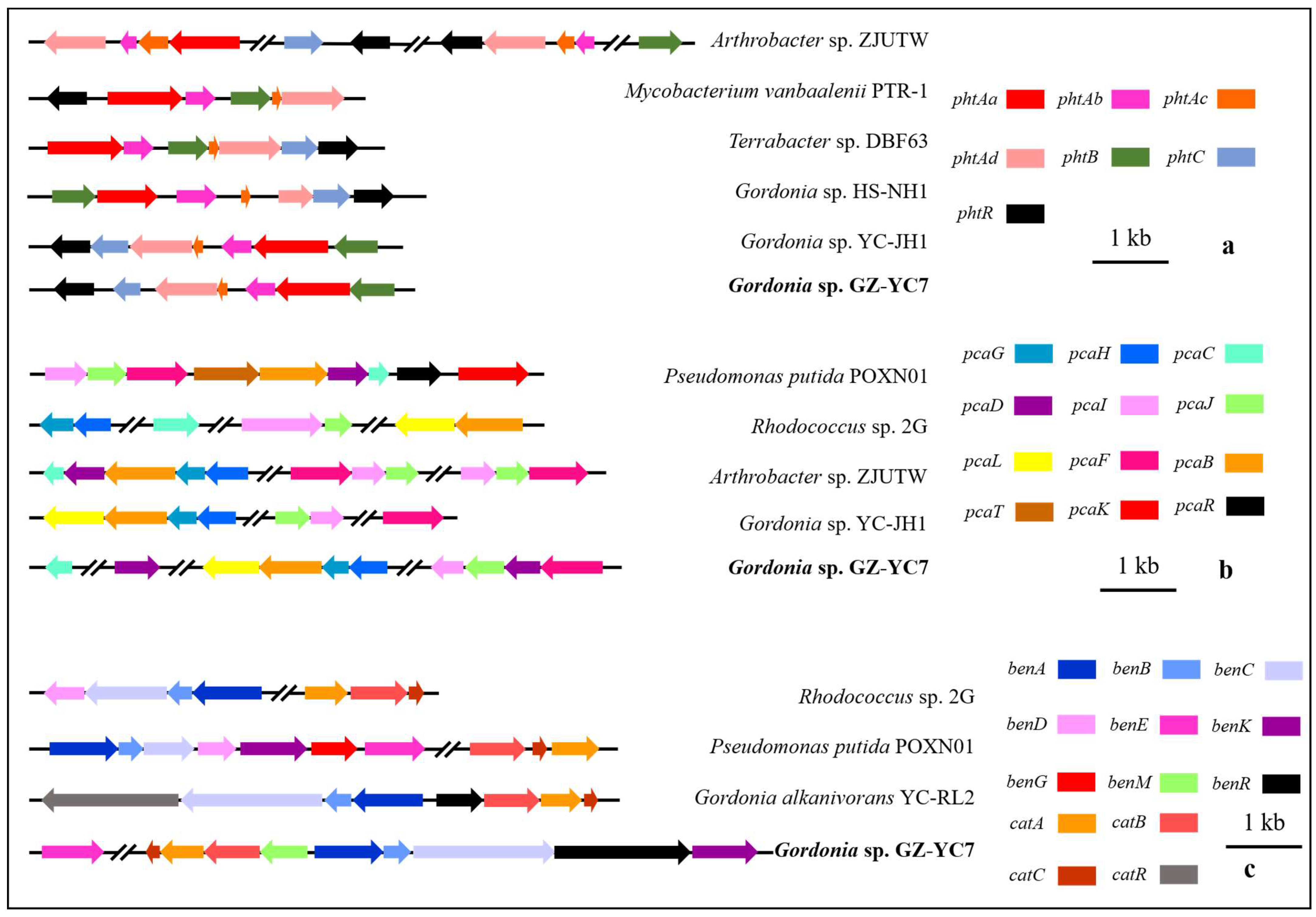
3.6. Genetic Peculiarities and Pathway of Phthalate Esters Degradation by GZ-YC7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Zeng, A.; Jiang, X.; Gu, X. Are Microplastics Correlated to Phthalates in Facility Agriculture Soil? J. Hazard. Mater. 2021, 412, 125164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Fan, X.; Qiu, Y.J.; Li, C.Y.; Xing, S.; Zheng, Y.T.; Xu, J.H. Newly Identified Thermostable Esterase from Sulfobacillus acidophilus: Properties and Performance in Phthalate Ester Degradation. Appl. Environ. Microbiol. 2014, 80, 6870–6878. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.Z.; Ma, Y.W.; Wang, Y.; Wan, J.Q.; Zhang, H.P. The Fate of Di-n-butyl Phthalate in A Laboratory-scale Anaerobic/Anoxic/Oxic Wastewater Treatment Process. Bioresour. Technol. 2010, 101, 7767–7772. [Google Scholar] [CrossRef] [PubMed]
- Chi, J. Phthalate Acid Esters in Potamogeton Crispus L. from Haihe River, China. Chemosphere 2009, 77, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pan, L.; Tsang, D.C.W.; Zhan, Y.; Zhu, L.; Li, X. Organic Contamination and Remediation in the Agricultural Soils of China: A Critical Review. Sci. Total. Environ. 2018, 615, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.J.; Huang, Y.H.; Chen, X.T.; Chen, X.H.; Mo, C.H.; Feng, Y.X.; Lv, H.X.; Xiang, L.; Li, Y.W.; Li, H.; et al. Prevalent Phthalates in Air-soil-vegetable Systems of Plastic Greenhouses in A Subtropical City And Health Risk Assessments. Sci. Total. Environ. 2020, 743, 140755. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Min, Y.; Liu, X.; Wang, P.; Zhou, Z.; Liu, D. Occurrence and Migration of Phthalates in Adhesive Materials to Fruits and Vegetables. J. Hazard. Mater. 2021, 418, 126277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, L.; Sun, Y.; Li, X.; Weng, L.; Li, Y. Contamination and Human Health Risks of Phthalate Esters in Vegetable and Crop Soils from the Huang-Huai-Hai Region of China. Sci. Total Environ. 2021, 778, 146281. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P. Micro- and Nanoplastics in the Environment: Research and policymaking. Curr Opin Environ. Sci. Health 2018, 1, 12–16. [Google Scholar] [CrossRef]
- Cheang, C.C.; Ma, Y.; Fok, L. Occurrence and Composition of Microplastics in the Seabed Sediments of the Coral Communities in Proximity of a Metropolitan Area. Int. J. Environ. Res. Public Health 2018, 15, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.; Lee, J.; An, Y. Towards Understanding the Impact of Plastics on Freshwater and Marine Microalgae: A Review of the Mechanisms and Toxicity Endpoints. J. Hazard. Mater. 2022, 423, 127174. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ren, L.; Jia, Y.; Fan, S.; Wang, J.; Wang, J.; Yan, Y. Biodegradation of Di-(2-ethylhexyl) Phthalate by Rhodococcus ruber YC-YT1 in Contaminated Water and Soil. Int. J. Environ. Res. Public Health 2018, 15, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lv, M.; Sun, A.; Ding, J.; Wang, Y.; Chang, X.; Chen, L. Exposure to Microplastics Reduces the Bioaccumulation of Sulfamethoxazole but Enhances its Effects on Gut Microbiota and the Antibiotic Resistome of Mice. Chemosphere 2022, 294, 133810. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, J.; Zhao, Y.; Zhang, M.; Zhou, S. Biodegradability of Bi-(2-ethylhexyl) Phthalate by a Newly Isolated Bacterium Achromobacter sp. RX. Sci. Total Environ. 2021, 755 Pt 1, 142476. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Lin, Z.; Liu, H.; Hu, H. Bacteria-mediated Phthalic Acid Esters Degradation and Related Molecular Mechanisms. Appl. Microbiol. Biotechno. 2018, 102, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wei, Z.; Chen, L.K.; Wu, L.; Luo, Y. Toxicity Effects of Di-(2-ethylhexyl) Phthalate to Eisenia Fetida at Enzyme, Cellular and Genetic Levels. PLoS ONE 2017, 12, e0173957. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.D.; Onesto, V.; Lagreca, E.; Vecchione, R.; Netti, P.A. Tunable Release of Curcumin with an In Silico-Supported Approach from Mixtures of Highly Porous PLGA Microparticles. Materials 2020, 13, 1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Zhang, X.Y.; Chen, T.L.; Zhao, Y.L.; Xu, D.S.; Bai, Y.P. Biodegradation of Structurally Diverse Phthalate Esters by a Newly Identified Esterase with Catalytic Activity toward Di(2-ethylhexyl) Phthalate. J. Agric. Food Chem. 2019, 67, 8548–8558. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Lin, C.; Bui, X.; Nguyen, M.K.; Cao, N.D.T.; Mukhtar, H.; Hoang, H.G.; Varjani, S.; Ngo, H.H.; Nghiem, L.D. Phthalates in the Environment: Characteristics, Fate and Transport, and Advanced Wastewater Treatment Technologies. Bioresour. Technol. 2022, 344, 126249. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhou, Y.; Wang, C.; Peng, Z.; Huang, Z. Development of a Whole-cell Biocatalyst for Diisobutyl Phthalate Degradation by Functional Display of a Carboxylesterase on the Surface of Escherichia coli. Microb. Cell Factories 2020, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Bai, Z.; Chang, D.; Hoefel, D.; Jin, B.; Wang, P.; Wei, D.; Zhuang, G. Biodegradation of Di-n-butyl Phthalate by an Isolated Gordonia sp. Strain QH-11: Genetic Identification and Degradation Linetics. J. Hazard. Mater. 2012, 221–222, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.X.; Feng, Y.X.; Liang, Q.F.; Chen, X.; Xiang, L.; Zhao, H.M.; Liu, L.B.; Cao, G.; Li, Y.W.; Li, H.; et al. Complete Biodegradation of Di-n-butyl Phthalate (DBP) by a Novel Pseudomonas sp. YJB6. Sci. Total Environ. 2021, 761, 143208. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Niu, C.; Lu, Z. Individual or Synchronous Biodegradation of Di-n-butyl Phthalate and Phenol by Rhodococcus Ruber Strain DP-2. J. Hazard. Mater. 2014, 273, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Hu, R.W.; Du, H.; Xin, X.P.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Liu, J.S.; Zhou, D.M.; et al. Functional Genomic Analysis of Phthalate Acid Ester (PAE) Catabolism Genes in the Versatile PAE-mineralising Bacterium Rhodococcus sp. 2G. Sci. Total Environ. 2018, 640–641, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Zhang, J. Status and Associated Human Health Risk of Zinc Accumulation in Agricultural Soils Across China. Process Saf. Environ. Prot. 2021, 146, 867–876. [Google Scholar] [CrossRef]
- Chao, W.L.; Cheng, C.Y. Effect of Introduced Phthalate-degrading Bacteria on the Diversity of Indigenous Bacterial Communities During Di-(2-ethylhexyl) Phthalate (DEHP) Degradation in a Soil Microcosm. Chemosphere 2007, 67, 482–488. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xiao, H.; Tang, L.; Min, H.; Lu, Z. Biodegradation of di-n-butyl phthalate by a stable bacterial consortium, HD-1, enriched from activated sludge. Bioresour. Technol. 2013, 128, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Jia, Y.; Ruth, N.; Qiao, C.; Wang, J.; Zhao, B.; Yan, Y. Biodegradation of Phthalic Acid Esters by a Newly Isolated Mycobacterium sp. YC-RL4 and the Bioprocess With Environmental Samples. Environ. Sci. Pollut. Res. Int. 2016, 23, 16609–16619. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.Y.; Chen, T.; Zhu, Y.; Teng, Y.; Luo, Y.M.; Christie, P. Isolation and Identification of a Di-(2-Ethylhexyl) Phthalate-Degrading Bacterium and Its Role in the Bioremediation of a Contaminated Soil. Pedosphere 2015, 25, 202–211. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Qiu, L.; Zhang, F.; Linhardt, R.J.; Zhong, W. Combined Genomic and Transcriptomic Analysis of the Dibutyl Phthalate Metabolic Pathway in Arthrobacter sp. ZJUTW. Biotechnol. Bioeng. 2020, 117, 3712–3726. [Google Scholar] [CrossRef] [PubMed]
- Nahurira, R.; Ren, L.; Song, J.; Jia, Y.; Wang, J.; Fan, S.; Wang, H.; Yan, Y. Degradation of Di(2-Ethylhexyl) Phthalate by a Novel Gordonia alkanivorans Strain YC-RL2. Curr. Microbiol. 2017, 74, 309–319. [Google Scholar] [CrossRef]
- Zhao, H.M.; Hu, R.W.; Chen, X.X.; Chen, X.B.; Lu, H.; Li, Y.W.; Wong, M.H. Biodegradation Pathway of Di-(2-ethylhexyl) Phthalate by a Novel Rhodococcus pyridinivorans XB and its Bioaugmentation for Remediation of DEHP Contaminated Soil. Sci. Total Environ. 2018, 640–641, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Du, H.; Lin, J.; Chen, X.B.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Qin, H.M.; Wong, M.H. Complete Degradation of the Endocrine Disruptor Di-(2-ethylhexyl) Phthalate by a Novel Agromyces sp. MT-O strain and Its Application to Bioremediation of Contaminated Soil. Sci. Total Environ. 2016, 562, 170–178. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Zhu, Y.; Li, J.; Li, X. Biodegradation of Seven Phthalate Esters by Bacillus mojavensis B1811. Int. Biodeterior. Biodegrad. 2018, 132, 200–207. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Chen, C.; Feng, L.; Dong, Y.; Chen, J.; Lan, J.; Hou, H. High-efficiency Degradation of Phthalic Acid Esters (PAEs) by Pseudarthrobacter defluvii E5: Performance, Degradative Pathway, and Key Genes. Sci. Total Environ. 2021, 794, 148719. [Google Scholar] [CrossRef]
- Ren, L.; Wang, G.; Huang, Y.; Guo, J.; Li, C.; Jia, Y.; Chen, S.; Zhou, J.; Hu, H. Phthalic Acid Esters Degradation by a Novel Marine Bacterial Strain Mycolicibacterium phocaicum RL-HY01: Characterization, Metabolic Aathway and Bioaugmentation. Sci. Total Environ. 2021, 791, 148303. [Google Scholar] [CrossRef]
- Meng, X.; Niu, G.; Yang, W.; Cao, X. Di-(2-ethylhexyl) Phthalate Biodegradation and Denitrification by a Pseudoxanthomonas sp. Strain. Bioresour. Technol. 2015, 180, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, W.; Ren, Q.; Cheng, S.; Wang, J.; Ma, X.; Zhang, C.; Wang, Y. Biodegradation of Di-(2-ethylhexyl) Phthalate by a Newly Isolated Gordonia sp. and its Application in the Remediation of Contaminated Soils. Sci. Total Environ. 2019, 689, 645–651. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yadav, M.P.; Li, X. Biodegradability and Biodegradation Pathway of Di-(2-ethylhexyl) Phthalate by Burkholderia Pyrrocinia B1213. Chemosphere 2019, 225, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.D.; Gao, D.W.; Wu, W.M. Biodegradation and Kinetic Analysis of Phthalates by an Arthrobacter Strain Isolated from Constructed Wetland Soil. Appl. Microbiol. Biotechnol. 2014, 98, 4683–4690. [Google Scholar] [CrossRef]
- Chen, S.; Geng, P.; Xiao, Y.; Hu, M. Bioremediation of Beta-cypermethrin and 3-phenoxybenzaldehyde Contaminated Soils Using Streptomyces aureus HP-S-01. Appl. Microbiol. Biotechnol. 2012, 94, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, Z.; Liu, B.; Wang, G.; Weng, L.; Zhou, J.; Hu, H.; He, H.; Huang, Y.; Chen, J.; et al. Bioremediation of Di-(2-ethylhexyl) Phthalate Contaminated Red Soil by Gordonia terrae RL-JC02: Characterization, Metabolic Pathway and Kinetics. Sci. Total Environ. 2020, 733, 139138. [Google Scholar] [CrossRef]
- Livny, J.; Brencic, A.; Lory, S.; Waldor, M.K. Identification of 17 Pseudomonas Aeruginosa sRNAs and Prediction of sRNA-encoding Genes in 10 Diverse Pathogens Using the Bioinformatic Tool sRNAPredict2. Nucleic Acids Res. 2006, 34, 3484–3493. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Iwata, M.; Imaoka, T.; Mutoh, M.; Egashira, Y.; Nishiyama, T.; Shin, T.; Fujii, T. A Mono-2-Ethylhexyl Phthalate Hydrolase from a Gordonia sp. that is Able To Dissimilate Di-2-Ethylhexyl Phthalate. Appl. Environ. Microbiol. 2006, 72, 2394–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, D.K.; Jang, S.H.; Lee, C. Gene Cloning and Characterization of a Psychrophilic Phthalate sterase With Organic Solvent Tolerance from an Arctic Bacterium Sphingomonas glacialis PAMC 26605. J. Mol. Catal. B Enzym. 2016, 133, S337–S345. [Google Scholar] [CrossRef]
- Hara, H.; Stewart, G.R.; Mohn, W.W. Involvement of a Novel ABC Aransporter and Monoalkyl Phthalate Ester Hydrolase in Phthalate Ester Catabolism by Rhodococcus jostii RHA1. Appl. Environ. Microbiol. 2010, 76, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Whangsuk, W.; Sungkeeree, P.; Nakasiri, M.; Thiengmag, S.; Mongkolsuk, S.; Loprasert, S. Two Endocrine Disrupting Dibutyl Phthalate Degrading Esterases and Their Compensatory Gene Expression in Sphingobium sp. SM42. Int. Biodeterior. Biodegrad. 2015, 99, 45–54. [Google Scholar] [CrossRef]
- Lei, B.; Ding, J.; Wang, C.; Xie, Z.; Li, J.; Yang, Y.; Mu, Y.; Tang, X.; Xu, B.; Zhou, J.; et al. Properties of a Newly Identified Esterase from Bacillus sp. K91 and Its Novel Function in Diisobutyl Phthalate Degradation. PLoS ONE 2015, 10, e0119216. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Wang, J.; Li, K.; Yang, T.; Jia, Y.; Zhao, B.; Yan, Y. Complete Genome Sequence of Gordonia sp. YC-JH1, a Bacterium Efficiently Degrading a Wide Range of Phthalic Acid Esters. J. Biotechnol. 2018, 279, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liao, X.; Yu, F.; Wei, Z.; Yang, L. Cloning of a dibutyl phthalate hydrolase gene from Acinetobacter sp. strain M673 and functional analysis of its expression product in Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 2483–2491. [Google Scholar] [CrossRef]

| Strain | Source | Degrading Substrates Spectrum | DEHP Degrading Efficiency | References |
|---|---|---|---|---|
| Rhodococcus sp. 2G | activated sludge | DEHP, DMP, DEP, DBP, BBP, DnOP, DiNP | 200 mg/L, 5 day, >95% | [24] |
| Rhodococcus ruber YC-YT1 | marine plastic debris | DEHP, DDP, DNP, DOP, DCHP, BBP, DHPP, DHP, DAP, DBP, DPrP, DEP, DMP | 100 mg/L, 3 day, >95% | [12] |
| Rhodococcus pyridinivorans XB | activated sludge | DEHP, DMP, DEP, DBP | 400 mg/L, 3 day, 100% | [32] |
| Agromyces sp. MT-O | landfill soil | DEHP, DMP, DEP, DBP, DnOP | 1000 mg/L, 7 day, 65% | [33] |
| Bacillus mojavensis B1811 | soil | DEHP, DEP, DMP, DBP, BBP, DnOP, DPP | 500 mg/L, 4 day, 100% | [34] |
| Pseudarthrobacter defluvii E5 | agricultural soil | DEHP, DMP, DEP, DBP, DHXP | 1200 mg/L, 2 day, >50% | [35] |
| Mycolicibacterium phocaicum RL-HY01 | wastewater | DEHP, DMP, DEP, DBP | 1000 mg/L, 3 day, 100% | [36] |
| Achromobacter sp. RX | activated sludge | DEHP | 300 mg/L, 4 day, 96% | [14] |
| Pseudoxanthomonas sp. N4 | Denitrification biofilter reactor | DEHP | 1250 mg/L, 5 day, 30% | [37] |
| Gordonia sp. Lff | river sludge | DEHP, DMP, DEP, DBP, DOP | 2000 mg/L, 3 day, 91.4% | [38] |
| Gordonia alkanivorans YC-RL2 | soil | DEHP, DCP, DEP, DMP, DBP | 1000 mg/L, 7 day, 68.3% | [31] |
| Gordonia sp. GZ-YC7 | landfill soil | DEHP, DEP, DPrP, DBP, BBP, DnOP, DiNP | 4000 mg/L, 5 day, 70.71%; 2000 mg/L, 3 day, 87.11%; 1000 mg/L, 2.5 day, 100%; 500 mg/L, 1 day, 100%; 200 mg/L, 1d, 100% | This study |
| Type | Ester | Strain | Gene Accession Number in GZ-YC7 | Similarity (%) | References |
|---|---|---|---|---|---|
| I | EstS1 | Sulfobacillus acidophilus DSM10332 | GZ-YC7GL004260 | 31.62 | [2] |
| I | EstSP1 | Sphingomonas glacialis PAMC 26605 | GZ-YC7GL000423 | 46.62 | [45] |
| II | mehpH | Gordonia sp. P8219 | GZ-YC7GL001189 | 98.94 | [44] |
| II | patE | Rhodococcus jostii RHA1 | GZ-YC7GL000190 | 38.92 | [46] |
| III | EstG | Sphingobium sp. SM42 | GZ-YC7GL000793 | 40.37 | [47] |
| III | CarEW | Bacillus sp. K91 | GZ-YC7GL000873 | 37.16 | [48] |
| III | pehA | Arthrobacter sp. ZJUTW | GZ-YC7GL001562 | 34.07 | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Yang, C.; Hou, Z.; Liu, T.; Mei, X.; Zheng, L.; Zhong, W. Phthalate Esters Metabolic Strain Gordonia sp. GZ-YC7, a Potential Soil Degrader for High Concentration Di-(2-ethylhexyl) Phthalate. Microorganisms 2022, 10, 641. https://doi.org/10.3390/microorganisms10030641
Hu T, Yang C, Hou Z, Liu T, Mei X, Zheng L, Zhong W. Phthalate Esters Metabolic Strain Gordonia sp. GZ-YC7, a Potential Soil Degrader for High Concentration Di-(2-ethylhexyl) Phthalate. Microorganisms. 2022; 10(3):641. https://doi.org/10.3390/microorganisms10030641
Chicago/Turabian StyleHu, Tong, Chen Yang, Zhengyu Hou, Tengfei Liu, Xiaotong Mei, Lianbao Zheng, and Weihong Zhong. 2022. "Phthalate Esters Metabolic Strain Gordonia sp. GZ-YC7, a Potential Soil Degrader for High Concentration Di-(2-ethylhexyl) Phthalate" Microorganisms 10, no. 3: 641. https://doi.org/10.3390/microorganisms10030641
APA StyleHu, T., Yang, C., Hou, Z., Liu, T., Mei, X., Zheng, L., & Zhong, W. (2022). Phthalate Esters Metabolic Strain Gordonia sp. GZ-YC7, a Potential Soil Degrader for High Concentration Di-(2-ethylhexyl) Phthalate. Microorganisms, 10(3), 641. https://doi.org/10.3390/microorganisms10030641







