Virological Characterization of Pigs with Erythema Multiforme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics Statement
2.3. Sample Collection and Histology
2.4. DNA Extraction
2.5. PERV Testing
2.6. Testing of PCMV, PLHV and PCV
2.7. PRRSV Testing
2.8. Treatment
3. Results
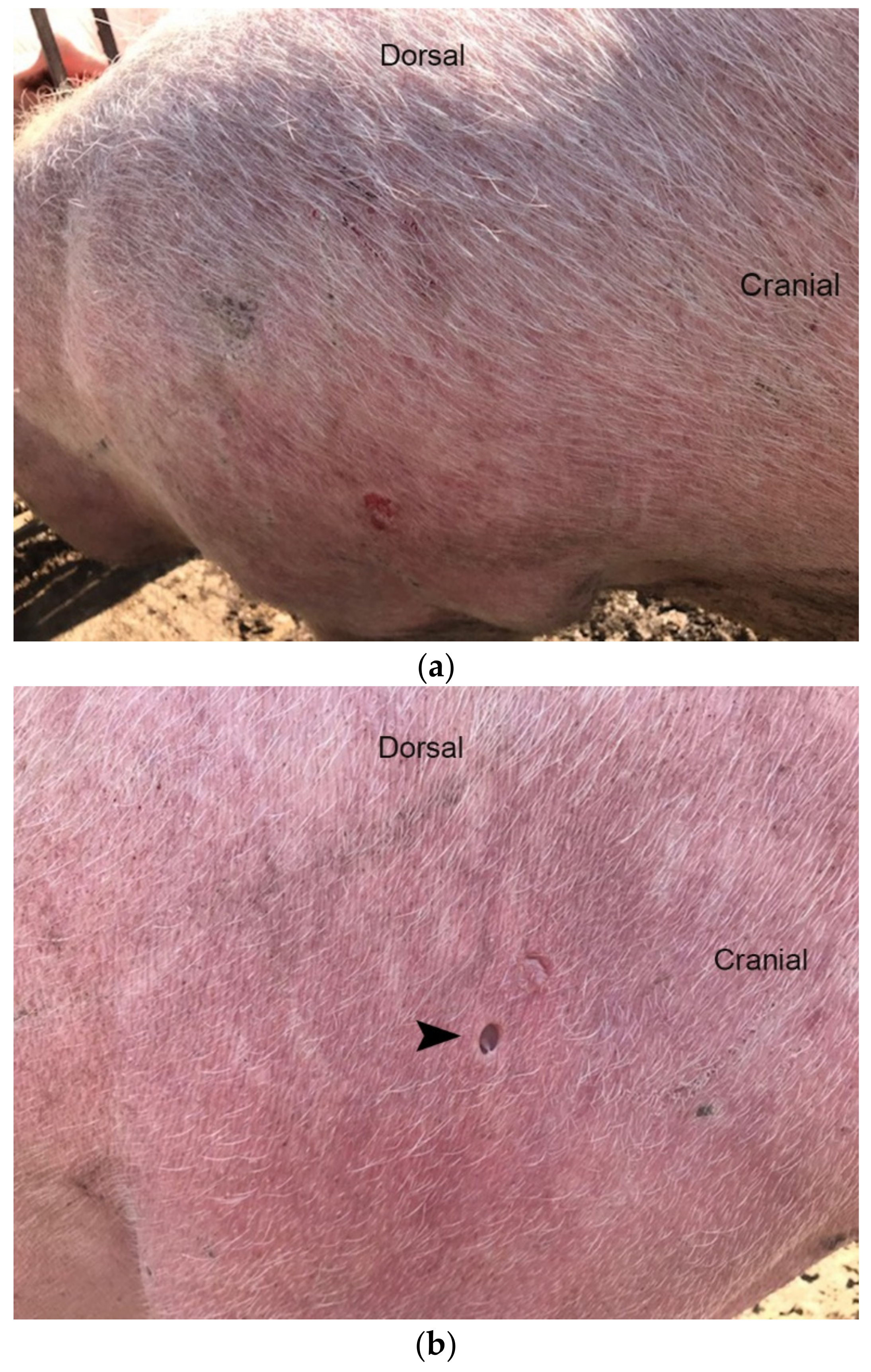
3.1. Diseased Animals and Histology of the Affected Skin
3.2. Results of Virus Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grünwald, P.; Mockenhaupt, M.; Panzer, R.; Emmert, S. Erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis-diagnosis and treatment. J. Dtsch. Dermatol. Ges. 2020, 18, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liang, G.; Liu, W.; Wang, X.; Sun, J. Erythema Multiforme Associated with Tinea of Vellus Hair Caused by Microsporum canis. Mycopathologia 2020, 185, 201–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, R. Diseases of the skin. In Diseases of Swine, 9th ed.; Straw, B., Zimmerman, J.J., D’Allaire, S., Taylor, D.J., Eds.; Blackwell: Oxford, UK, 2006; pp. 1131–1143. [Google Scholar]
- Straw, B.E.; Dewey, C.E.; Wilson, M.R. Differential diagnosis of disease. In Diseases of Swine, 9th ed.; Straw, B., Zimmerman, J.J., D’Allaire, S., Taylor, D.J., Eds.; Blackwell: Oxford, UK, 2006; pp. 241–286. [Google Scholar]
- Thibault, S.; Drolet, R.; Germain, M.-C.; D’Allaire, S.; LaRochelle, R.; Magar, R. Cutaneous and systemic necrotizing vasculitis in swine. Vet. Pathol. 1998, 35, 108–116. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Athanasiou, L.V.; Psalla, D.; Petridou, E.; Maragkakis, G.G.; Papatsas, I.; Arsenakis, I.; Maes, D. Erythema Multiforme Associated with Respiratory Disease in a Commercial Breeding Pig Herd. Viral Immunol. 2015, 28, 464–471. [Google Scholar] [CrossRef]
- Woldemeskel, M.; Liggett, A.; Ilha, M.; Saliki, J.T.; Johnson, L.P. Canine parvovirus-2b-associated erythema multiforme in a litter of English Setter dogs. J. Vet. Diagn. Investig. 2011, 23, 576–580. [Google Scholar] [CrossRef] [Green Version]
- Fisher, P.G. Erythema multiforme in a ferret (Mustela putorius furo). Vet. Clin. N. Am. Exot. Anim. Pract. 2013, 16, 599–609. [Google Scholar] [CrossRef]
- Hanley, C.S.; Simmons, H.A.; Wallace, R.S.; Clyde, V.L. Erythema multiforme in a spotted hyena (Crocuta crocuta). J. Zoo Wildl. Med. 2005, 36, 515–519. [Google Scholar] [CrossRef]
- Grand, N. Diseases of minipigs. In The Minipig in Biomedical Research; McAnulty, P.A., Dayan, A.D., Ganderup, N.C., Hastings, K.L., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Halecker, S.; Metzger, J.; Strube, C.; Krabben, L.; Kaufer, B.; Denner, J. Virological and Parasitological Characterization of Mini-LEWE Minipigs Using Improved Screening Methods and an Overview of Data on Various Minipig Breeds. Microorganisms 2021, 9, 2617. [Google Scholar] [CrossRef]
- Denner, J. Sensitive detection systems for infectious agents in xenotransplantation. Xenotransplantation 2020, 18, e12594. [Google Scholar] [CrossRef] [Green Version]
- Krüger, L.; Kristiansen, Y.; Reuber, E.; Möller, L.; Laue, M.; Reimer, C.; Denner, J. A Comprehensive Strategy for Screening for Xenotransplantation-Relevant Viruses in a Second Isolated Population of Göttingen Minipigs. Viruses 2019, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Doster, A.R. Skin diseases of swine. J. Swine Health Prod. 1995, 3, 256–261. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: München, Germany, 2018. [Google Scholar]
- Takeuchi, Y.; Patience, C.; Magre, S.; Weiss, R.A.; Banerjee, P.T.; Le Tissier, P.; Stoye, J.P. Host range and interference studies of three classes of pig endogenous retrovirus. J. Virol. 1998, 72, 9986–9991. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.C.; Quinn, G.; Suling, K.M.; Oldmixon, B.A.; Van Tine, B.A.; Cina, R.; Arn, S.; Huang, C.A.; Scobie, L.; Onions, D.E.; et al. Identification of exogenous forms of human-tropic porcine endogenous retrovirus in miniature Swine. J. Virol. 2004, 78, 2494–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, N.J.; Barth, R.; Yamamoto, S.; Kitamura, H.; Patience, C.; Yamada, K.; Cooper, D.K.C.; Sachs, D.H.; Kaur, A.; Fishman, J.A. Activation of Cytomegalovirus in Pig-to-Primate Organ Xenotransplantation. J. Virol. 2002, 76, 4866–4872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielewicz, B.; Goltz, M.; Franz, T.; Bauer, C.; Brema, S.; Ellerbrok, H.; Beckmann, S.; Rziha, H.-J.; Lahrmann, K.-H.; Romero, C.; et al. A novel porcine gammaherpesvirus. Virology 2003, 308, 317–329. [Google Scholar] [CrossRef] [Green Version]
- McMahon, K.J.; Minihan, D.; Campion, E.M.; Loughran, S.T.; Allan, G.; McNeilly, F.; Walls, D. Infection of pigs in Ireland with lymphotropic gamma-herpesviruses and relationship to postweaning multisystemic wasting syndrome. Vet. Microbiol. 2006, 116, 60–68. [Google Scholar] [CrossRef]
- Chen, N.; Xiao, Y.; Li, X.; Li, S.; Xie, N.; Yan, X.; Li, X.; Zhu, J. Development and application of a quadruplex real-time PCR assay for differential detection of porcine circoviruses (PCV1 to PCV4) in Jiangsu province of China from 2016 to 2020. Transbound. Emerg. Dis. 2021, 68, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef] [Green Version]
- Duvigneau, J.; Hartl, R.; Groiss, S.; Gemeiner, M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J. Immunol. Methods 2005, 306, 16–27. [Google Scholar] [CrossRef]
- Maden, C.L.; Ah-Kye, L.; Alfallouji, Y.; Kulakov, E.; Ellery, P.; Papamichael, E. Erythema multiforme major with ocular involvement following COVID-19 infection. Oxf. Med. Case Rep. 2021, 2021, omab120. [Google Scholar] [CrossRef]
- Bennardo, L.; Nisticò, S.P.; Dastoli, S.; Provenzano, E.; Napolitano, M.; Silvestri, M.; Passante, M.; Patruno, C. Erythema Multiforme and COVID-19: What Do We Know? Medicina 2021, 57, 828. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.Q.; Mahmmod, Z.; Mathkhor, A.J. Adalimumab-Induced Erythema Multiforme in a Patient With Rheumatoid Arthritis: A Case Report. Cureus 2022, 14, e21126. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, M.; Nguyen, N.M.; Parikh, S.A.; Sinai, M.; Goldberg, L. Erythema multiforme following exposure to the herbicide atrazine. Bayl. Univ. Med. Cent. Proc. 2020, 34, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zang, P.; Miller, M.; Cutler, L.; Worswick, S. Herpes associated erythema multiforme: A retrospective study. Am. J. Emerg. Med. 2020, 38, 2761.e1–2761.e3. [Google Scholar] [CrossRef] [PubMed]
- Bouabdella, S.; Benkaraache, M.; Almheirat, Y.; Zizi, N.; Dikhaye, S. Erythema multiforme eruption due to SARS-CoV 2: Case report. Ann. Med. Surg. 2021, 68, 102591. [Google Scholar] [CrossRef]
- Neri, I.; Evangelista, V.; Guglielmo, A.; Sechi, A.; Virdi, A. A Case of Bullous Rash Apparently Triggered by Meningococcal and Rotavirus Vaccines in an Infant: Focus on Infantile Bullous Pemphigoid. Dermatopathology 2021, 8, 33–36. [Google Scholar] [CrossRef]
- Kano, Y.; Kasami, S.; Murata, K.; Kato, M. Erythema multiforme after SARS-CoV-2 messenger RNA vaccination. QJM 2021, 1, hcab303. [Google Scholar]
- Saibene, A.M.; Alliata, A.; Cozzi, A.T.; Ottavi, A.; Spagnolini, S.; Pipolo, C.; Maccari, A.; Felisati, G. Erythema Multiforme Major following SARS-CoV-2 vaccine. Clin. Case Rep. 2021, 9, e04947. [Google Scholar] [CrossRef]
- Prunier, A.; Averos, X.; Dimitrov, I.; Edwards, S.A.; Hillmann, E.; Holinger, M.; Ilieski, V.; Leming, R.; Tallet, C.; Turner, S.P.; et al. Review: Early life predisposing factors for biting in pigs. Animal 2020, 14, 570–587. [Google Scholar] [CrossRef] [Green Version]
- Dor, J.; Doucette, K.E.; Mueller, N.J.; Wilkinson, R.A.; Bajwa, J.A.; McMorrow, I.M.; Tseng, Y.L.; Kuwaki, K.; Houser, S.L.; Fishman, J.A. Posttransplant lymphoproliferative disease after allogeneic transplantation of the spleen in miniature swine. Transplantation 2004, 78, 286–291. [Google Scholar] [CrossRef]
- Doucette, K.; Dor, F.J.; Wilkinson, R.A.; Martin, S.I.; Huang, C.A.; Cooper, D.K.; Sachs, D.H.; Fishman, J.A. Gene expression of porcine lymphotrophic herpesvirus-1 in miniature Swine with posttransplant lymphoproliferative disorder. Transplantation 2007, 83, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fuchimoto, Y.; Gleit, Z.; Ericsson, T.; Griesemer, A.; Scheier-Dolberg, R.; Melendy, E.; Kitamura, H.; Fishman, J.; Ferry, J. Post-trans-plantation lymphoproliferative disease in miniature swine after allogeneic hematopoietic cell transplantation: Similarity to human PTLD and association with a porcine gammaherpesvirus. Blood 2001, 97, 1467–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denner, J. Porcine Lymphotropic Herpesviruses (PLHVs) and Xenotranplantation. Viruses 2021, 13, 1072. [Google Scholar] [CrossRef] [PubMed]
- Denner, J.; Längin, M.; Reichart, B.; Krüger, L.; Fiebig, U.; Mokelke, M.; Radan, J.; Mayr, T.; Milusev, A.; Luther, F.; et al. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci. Rep. 2020, 10, 17531. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Reduction of the survival time of pig xenotransplants by porcine cytomegalovirus. Virol. J. 2018, 15, 171. [Google Scholar] [CrossRef] [Green Version]
- Denner, J.; Tönjes, R.R. Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin. Microbiol. Rev. 2012, 25, 318–343. [Google Scholar] [CrossRef] [Green Version]
- Denner, J.; Schuurman, H.J. High Prevalence of Recombinant Porcine Endogenous Retroviruses (PERV-A/Cs) in Minipigs: A Review on Origin and Presence. Viruses 2021, 13, 1869. [Google Scholar] [CrossRef]
- Denner, J. How Active Are Porcine Endogenous Retroviruses (PERVs)? Viruses 2016, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Denner, J. Porcine Endogenous Retroviruses and Xenotransplantation, 2021. Viruses 2021, 13, 2156. [Google Scholar] [CrossRef]

| Animal ID | Parity | Age | Animal Status |
|---|---|---|---|
| 1 | 3 | 23.5 months | breeding sow |
| 2 | 4 | 26 months | breeding sow |
| 3 | 2 | 18.5 months | breeding sow |
| 4 | 3 | 23 months | breeding sow |
| 5 | 1 | 14 months | breeding sow |
| PCR Assay | Primer/Probe | Sequence 5’-3’ | Reference |
|---|---|---|---|
| PCMV | PCMV-Fwd | ACT TCG TCG CAG CTC ATC TGA | Mueller et al. [18], modified |
| PCMV-Rev | GTT CTG GGA TTC CGA GGT TG | ||
| PCMV-Probe | 6FAM-CAG GGC GGC GGT CGA GCT C-BHQ | ||
| PLHV-1 | PLHV-1 (1125)-Fwd | CTC ACC TCC AAA TAC AGC GA | Chmielewicz et al. [19] |
| PLHV-1 (1125)-Rev | GCT TGA ATC GTG TGT TCC ATA G | ||
| PLHV-1 (1125)-Probe | 6FAM-CTG GTC TAC TGA ATC GCC GCT AAC AG-TAMRA | ||
| PLHV-2 | PLHV-2 (1155)-Fwd | GTC ACC TGC AAA TAC ACA GG | Chmielewicz et al. [19] |
| PLHV-2 (1155)-Rev | GGC TTG AAT CGT ATG TTC CAT AT | ||
| PLHV-2 (1155)-Probe | 6FAM-CTG GTC TAC TGA AGC GCT GCC AAT AG-TAMRA | ||
| PLVH-3 | PLHV-3 (210s)-Fwd | AAC AGC GCC AGA AAA AAA GG | McMahon et al. [20] |
| PLHV-3 (210as)-Rev | GGA AAG GTA GAA GGT GAA CCA TAA AA | ||
| PLHV-3 (210)-Probe | 6-FAM CCA AAG AGG AAA ATC-MGB | ||
| PCV1 | PCV1 (F2020)-Fwd | AAC CCC ATA AGA GGT GGG TGT T | Chen et al. [21], modified |
| PCV1 (F2020)-Rev | TTC TAC CCT CTT CCA AAC CTT CCT | ||
| PCV1 (F2020)-Probe | 6FAM-TCC GAG GAG GAG AAA AAC AAA ATA CGGGA-BHQ1 | ||
| PCV2 | PCV2 (F2020)-Fwd | CTG AGT CTT TTT TAT CAC TTC GTA ATG GT | Chen et al. [21], modified |
| PCV2 (F2020)-Rev | ACT GCG TTC GAA AAC AGT ATA TAC GA | ||
| PCV2 (F2020)-Probe | 6FAM-TTA AGT GGG GGG TCT TTA AGA TTA AAT TCT CTG AAT TGT-TAMRA | ||
| PCV3 | PCV3-Fwd | AGT GCT CCC CAT TGA ACG | Palinski et al. [22] |
| PCV3-Rev | ACA CAG CCG TTA CTT CAC | ||
| PCV3-Probe | 6FAM-ACC CCA TGG CTC AAC ACA TAT GAC C-BHQ1 | ||
| PCV4 | PCV4 (F2020)-Fwd | ATT ATT AAA CAG ACT TTA TTT GTG TCA TCA CTT | Chen et al. [21] |
| PCV4 (F2020)-Rev | ACA GGG ATA ATG CGT AGT GAT CAC T | ||
| PCV4 (F2020)-Probe | 6FAM-ATA CTA CAC TTG ATC TTA GCC AAA AGG CTC GTT GA-BHQ1 | ||
| PERV-C | PERV envC-Fwd | CTGACCTGGATTAGAACTGG | Takeuchi et al. [16] |
| PERV envC-Rev | ATGTTAGAGGATGGTCCTGG | ||
| PERV envC-Probe | 6FAM-CTC TAA CAT AAC TTC TGG ATC AGA CCC-BHQ1 | ||
| PERV-A/C | PERV-A env VRBF-Fwd | CCT ACC AGT TAT AAT CAA TTT AAT TAT GGC | Wood et al. [17] |
| PERV-C env TMR-Rev | CTC AAA CCA CCC TTG AGT AGT TTC C | ||
| pGAPDH | pGAPDH-Fwd | ACA TGG CCT CCA AGG AGT AAG A | Duvigneau et al. [23] |
| pGAPDH-Rev | GAT CGA GTT GGG GCT GTG ACT | ||
| pGAPDH-Probe | HEX-CCA CCA ACC CCA GCA AGA GCA CGC-BHQ1 |
| Material Tested | Animal ID | Ct Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCMV | PLHV-1 | PLHV-2 | PLHV-3 | PCV1 | PCV2 | PCV3 | PCV4 | PRRSV-1 | PRRSV-2 | PERV-C | PERV-A/C | ||
| Skin | 1 | No Ct | 33.50 | No Ct | 32.70 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 13.68 | - |
| samples | 2 | No Ct | 31.87 | No Ct | 32.95 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 14.56 | - |
| 3 | No Ct | 33.63 | No Ct | 31.28 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 14.38 | - | |
| 4 | No Ct | 31.82 | 33.43 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 15.17 | - | |
| 5 | No Ct | 32.48 | No Ct | 37.36 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 14.83 | - | |
| Blood | 1 | No Ct | 32.84 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 16.00 | - |
| 2 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 21.08 | - | |
| 3 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | 19.44 | - | |
| 4 | No Ct | 31.62 | 31.43 | No Ct | No Ct | 37.33 | 39.22 | No Ct | No Ct | No Ct | 17.79 | - | |
| 5 | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | No Ct | nt | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halecker, S.; Papatsiros, V.; Psalla, D.; Krabben, L.; Kaufer, B.; Denner, J. Virological Characterization of Pigs with Erythema Multiforme. Microorganisms 2022, 10, 652. https://doi.org/10.3390/microorganisms10030652
Halecker S, Papatsiros V, Psalla D, Krabben L, Kaufer B, Denner J. Virological Characterization of Pigs with Erythema Multiforme. Microorganisms. 2022; 10(3):652. https://doi.org/10.3390/microorganisms10030652
Chicago/Turabian StyleHalecker, Sabrina, Vasileios Papatsiros, Dimitra Psalla, Ludwig Krabben, Benedikt Kaufer, and Joachim Denner. 2022. "Virological Characterization of Pigs with Erythema Multiforme" Microorganisms 10, no. 3: 652. https://doi.org/10.3390/microorganisms10030652
APA StyleHalecker, S., Papatsiros, V., Psalla, D., Krabben, L., Kaufer, B., & Denner, J. (2022). Virological Characterization of Pigs with Erythema Multiforme. Microorganisms, 10(3), 652. https://doi.org/10.3390/microorganisms10030652







