Effect of Rhizobacteria Inoculation via Soil and Seeds on Glycine max L. Plants Grown on Soils with Different Cropping History
Abstract
:1. Introduction
2. Materials and Methods
2.1. Multifunctional Rhizobacteria Collection and Inoculum Preparation
2.2. Preparation, Installation, and Conduction of Field Experiments
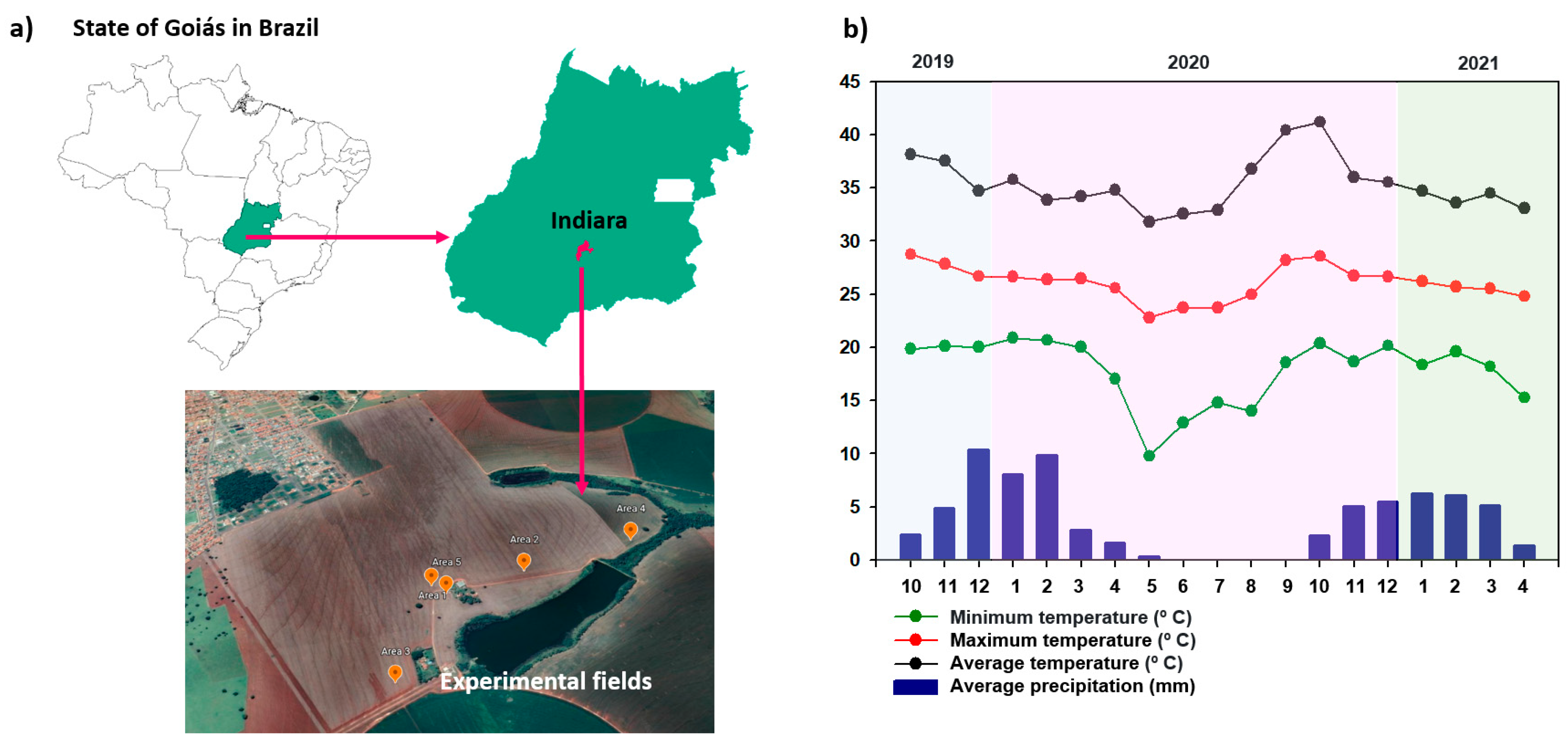
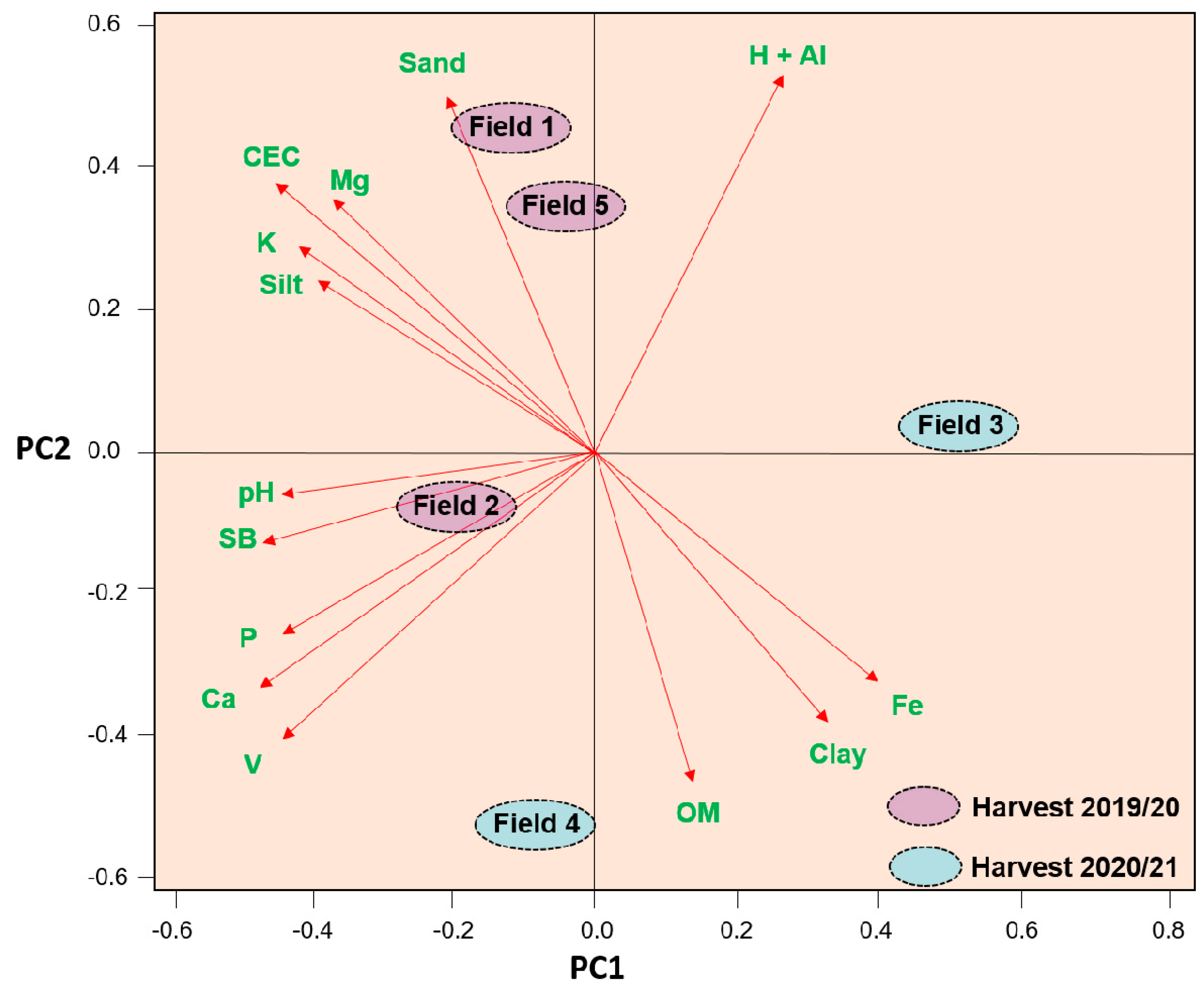
2.2.1. Characterization of the Experimental Fields
2.2.2. Experimental Procedures in the Summer Crops of 2019/2020 and 2020/2021
2.3. Data Collection
2.4. Experimental Design and Statistical Analyses
3. Results
3.1. Results for the 2019/2020 Summer Crop
3.2. Results for the Summer Crop 2020/2021
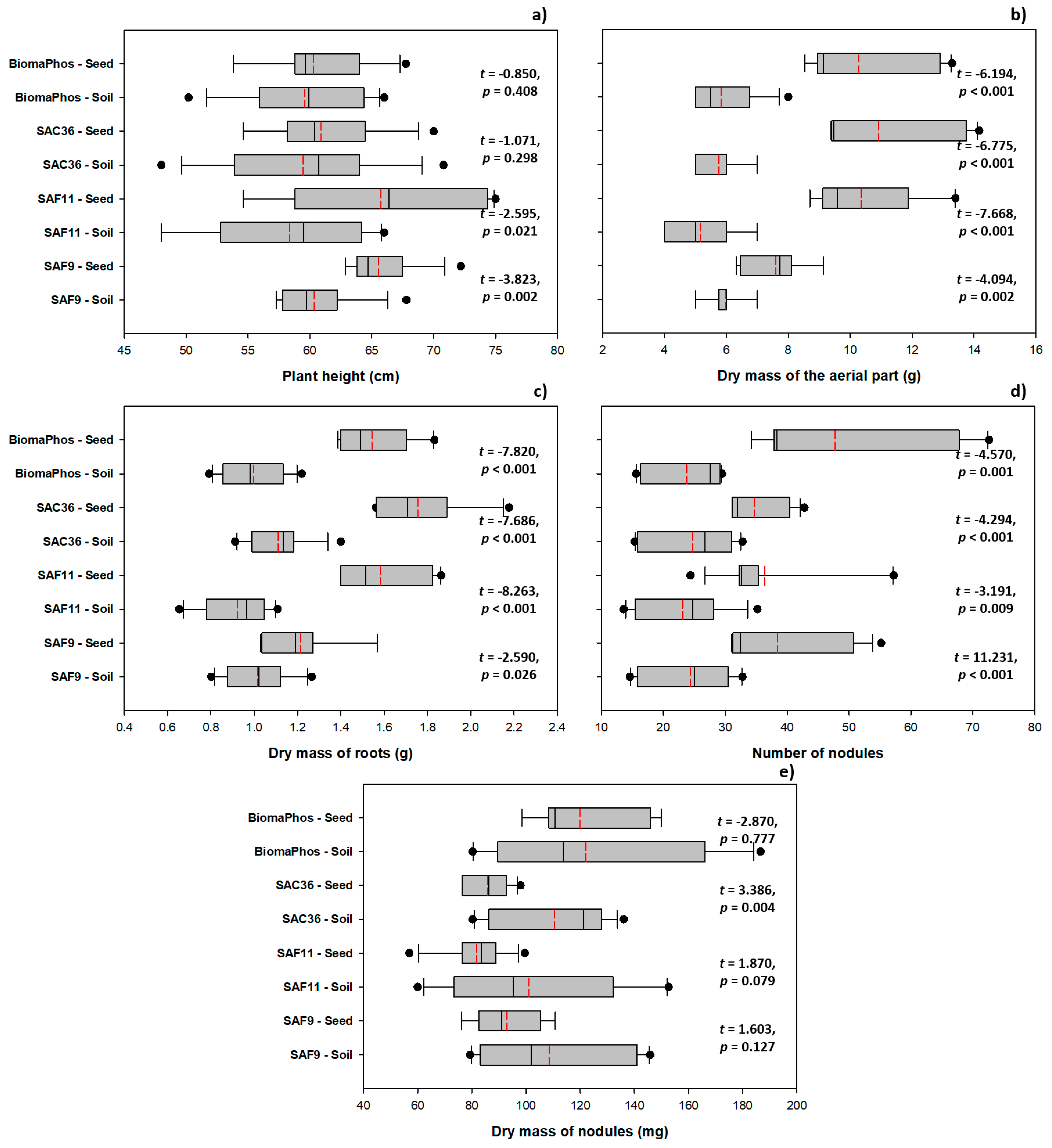
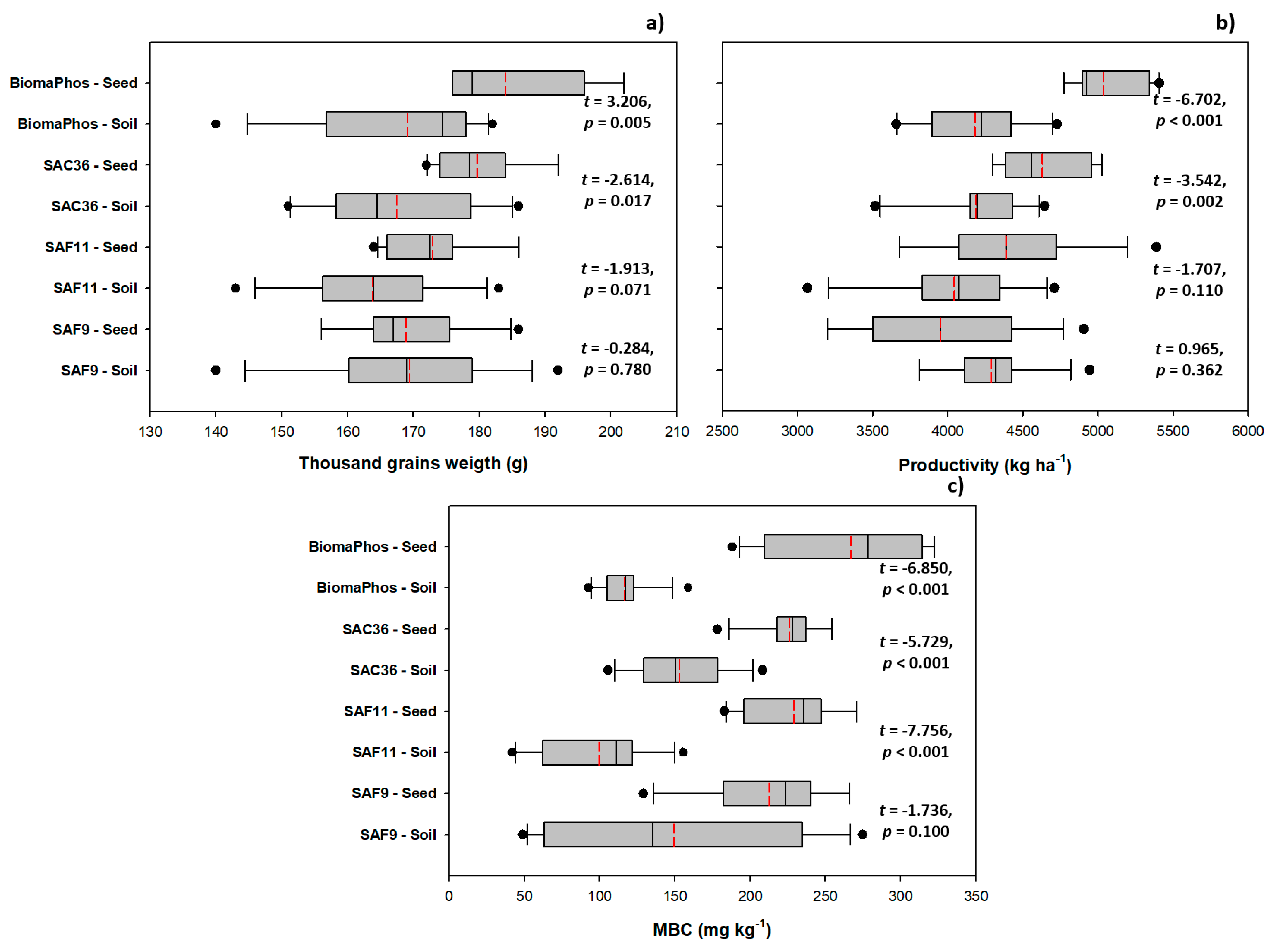
3.3. Comparing Inoculation Methods: Soil vs. Seed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy Phosphorus and No Tillage Agriculture in Tropical Oxisols of the Brazilian Savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- CONAB. Acompanhamento Da Safra Brasileira. In 12o Grain Lifting. Setember 2021; CONAB: Brasília, Brazil, 2021. [Google Scholar]
- CONAB. Perspectivas Para a Agropecuária Safra 2021/22. In Perspectives for Agriculture, Brasília, Harvest 2020/21; CONAB: Brasília, Brazil, 2021; pp. 1–85. [Google Scholar]
- de Oliveira Junior, A.; de Castro, C.; de Oliveira, F.A.; Klepker, D. (Eds.) The Economic Context of Soy Production. In Soy Production Technologies; Cap. 7. Prodution System 17; EMBRAPA: Brasília, Brazil, 2020; Volume 6, pp. 133–184. [Google Scholar]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus Activators Contribute to Legacy Phosphorus Availability in Agricultural Soils: A Review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of Phosphate Solubilizing Bacteria on Belowground Crop Performance for Improved Crop Acquisition of Phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J.B.N. Use of Compost and Phosphate-Solubilizing Bacteria Affect Sugarcane Mineral Nutrition, Phosphorus Availability, and the Soil Bacterial Community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Lucero, C.T.; Lorda, G.S.; Anzuay, M.S.; Ludueña, L.M.; Taurian, T. Peanut Endophytic Phosphate Solubilizing Bacteria Increase Growth and P Content of Soybean and Maize Plants. Curr. Microbiol. 2021, 78, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate Bacterial Solubilization: A Key Rhizosphere Driving Force Enabling Higher P Use Efficiency and Crop Productivity. J. Adv. Res. 2021. [Google Scholar] [CrossRef]
- Maldonado, S.; Rodríguez, A.; Ávila, B.; Morales, P.; González, M.P.; Araya Angel, J.P.A.; Olalde, V.; Bravo, J.; Jana, C.; Sierra, C.; et al. Enhanced Crop Productivity and Sustainability by Using Native Phosphate Solubilizing Rhizobacteria in the Agriculture of Arid Zones. Front. Sustain. Food Syst. 2020, 4, 263. [Google Scholar] [CrossRef]
- Rathinasabapathi, B.; Liu, X.; Cao, Y.; Ma, L.Q. Phosphate-Solubilizing Pseudomonads for Improving Crop Plant Nutrition and Agricultural Productivity. In Crop Improvement Through Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 363–372. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, M.S. Role of Phosphate-Solubilizing Bacteria in Improving the Soil Fertility and Crop Productivity in Organic Farming. Arch. Agron. Soil Sci. 2014, 60, 549–564. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Cota, L.V.; Marriel, I.E.; Gomes, E.A.; de Sousa, S.M.; Lana, U.G.D.P.; dos Santos, F.C.; Pinto Junior, A.S.; Alves, V.M.C. Technical and Economic Feasibility of Biomaphos® (Bacillus Subtilis CNPMS B2084 and Bacillus Megaterium CNPMS B119) in Corn and Soybean Crops. Embrapa Milho e Sorgo-Boletim Pesqui. e Desenvolv. 2020, 210, 1–21. [Google Scholar]
- Ma, M.; Jiang, X.; Wang, Q.; Guan, D.; Li, L.; Ongena, M.; Li, J. Isolation and Identification of PGPR Strain and Its Effect on Soybean Growth and Soil Bacterial Community Composition. Int. J. Agric. Biol. 2018, 20, 1289–1297. [Google Scholar] [CrossRef]
- Breedt, G.; Labuschagne, N.; Coutinho, T.A. Seed Treatment with Selected Plant Growth-Promoting Rhizobacteria Increases Maize Yield in the Field. Ann. Appl. Biol. 2017, 171, 229–236. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, G.; Schwinghamer, T.D.; Smith, D.L. Rhizobacteria from Root Nodules of an Indigenous Legume Enhance Salinity Stress Tolerance in Soybean. Front. Sustain. Food Syst. 2021, 4, 617978. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-Inoculation of Soybeans and Common Beans with Rhizobia and Azospirilla: Strategies to Improve Sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Jabborova, D.; Räsänen, L.A.; Liao, H. Coordination between Bradyrhizobium and Pseudomonas Alleviates Salt Stress in Soybean through Altering Root System Architecture. J. Plant Interact. 2017, 12, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Moraes, C.; dos Santos, R.M.; Rigobelo, E.C. Rock Phosphate Fertilization Harms Azospirillum brasilense Selection by Maize. Aust. J. Crop Sci. 2019, 13, 1967–1974. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of Plant Growth Promoting Rhizobacteria in Sustainable Production of Vegetables: Current Perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A. Synergistic Effects of the Inoculation with Plant Growth-Promoting Rhizobacteria and an Arbuscular Mycorrhizal Fungus on the Performance of Wheat. Turkish J. Agric. For. 2007, 31, 355–362. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Zaidi, A.; Musarrat, J. (Eds.) Phosphate Solubilizing Microorganisms. In Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 31–62. [Google Scholar] [CrossRef]
- Bissett, A.; Richardson, A.E.; Baker, G.; Thrall, P.H. Long-Term Land Use Effects on Soil Microbial Community Structure and Function. Appl. Soil Ecol. 2011, 51, 66–78. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of Agricultural Management Practices on Soil Quality: A Review of Long-Term Experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Fageria, N.K. Soil Quality vs. Environmentally-Based Agricultural Management Practices. Commun. Soil Sci. Plant Anal. 2002, 33, 2301–2329. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil Quality: A Concept, Definition, and Framework for Evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Delgado, A.; Quemada, M.; Villalobos, F.J.; Mateos, L. Fertilization with Phosphorus, Potassium and Other Nutrients. In Principles of Agronomy for Sustainable Agriculture; Springer: Cham, Switzerland, 2016; pp. 381–405. [Google Scholar]
- Manlay, R.J.; Feller, C.; Swift, M.J. Historical Evolution of Soil Organic Matter Concepts and Their Relationships with the Fertility and Sustainability of Cropping Systems. Agric. Ecosyst. Environ. 2007, 119, 217–233. [Google Scholar] [CrossRef]
- Reeves, D.W. The Role of Soil Organic Matter in Maintaining Soil Quality in Continuous Cropping Systems. Soil Tillage Res. 1997, 43, 131–167. [Google Scholar] [CrossRef]
- Turner, B.L.; Lambers, H.; Condron, L.M.; Cramer, M.I.D.; Leake, J.R.; Richardson, A.E.; Smith, S.E. Soil Microbial Biomass and the Fate of Phosphorus during Long-Term Ecosystem Development. Plant Soil 2013, 367, 225–234. [Google Scholar] [CrossRef]
- de Carvalho Mendes, I.; Fernandes, M.F.; Chaer, G.M.; Bueno dos Reis Junior, F. Biological Functioning of Brazilian Cerrado Soils under Different Vegetation Types. Plant Soil 2012, 359, 183–195. [Google Scholar] [CrossRef]
- Gonçalves, V.A.; Melo, C.A.D.; de Assis, I.R.; Ferreira, L.R.; Saraiva, D.T. Biomass and Soil Microbial Activity Under Different Planting Systems and Crop Successions. Rev. Ciências Agrárias 2019, 62, 1–8. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial Inoculation of Seed for Improved Crop Performance: Issues and Opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The Inoculation Method Affects Colonization and Performance of Bacterial Inoculant Strains in the Phytoremediation of Soil Contaminated with Diesel Oil. Int. J. Phytoremed. 2012, 14, 35–47. [Google Scholar] [CrossRef]
- Tefera, T.; Vidal, S. Effect of Inoculation Method and Plant Growth Medium on Endophytic Colonization of Sorghum by the Entomopathogenic Fungus Beauveria bassiana. BioControl 2009, 54, 663–669. [Google Scholar] [CrossRef] [Green Version]
- SBCS-Brazilian System of Soil Classification, 5th ed.; EMBRAPA SOLOS: Brasília, Brazil, 2018; p. 356.
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Assessment of the Nutritional Status of Plants. In Associação Brasileira Para Pesquisa da Potassa e Do Fosfato; Instituto da Potassa e Fosfato: Viçosa, Brazil, 1997; p. 201. [Google Scholar]
- Da Silva, E.E.; de Azevedo, P.H.S.; De-Polli, H. Determination of Soil Microbial Biomass Carbon (BMS-C). In Technical Communication 98; EMBRAPA: Seropédica, Brazil, 2007; pp. 1–6. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Adeniji, A.A.; Loots, D.T.; Babalola, O.O. Bacillus velezensis: Phylogeny, Useful Applications, and Avenues for Exploitation. Appl. Microbiol. Biotechnol. 2019, 103, 3669–3682. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Jiang, H.; Hao, J.J. Effects of Bacillus velezensis Strain BAC03 in Promoting Plant Growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Balderas-Ruíz, K.A.; Bustos, P.; Santamaria, R.I.; González, V.; Cristiano-Fajardo, S.A.; Barrera-Ortíz, S.; Mezo-Villalobos, M.; Aranda-Ocampo, S.; Guevara-García, Á.A.; Galindo, E.; et al. Bacillus Velezensis 83 a Bacterial Strain from Mango Phyllosphere, Useful for Biological Control and Plant Growth Promotion. AMB Express 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for Bio-Control Activities against Fungal Diseases and Potential Plant Growth Promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K. Molecules Bacillus velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Shi, H.; Heng, J.; Wang, D.; Bian, K. Antimicrobial, Plant Growth-Promoting and Genomic Properties of the Peanut Endophyte Bacillus velezensis LDO2. Microbiol. Res. 2019, 218, 41–48. [Google Scholar] [CrossRef]
- Li, C.; Shi, W.; Wu, D.; Tian, R.; Wang, B.; Lin, R.; Zhou, B.; Gao, Z. Biocontrol of Potato Common Scab by Brevibacillus laterosporus BL12 Is Related to the Reduction of Pathogen and Changes in Soil Bacterial Community. Biol. Control 2021, 153. [Google Scholar] [CrossRef]
- Nehra, V.; Saharan, B.S.; Choudhary, M. Evaluation of Brevibacillus brevis as a Potential Plant Growth Promoting Rhizobacteria for Cotton (Gossypium hirsutum) Crop. Springerplus 2016, 5, 948. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Wang, C.; Hou, X.; Xia, Z.; Ye, J.; Liu, K.; Liu, H.; Wang, J.; Guo, H.; Yu, X.; et al. Draft Genome Sequence of Brevibacillus brevis DZQ7, a Plant Growth-Promoting Rhizobacterium with Broad-Spectrum Antimicrobial Activity. Genome Announc. 2015, 3, e00831-15. [Google Scholar] [CrossRef] [Green Version]
- Wani, P.A.; Rafi, N.; Wani, U.; Biliki, A.H.; Khan, M.S.A. Simultaneous Bioremediation of Heavy Metals and Biodegradation of Hydrocarbons by Metal Resistant Brevibacillus parabrevis OZF5 Improves Plant Growth Promotion. Bioremediat. J. 2021, 1–12. [Google Scholar] [CrossRef]
- Ray, S.; Patel, N.; Amin, D. Brevibacillus. In Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020; pp. 149–167. [Google Scholar] [CrossRef]
- Chakra, P.S.; Kumar, P.G.V.; Swamy, C. Isolation and Biochemical Characterization of Plant Growth Promoting Bacteria from a Maize Crop Field. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1415–1422. [Google Scholar] [CrossRef]
- Lauthate, D.M.; Quisinski, A.; Hauschild, F.E.G.; Portela, E.F.M. Evaluation of the Efficiency of the Use of Phosphorus Solubilizer in the Development of Soybean Culture in São Luiz Gonzaga-RS. In 10° Siepex Salão Integrado de Ensino, Pesquisa e Extensão da Uergs; UERGS: Porto Alegre, Brazil, 2021; pp. 10–13. [Google Scholar]
- De Oliveira-Paiva, C.A.; Bini, D.; Marriel, I.E.; Gomes, E.A.; dos Santos, F.C.; de Sousa, S.M.; Alves, V.M.C.; Lana, U.G.D.P.; de Souza, F.F. Phosphate Solubilizing Bacteria-Based Inoculator in Corn and Soybean Crops (BiomaPhos®): Frequently Asked Questions and Good Inoculation Practices; Technical Communication, 252; Embrapa: Milho e Sorgo, Brazil, 2021. [Google Scholar]
- Kuzmicheva, Y.V.; Shaposhnikov, A.I.; Petrova, S.N.; Makarova, N.M.; Tychinskaya, I.L.; Puhalsky, J.V.; Parahin, N.V.; Tikhonovich, I.A.; Belimov, A.A. Variety Specific Relationships between Effects of Rhizobacteria on Root Exudation, Growth and Nutrient Uptake of Soybean. Plant Soil 2017, 419, 83–96. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Arshad, M. The Combined Application of Rhizobial Strains and Plant Growth Promoting Rhizobacteria Improves Growth and Productivity of Mung Bean (Vigna Radiata L.) under Salt-Stressed Conditions. Ann. Microbiol. 2012, 62, 1321–1330. [Google Scholar] [CrossRef]
- Pagnani, G.; Galieni, A.; Stagnari, F.; Pellegrini, M.; Del Gallo, M.; Pisante, M. Open Field Inoculation with PGPR as a Strategy to Manage Fertilization of Ancient Triticum Genotypes. Biol. Fertil. Soils 2020, 56, 111–124. [Google Scholar] [CrossRef]
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.K.; Kumar, A. Plant Growth Promoting Rhizobacteria: Application in Biofertilizers and Biocontrol of Phytopathogens. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Sawston, UK, 2019; pp. 41–66. [Google Scholar]
- Bai, Y.; Zhou, X.; Smith, D.L. Crop Ecology, Management and Quality: Enhanced Soybean Plant Growth Resulting from Coinoculation of Bacillus Strains with Bradyrhizobium japonicum. Crop Sci. 2003, 43, 1774–1781. [Google Scholar] [CrossRef]
- Parmar, N.; Dadarwal, K.R. Stimulation of Nitrogen Fixation and Induction of Flavonoid-like Compounds by Rhizobacteria. J. Appl. Microbiol. 1999, 86, 36–44. [Google Scholar] [CrossRef]
- Aung, T.T.; Buranabanyat, B.; Piromyou, P.; Longtonglang, A. Enhanced Soybean Biomass by Co-Inoculation of Bradyrhizobium japonicum and Plant Growth Promoting Rhizobacteria and Its Effects on Microbial Community Structures. African J. Microbiol. Res. 2013, 7, 3858–3873. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Berg, G. Synergistic Interactions between Bradyrhizobium japonicum and the Endophyte Stenotrophomonas rhizophila and Their Effects on Growth, and Nodulation of Soybean under Salt Stress. Plant Soil 2016, 405, 35–45. [Google Scholar] [CrossRef]
- Marinkovic, J.; Bjelic, D.; Tintor, B.; Djordjevic, V.B.; Balesevic-Tubic, S.; Djukic, V.; Ceran, M. Soil Microbial Properties under Different Management Systems in Soybean Production. Zb. Matice Srp. Za Prir. Nauk. 2020, 138, 41–49. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Fantin, L.H.; Koltun, A.; de Oliveira, A.L.M.; Nunes, M.P.B.A.; Canteri, M.G.; Gonçalves, L.S.A. Effects of Plant Growth-Promoting Rhizobacteria on Co-Inoculation with Bradyrhizobium in Soybean Crop: A Meta-Analysis of Studies from 1987 to 2018. PeerJ 2020, 8, e7905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masciarelli, O.; Llanes, A.; Luna, V. A New PGPR Co-Inoculated with Bradyrhizobium japonicum Enhances Soybean Nodulation. Microbiol. Res. 2014, 169, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Gupta, V.K. Soil Microbial Biomass: A Key Soil Driver in Management of Ecosystem Functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Bloom, A.P. Mineral Nutrition of Plants-Principles and Perspectives; Planta: Londrina, Brazil, 2006. [Google Scholar]
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global Meta-Analysis of the Relationship between Soil Organic Matter and Crop Yields. SOIL 2019, 5, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Kaschuk, G.; Alberton, O.; Hungria, M. Three Decades of Soil Microbial Biomass Studies in Brazilian Ecosystems: Lessons Learned about Soil Quality and Indications for Improving Sustainability. Soil Biol. Biochem. 2010, 42, 1–13. [Google Scholar] [CrossRef]
- Junior, F.B.D.R.; Mendes, I.D.C. Biomassa Microbiana Do Solo. Embrapa Cerrados. 2007, 1, 1517–5111. [Google Scholar]
- Jain, R.; Saxena, J.; Sharma, V. Differential Effects of Immobilized and Free Forms of Phosphate-Solubilizing Fungal Strains on the Growth and Phosphorus Uptake of Mung Bean Plants. Ann. Microbiol. 2014, 64, 1523–1534. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]

| Functional Traits | Brevibacillus sp. | Brevibacillus sp. | Bacillus velezensis | |
|---|---|---|---|---|
| (SAF9) | (SAF11) | (SAC36) | ||
| Solubilization of CaHPO4 | (mg L−1) | 12.2 | 10.5 | 10.8 |
| Solubilization of FePO4 | (mg L−1) | 8.4 | 7.4 | 6.6 |
| IAA synthesis | (µg mL−1) | 11.4 | 16.9 | 13.7 |
| GA3 synthesis | (µg mL−1) | 211.2 | 385.6 | 226.6 |
| Fusarium sp. Sclerotinia sclerotiorum | (R.I.%) | 33.6 | 44.2 | 37.2 |
| (R.I.%) | 51.8 | 38.2 | 37.2 | |
| Production of siderophores | − | − | + | |
| Field 1 | Field 2 | Field 5 | Field 1 | Field 2 | Field 5 | Field 1 | Field 2 | Field 5 | ||||
| Treatments | APH (cm) | CV (%) | APDM (g) | CV (%) | RDM (g) | CV (%) | ||||||
| Control | 54.48 ± 0.52 NSns | 59.50 ± 2.11 NSns | 59.21 ± 1.62 NSns | 7.03 | 5.25 ± 0.12 NSns | 5. 50 ± 0.43 NSns | 4.13 ± 0.36 Bns | 19.75 | 0. 87 ± 0.03 NSns | 0.87 ± 0.05 NSns | 0.92 ± 0.03 Bns | 10.77 |
| SAF9 | 58.50 ± 0.90 NSns | 60.85 ± 2.01 NSns | 61.69 ns ± 0.47 NSns | 4.62 | 5.50 ± 0.25 NSns | 6.13 ± 0.25 NSns | 6.25 ± 0.21 Ans | 8.59 | 0.91 ± 0.03 NSns | 1.08 ± 0.05 NSns | 1.05 ± 0.07 ABns | 15.19 |
| SAF11 | 52.85 ± 1.60 NSns | 58.85 ± 3.56 NSns | 63.35 ± 0.60 NSns | 7.09 | 4.75 ± 0.64 NSns | 5.50 ± 0.55 NSns | 5.25 ± 0.21 ABns | 15.93 | 0.81 ± 0.07 NSns | 0.97 ± 0.05 NSns | 0.99 ± 0.04 ABns | 11.79 |
| SAC36 | 52.70 ± 1.42 NSns | 65.95 ± 1.41 NSns | 59.70 ± 1.50 NSns | 5.05 | 5.50 ± 0.25 NSns | 5.50 ± 0.43 NSns | 6.25 ± 0.21 Ans | 11.79 | 1.02 ± 0.04 NSns | 1.08 ± 0.04 NSns | 1.23 ± 0.04 Ans | 9.40 |
| BiomaPhos | 54.30 ± 20 NSns | 66.45 ± 0.34 NSns | 59.65 ± 0.38 NSns | 1.42 | 5.50 ± 0.43 NSns | 6.50 ± 0.43 NSns | 5.50 ± 0.43 ABns | 17.14 | 0.84 ± 0.01 NSns | 1.08 ± 0.04 NSns | 1.07 ± 0.05 ABns | 11.31 |
| CV (%) | 5.06 | 8.05 | 4.04 | 16.88 | 17.20 | 12.86 | 11.92 | 11.54 | 11.99 | |||
| Field 1 | Field 2 | Field 5 | Field 1 | Field 2 | Field 5 | |||||||
| Treatments | NN | CV (%) | NDM (mg) | CV (%) | ||||||||
| Control | 14.40 ± 0.68 Cb | 19.29 ± 0.55 Ba | 5.30 ± 0.15 Bc | 7.93 | 32.50 ± 1.14 Cb | 70.00 ± 0.60 Ba | 33.00 ± 0.90 Cb | 20.14 | ||||
| SAF9 | 31.33 ± 0.64 Aa | 2.25 ± 1.26 ABa | 15.47 ± 0.39 Ab | 9.66 | 134.52 ± 2.27 ABa | 111.50 ± 0.80 Ab | 82.00 ± 0.10 ABc | 13.49 | ||||
| SAF11 | 25.65 ± 1.29 Ba | 28.90 ± 1.93 Aa | 14.85± 0.38 Ab | 13.71 | 110.95 ± 2.85 Ba | 120.00 ± 0.92 Aa | 71.00 ± 0.40 Bb | 16.42 | ||||
| SAC36 | 27.85 ± 0.80 ABa | 30.60 ± 1.18 Aa | 15.76 ± 0.18 Ab | 7.85 | 122.50 ± 2.31 ABa | 120.00 ± 0.14 Aa | 85.00 ± 0.22 Ab | 4.71 | ||||
| BiomaPhos | 27.94 ± 0.20 ABa | 27.43 ± 1.73 Aa | 16.03 ± 0.16 Ab | 11.81 | 169.40 ± 0.60 Aa | 111.00 ± 0.49 Ab | 85.00 ± 0.25 Ac | 8.65 | ||||
| CV (%) | 7.31 | 12.37 | 4.82 | 19.57 | 14.85 | 8.01 | ||||||
| Field 1 | Field 2 | Field 5 | Field 1 | Field 2 | Field 5 | |||
| Treatments | N Aerial Part (g kg−1) | CV (%) | P Aerial Part (g kg−1) | CV (%) | ||||
| Control | 35.50 ± 0.25 NSa | 35.00 ± 0.75 Ba | 33.50 ± 0.75 NSb | 4.09 | 3.10 ± 0.15 Bns | 3.00 ± 0.10 NSns | 3.00 ± 0.00 NSns | 7.75 |
| SAF9 | 35.50 ± 0.50 NSns | 35.50 ± 0.25 ABns | 35.00 ± 0.50 NSns | 2.84 | 3.20 ± 0.06 Aa | 2.90 ± 0.05 NSb | 2.90 ± 0.05 NSb | 5.44 |
| SAF11 | 34.50 ± 0.25 NSns | 34.00 ± 0.00 Cns | 35.00 ± 0.50 NSns | 2.16 | 2.90 ± 0.05 Bns | 3.10 ± 0.15 NSns | 3.10 ± 0.15 NSns | 9.59 |
| SAC36 | 36.00 ± 0.10 NSa | 37.00 ± 0.50 Aa | 34.50 ± 0.25 NSb | 5.76 | 3.20 ± 0.10 Ans | 3.10 ± 0.15 NSns | 3.20 ± 0.10 NSns | 8.68 |
| BiomaPhos | 36.00 ± 0.25 NSns | 35.00 ± 0.50 Bns | 35.50 ± 0.25 NSns | 2.29 | 3.10 ± 0.15 Bab | 3.40 ± 0.00 NSa | 2.90 ± 0.05 NSb | 6.73 |
| CV (%) | 3.71 | 2.93 | 3.37 | 8.97 | 7.71 | 7.35 | ||
| Field 1 | Field 2 | Field 5 | Field 1 | Field 2 | Field 5 | |||
| Treatments | N grain (g kg−1) | CV (%) | P grain (g kg−1) | CV (%) | ||||
| Control | 61.00 ± 1.00 Ba | 60.50 ± 0.25 Ba | 57.00 ± 0.25 Bb | 2.75 | 4.00 ± 0.05 NSns | 4.10 ± 0.00 Bns | 4.30 ± 0.15 NSns | 5.76 |
| SAF9 | 60.50 ± 0.40 Bb | 63.50 ± 0.25 Aa | 62.00 ± 0.10 Aab | 2.15 | 4.40 ± 0.00 NSa | 4.50 ± 0.05 Aa | 4.10 ± 0.05 NSb | 2.18 |
| SAF11 | 62.00 ± 0.30 Ba | 62.00 ± 0.20 ABa | 60.50 ± 0.12 ABb | 3.11 | 4.00 ± 0.00 NSb | 4.20 ± 0.10 ABb | 4.50 ± 0.05 NSa | 3.52 |
| SAC36 | 64.00 ± 0.20 Aa | 61.00 ± 0.10 Bb | 61.00 ± 0.10 ABb | 3.10 | 4.00 ± 0.02 NSns | 3.90 ± 0.15 Cns | 4.30 ± 0.15 NSns | 9.56 |
| BiomaPhos | 61.00 ± 0.20 Bb | 63.50 ± 0.20 Aa | 61.50 ± 0.50 ABab | 2.01 | 4.50 ± 0.00 NSns | 4.40 ± 0.00 ABns | 4.50 ± 0.05 NSns | 2.11 |
| CV (%) | 2.35 | 2.18 | 3.68 | 5.40 | 5.78 | 5.32 | ||
| Field 1 | Field 2 | Field 5 | Field 1 | Field 2 | Field 5 | |||
| Treatments | Mass of One Thousand Grains (g) | CV (%) | Productivity (kg ha−1) | CV (%) | ||||
| Control | 164 ± 1.14 Bns | 155 ± 8.28 NSns | 174 ± 2.13 Bns | 7.61 | 4133.93 ± 38 66 NSa | 3440.63 ± 30.80 Cb | 4242.50 ± 103.30 Ba | 6.68 |
| SAF9 | 169 ± 2.27 ABa | 155 ± 4.86 NSb | 180 ± 0.8 Aa | 4.78 | 4337.50 ± 68.80 NSa | 4007.29 ± 99.71 Ab | 4526.25 ± 28.08 Aa | 6.33 |
| SAF11 | 164 ± 2.85 Ba | 153 ± 3.20 NSb | 174 ± 2.10 Ba | 4.35 | 4133.93 ± 109.13 NSa | 3645.83 ± 99.21 Bb | 4348.75 ± 48.30 Ba | 9.38 |
| SAC36 | 165 ± 2.31 ABa | 156 ± 2.24 NSb | 178 ± 0.50 ABa | 2.41 | 4206.25 ± 27.58 NSa | 4070.83 ± 33.34 Ab | 4286.87 ± 24.40 Ba | 10.89 |
| BiomaPhos | 177 ± 0.61 Aa | 153 ± 3.73 NSb | 180 ± 0.80 Aa | 3.06 | 4436.07 ± 141.61 NSa | 3779.17 ± 58.35 Bb | 4328.13 ± 81.73 Ba | 4.16 |
| CV (%) | 2.77 | 7.37 | 3.24 | 4.80 | 8.08 | 7.74 | ||
| Field 1 | Field 2 | Field 5 | ||||||
| Treatments | BC (mg Kg−1) | CV (%) | ||||||
| Control | 103.96 ±4.61 Bb | 71.39 ± 6.76 Bc | 162.41 ± 21.86 Ba | 16.73 | ||||
| SAF9 | 142.87 ± 15.02 Bb | 59.43 ± 4.46 Bc | 247.06 ± 20.04 Aa | 15.93 | ||||
| SAF11 | 119.89 ±15.15 Ba | 56.66 ± 9.26 Bb | 122.65 ± 10.54 Ba | 23.04 | ||||
| SAC36 | 186.25 ± 22.53 Aa | 147.98 ± 4.53 Aab | 123.26 ± 19.78 ABb | 12.48 | ||||
| BiomaPhos | 110.66 ± 5.33 Bns | 114.63 ± 4.65 Ans | 124.65 ± 27.18 ABns | 18.07 | ||||
| CV (%) | 10.84 | 12.40 | 9.47 | |||||
| Field 3 | Field 4 | Field 3 | Field 4 | Field 3 | Field 4 | ||||
| Treatments | APH (cm) | Test t | APDM (g) | Test t | RDM (g) | Test t | |||
| Control | 52.34 ± 1.99 B | 64.10 ± 1.14 NS | t = −4.42, p = 0.007 | 7.16 ± 0.30 B | 6.19 ± 0.35 C | t = 34.42, p < 0.001 | 1.05 ± 0.03 B | 1.15 ± 0.04 B | t = 1.43, p = 0.205 |
| SAF9 | 64.01 ± 0.45 A | 68.33 ± 1.14 N | t = −3.02, p = 0.039 | 7.11 ± 0.37 B | 8.25 ± 0.26 B | t = −2.15, p = 0.079 | 1.11 ± 0.04 B | 1.36 ± 0.06 B | t = −2.94, p = 0.030 |
| SAF11 | 63.60 ± 3.81 A | 70.00 ± 2.21 NS | t = −1.26, p = 0.266 | 9.24 ± 0.18 A | 12.57 ± 0.41 A | t = 6.34, p = 0.002 | 1. 46 ± 0.04 A | 1.83 ± 0.01 A | t = −6.77, p = 0.002 |
| SAC36 | 58.40 ± 1.34 AB | 65.95 ± 1.30 NS | t = −3.48, p = 0.013 | 9.43 ± 0.02 A | 13.88 ± 0.09 A | t = 37.74, p < 0.001 | 1. 64 ± 0.04 A | 2.00 ± 0.07 A | t = −3.66, p = 0.014 |
| BiomaPhos | 58.01 ± 1.25 AB | 64.82 ± 1.40 NS | t = −3.12, p = 0.020 | 8.94 ± 0.13 A | 12.98 ± 0.17 A | t = 16.36, p < 0.001 | 1. 44 ± 0.02 A | 1.75 ± 0.04 A | t = −5.09, p = 0.005 |
| CV (%) | 8.20 | 6.99 | 6.60 | 6.12 | 6.57 | 7.47 | |||
| Field 3 | Field 4 | Field 3 | Field 4 | ||||||
| Treatments | NN | Test t | NDM (mg) | Test t | |||||
| Control | 13.23 ± 0.52 B | 22.16 ± 0.28C | t = −13.01, p < 0.001 | 30.00 ± 2.50 C | 43.00 ± 0.80 D | t = 21.76, p < 0.001 | |||
| SAF9 | 31.76 ± 0.38 B | 55.15 ± 2.21 AB | t = −7.38, p = 0.004 | 87.00 ± 5.50 B | 102.00 ± 2.46 B | t = 71.13, p < 0.001 | |||
| SAF11 | 32.79 ± 0.30 B | 43.54 ± 7.02 B | t = −1.32, p = 0.276 | 83.00 ± 2.34 B | 79.00 ± 8.60 C | t = 3.35, p = 0.043 | |||
| SAC36 | 31.55 ± 0.23 B | 40.95 ± 0.54 B | t = −13.73, p < 0.001 | 83.00 ± 3.35 B | 92.00 ± 1.99 BC | t = 115.51, p < 0.001 | |||
| BiomaPhos | 37.00 ± 0.88 A | 68.00 ± 2.50 A | t = −10.09, p < 0.001 | 107.00 ± 2.53 A | 146.00 ± 3.54 A | t = 36.24, p < 0.001 | |||
| CV (%) | 4.10 | 17.86 | 10.29 | 11.11 | |||||
| Field 3 | Field 4 | Field 3 | Field 4 | |||
| Treatments | N Aerial Part (g kg−1) | Test t | P Aerial Part (g kg−1) | Test t | ||
| Control | 34.00 ± 0.00 C | 35.30 ± 0.21 NS | t = −5.00, p = 0.015 | 3.00 ± 0.01 BC | 2.90 ± 0.05 B | t = 1.73, p = 0.187 |
| SAF9 | 35.50 ± 0.25 B | 35.00 ± 0.50 NS | t = 0.77, p = 0.478 | 2.90 ± 0.05 BC | 3.30 ± 0.12 A | t = 2.17, p = 0.097 |
| SAF11 | 35.00 ± 0.50 BC | 35.00 ± 0.50 NS | t = 0.00, p = 0.875 | 3.30 ± 0.12 A | 3.30 ± 0.08 A | t = 0.27, p = 0.792 |
| SAC36 | 38.00 ± 0.00 A | 35.50 ± 0.25 NS | t = 8.66, p = 0.003 | 3.20 ± 0.04 AB | 2.90 ± 0.05 B | t = −3.27, p = 0.017 |
| BiomaPhos | 35.50 ± 0.25 B | 36.00 ± 0.43 NS | t = −1.73, p = 0.146 | 2.80 ± 0.02 C | 3.00 ± 0.12 B | t = 1.73, p = 0.174 |
| CV (%) | 1.78 | 2.60 | 5.07 | 7.09 | ||
| Field 3 | Field 4 | Field 3 | Field 4 | |||
| Treatments | N grain (g kg−1) | Test t | P grain (g kg−1) | Test t | ||
| Control | 58.00 ± 0.01 NS | 55.80 ± 0.64 B | t = −3.00, p = 0.057 | 4.00 ± 0.01 B | 4.40 ± 0.01 AB | t = 0.10, p = 0.920 |
| SAF9 | 62.000 ± 0.08 NS | 62.00 ± 0.86 A | t = 0.00, p = 0.848 | 4.60 ± 0.04 A | 4.60 ± 0.04 AB | t = 0.00, p = 0.965 |
| SAF11 | 59.80 ± 0.21 NS | 62.00 ± 0.86 A | t = 2.18, p = 0.107 | 4.50 ± 0.12 A | 4.30 ± 0.15 AB | t = −0.65, p = 0.537 |
| SAC36 | 61.50 ± 1.20 NS | 60.50 ± 1.75 AB | t = −0.39, p = 0.706 | 4.00 ± 0.01 B | 4.70 ± 0.01 A | t = 27.00, p < 0.001 |
| BiomaPhos | 60.50 ± 1.25 NS | 60.00 ± 0.86 AB | t = −0.28, p = 0.786 | 4.30 ± 0.05 AB | 4.00 ± 0.02 B | t = −1.26, p = 0.287 |
| CV (%) | 3.44 | 4.12 | 3.52 | 5.57 | ||
| Field 3 | Field 4 | Field 3 | Field 4 | |||
| Treatments | Mass of One Thousand Grains (g) | Test t | Productivity (kg ha−1) | Test t | ||
| Control | 167 ± 4.09 AB | 172 ± 1.73 B | t = −0. 97, p = 0.384 | 3138.19 ± 100.36 B | 3721.18 ± 205.77 B | t = −2. 20, p = 0.086 |
| SAF9 | 164 ± 2.28 B | 177 ± 3.78 B | t = −2.65, p = 0.043 | 3652.08 ± 168.81 AB | 4561.46 ± 99.55 A | t = −4.01, p = 0.010 |
| SAF11 | 175 ± 3.54 AB | 167 ± 1.29 B | t = 1.89, p = 0.135 | 4299.66 ± 205.85 AB | 4561.11 ± 265.70 A | t = −0.67, p = 0.527 |
| SAC36 | 182 ± 3.32 A | 174 ± 1.24 B | t = 1.86, p = 0.139 | 4522.92 ± 145.93 A | 4843.58 ± 49.99 A | t = −1.80, p = 0.152 |
| BiomaPhos | 177 ± 0.82 AB | 197 ± 3.77 A | t = −4.36, p = 0.018 | 4879.41 ± 31.53 A | 5347.40 ± 48.20 A | t = −7. 03, p < 0.001 |
| CV (%) | 4.10 | 3.43 | 8.10 | 8.01 | ||
| Field 3 | Field 4 | |||||
| Treatments | MBC (mg Kg−1) | Test t | ||||
| Control | 155.01 ± 9.96 B | 154.20 ± 13.18 NS | t = −0.03, p = 0.970 | |||
| SAF9 | 238.58 ± 13.54 A | 154.48 ± 12.59 NS | t = −3.71, p = 0.020 | |||
| SAF11 | 247.62 ± 9.93 A | 192.18 ± 5.97 NS | t = −3.90, p = 0.025 | |||
| SAC36 | 237.10 ± 7.72 A | 204.67 ± 10.77 NS | t = −1.99, p = 0.123 | |||
| BiomaPhos | 298.43 ± 16.47 A | 204.40 ± 7.84 NS | t = −4. 18, p = 0.027 | |||
| CV (%) | 11.41 | 13.40 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiuza, D.A.F.; Vitorino, L.C.; Souchie, E.L.; Neto, M.R.; Bessa, L.A.; Silva, C.F.d.; Trombela, N.T. Effect of Rhizobacteria Inoculation via Soil and Seeds on Glycine max L. Plants Grown on Soils with Different Cropping History. Microorganisms 2022, 10, 691. https://doi.org/10.3390/microorganisms10040691
Fiuza DAF, Vitorino LC, Souchie EL, Neto MR, Bessa LA, Silva CFd, Trombela NT. Effect of Rhizobacteria Inoculation via Soil and Seeds on Glycine max L. Plants Grown on Soils with Different Cropping History. Microorganisms. 2022; 10(4):691. https://doi.org/10.3390/microorganisms10040691
Chicago/Turabian StyleFiuza, Denise Almeida Fonseca, Luciana Cristina Vitorino, Edson Luiz Souchie, Moacir Ribeiro Neto, Layara Alexandre Bessa, Cintia Faria da Silva, and Natasha Taline Trombela. 2022. "Effect of Rhizobacteria Inoculation via Soil and Seeds on Glycine max L. Plants Grown on Soils with Different Cropping History" Microorganisms 10, no. 4: 691. https://doi.org/10.3390/microorganisms10040691
APA StyleFiuza, D. A. F., Vitorino, L. C., Souchie, E. L., Neto, M. R., Bessa, L. A., Silva, C. F. d., & Trombela, N. T. (2022). Effect of Rhizobacteria Inoculation via Soil and Seeds on Glycine max L. Plants Grown on Soils with Different Cropping History. Microorganisms, 10(4), 691. https://doi.org/10.3390/microorganisms10040691







