Antiseptic Effects and Biosafety of a Controlled-Flow Electrolyzed Acid Solution Involve Electrochemical Properties, Rather than Free Radical Presence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Minimum Inhibitory Concentration Assay
2.3. In Vitro Eradication of Single-Species Biofilms
2.4. Cell Cultures
2.5. Cytotoxicity Assessment on Eukaryotic Cells
2.6. Superoxide Ion Activity Assessment
2.7. Human-Infected Subacute and Chronic Wounds Treated with CFEAS: A Pilot Trial
2.8. Determination of the Bacterial Colony Forming Units per Gram of Tissue
2.9. Statistical Analysis
3. Results
3.1. CFEAS Exhibits Bactericidal and Antibiofilm Effects
3.2. Fibroblasts Are Less Sensitive to CFEAS Induced Cytotoxicity than Macrophages
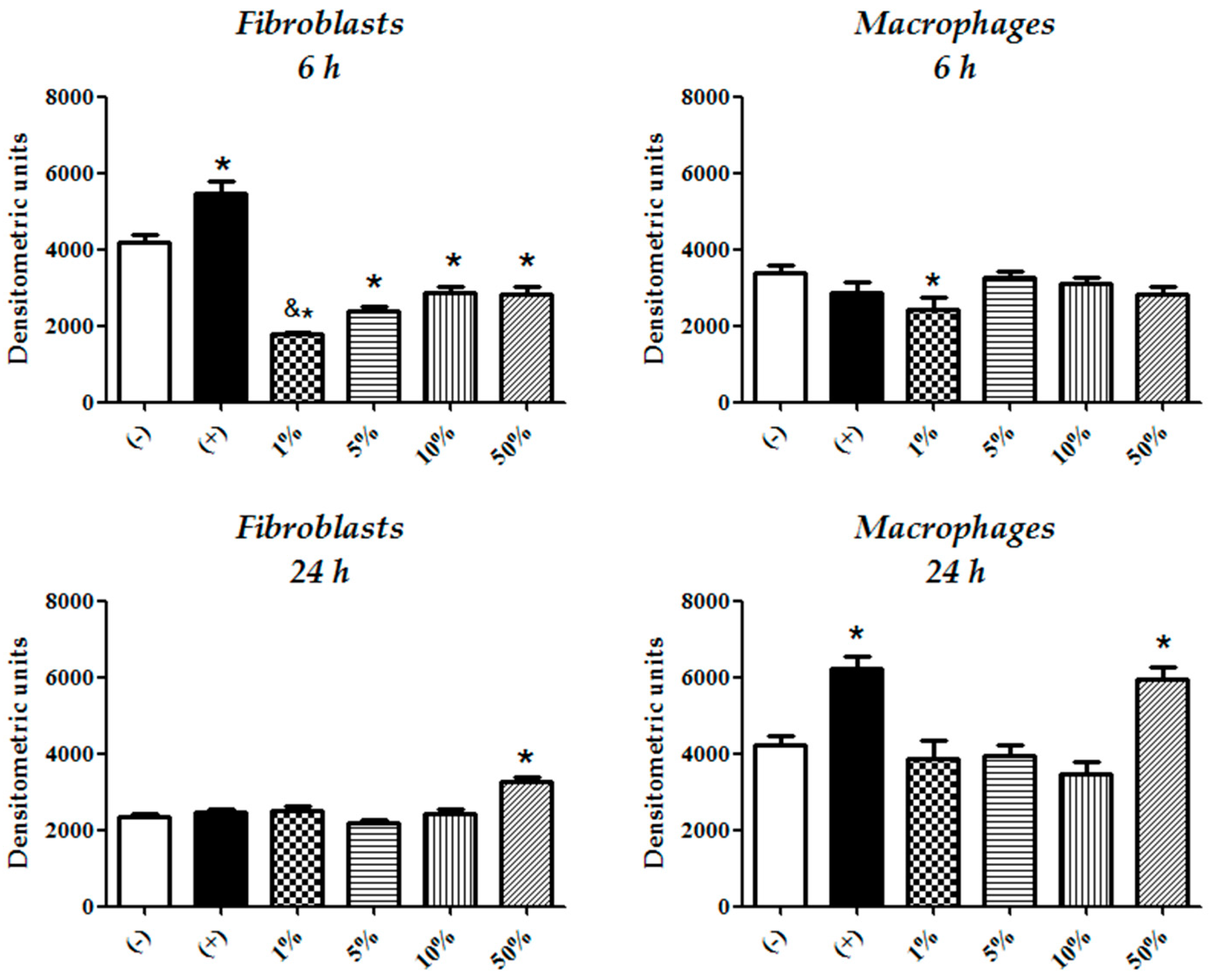
3.3. Fibroblasts Compensate More Efficiently CFEAS Derived Oxidizing Radicals than Macrophages
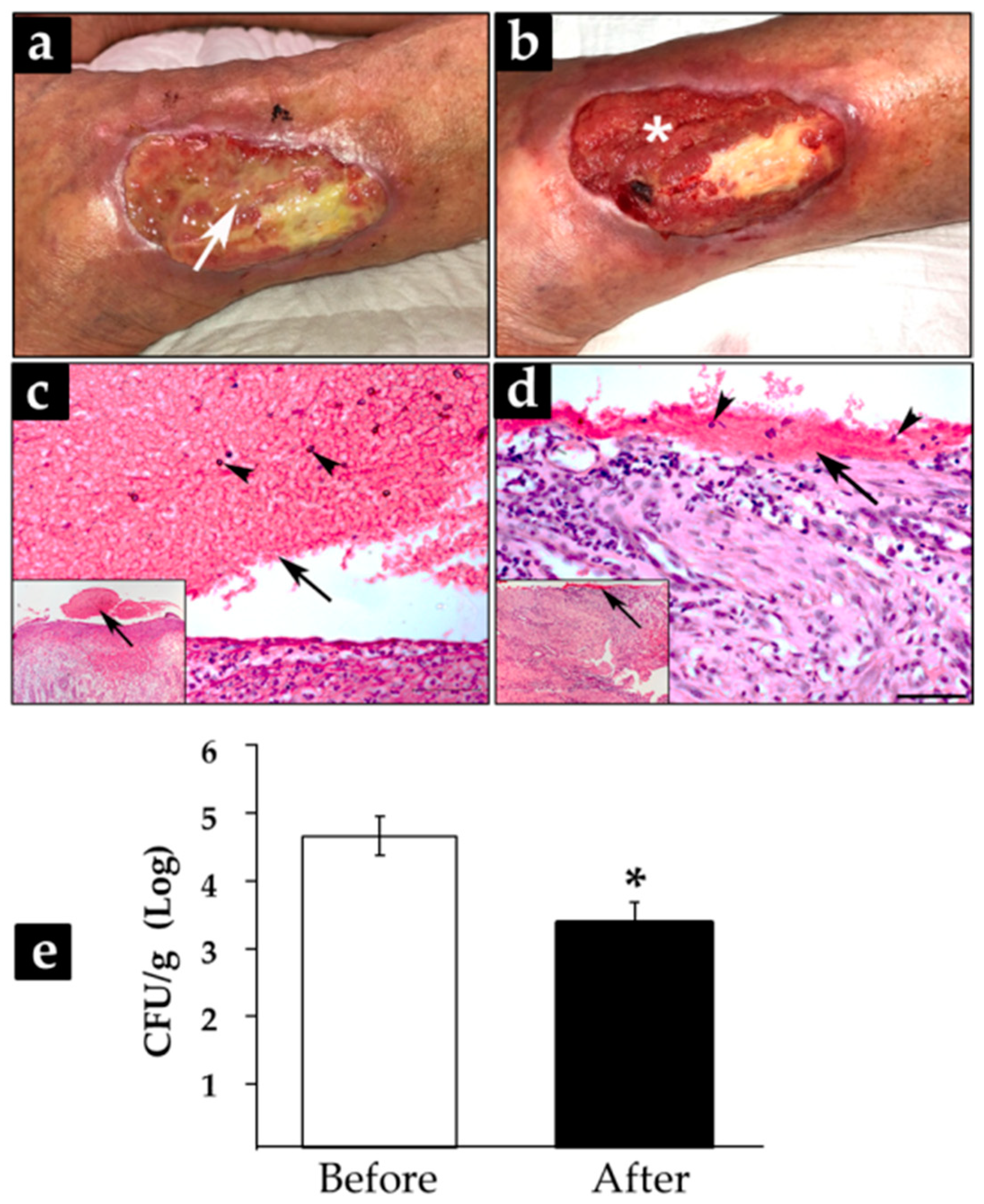
3.4. Treatment with CFEAS of Subacute and Chronic-Infected Wounds Reduced Significantly Biofilm Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, G.S.; Barillo, D.J.; Mozingo, D.W.; Chin, A.G.; Wound Bed Advisory Board Members. Wound bed preparation and a brief history of TIME. Int. Wound J. 2004, 1, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.B.; Bartolo, P.J.D.S. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Peña, S.; Hidalgo-González, C.; Robson, M.C.; Krötzsch, E. In vitro microbicidal, anti-biofilm and cytotoxic effects of different commercial antiseptics. Int. Wound J. 2016, 14, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.I.; Sulaiman, S.; Ab Aziz, N.; Taip, F.S.; Sobri, S.; Nor-Khaizura, M.A.R. Electrolyzed water as a green cleaner: Chemical and physical characterization at different electrolysing parameters. Food Res. 2018, 2, 512–519. [Google Scholar] [CrossRef]
- Sipahi, H.; Reis, R.; Dinc, O.; Kavaz, T.; Dimoglo, A.; Aydın, A. In vitro biocompatibility study approaches to evaluate the safety profile of electrolyzed water for skin and eye. Hum. Exp. Toxicol. 2019, 38, 1314–1326. [Google Scholar] [CrossRef]
- Thorn, R.; Lee, S.W.H.; Robinson, G.M.; Greenman, J.; Reynolds, D.M. Electrochemically activated solutions: Evidence for antimicrobial efficacy and applications in healthcare environments. Eur. J. Clin. Microbiol. 2011, 31, 641–653. [Google Scholar] [CrossRef]
- Cai, L.-L.; Hu, H.-J.; Lu, Q.; Wang, H.-H.; Xu, X.-L.; Zhou, G.-H.; Kang, Z.-L.; Ma, H.-J. Morphophysiological responses of detached and adhered biofilms of Pseudomonas fluorescens to acidic electrolyzed water. Food Microbiol. 2019, 82, 89–98. [Google Scholar] [CrossRef]
- Mokudai, T.; Nakamura, K.; Kanno, T.; Niwano, Y. Presence of Hydrogen Peroxide, a Source of Hydroxyl Radicals, in Acid Electrolyzed Water. PLoS ONE 2012, 7, e46392. [Google Scholar] [CrossRef] [Green Version]
- Mokudai, T.; Kanno, T.; Niwano, Y. Involvement of reactive oxygen species in the cytotoxic effect of acid-electrolyzed water. J. Toxicol. Sci. 2015, 40, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, L.; Lee, J.; Pascall, M.A. Transmission Electron Microscopic Analysis Showing Structural Changes to Bacterial Cells Treated with Electrolyzed Water and an Acidic Sanitizer. J. Food Sci. 2012, 77, M182–M187. [Google Scholar] [CrossRef]
- Yahagi, N.; Kono, M.; Kitahara, M.; Ohmura, A.; Sumita, O.; Hashimoto, T.; Hori, K.; Chen, N.-J.; Woodson, P.; Kubota, S.; et al. Effect of Electrolyzed Water on Wound Healing. Artif. Organs 2000, 24, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Okubo, K.; Urakami, H.; Tamura, A. Cytotoxicity and microbicidal activity of electrolyzed strong acid water and acidic hypochlorite solution under isotonic conditions. Kansenshogaku Zasshi 1999, 73, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Makino, T.; Suzuki, S.; Hasegawa, M.; Maeda, N.; Arai, T. Microbicidal and cytotoxic effects of functional water in vitro. Quintessence Int. 2010, 41, e166–e172. [Google Scholar] [PubMed]
- Nuñez-Montalvo, J.M.; Laboratorios Naturales EJ SA de CV. Apparatus for the Production of an Acid Super Oxidation Solution and an Alkaline Solution with Independent Flows with ORP from Aqueous Solutions. U.S. Patent 2,017,268,117, 21 September 2017. [Google Scholar]
- Martínez-Nova, A.; Sánchez-Rodríguez, R.; Escamilla-Martínez, E.; Gómez-Martín, B. The effect of controlled flux electrolyzed acid solution (SAEFC) protocol in the recovery of phenol total matricectomy: A randomized controlled trial. Rev. Esp. Pod. 2019, 30, 10–14. [Google Scholar] [CrossRef]
- CLSI. Performance for Antimicrobial Susceptibility Testing. Twenty-Second Informational Supplement; CLSI Document M100-S22; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Lima, E.; Flores, J.; Cruz, A.S.; Leyva-Gómez, G.; Krötzsch, E. Controlled release of ferulic acid from a hybrid hydrotalcite and its application as an antioxidant for human fibroblasts. Microporous Mesoporous Mater. 2013, 181, 1–7. [Google Scholar] [CrossRef]
- Sekiya, S.; Ohmori, K.; Harii, K. Treatment of Infectious Skin Defects or Ulcers with Electrolyzed Strong Acid Aqueous Solution. Artif. Organs 2008, 21, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yanik, K.; Karadag, A.; Unal, N.; Odabasi, H.; Esen, S.; Gunaydin, M. An investigation into the in-vitro effectiveness of electrolyzed water against various microorganisms. Int. J. Clin. Exp. Med. 2015, 8, 11463–11469. [Google Scholar]
- Takesue, Y.; Takahashi, Y.; Ichiki, K.; Nakajima, K.; Tsuchida, T.; Uchino, M.; Ikeuchi, H. Application of an Electrolyzed Strongly Acidic Aqueous Solution before Wound Closure in Colorectal Surgery. Dis. Colon Rectum 2011, 54, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Hati, S.; Mandal, S.; Minz, P.S.; Vij, S.; Khetra, Y.; Singh, B.P.; Yadav, D. Electrolyzed Oxidized Water (EOW): Non-Thermal Approach for Decontamination of Food Borne Microorganisms in Food Industry. Food Nutr. Sci. 2012, 3, 760–768. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Hung, Y.-C.; Brackett, R.E. Roles of Oxidation–Reduction Potential in Electrolyzed Oxidizing and Chemically Modified Water for the Inactivation of Food-Related Pathogens. J. Food Prot. 2000, 63, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.-S.W.; Lu, Y.-F.; Hsu, S.-Y. Effects of electrolysis time and electric potential on chlorine generation of electrolyzed deep ocean water. J. Food Drug Anal. 2017, 25, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.B.; Wood, M.L.; Baldwin, I.L. The Relation of Oxidation-Reduction Potential to the Growth of an Aerobic Microorganism. J. Bacteriol. 1935, 30, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Han, S.; Wang, Y.; Zhao, G.; Qian, W.; Dong, J. Detection of Redox State Evolution during Wound Healing Process Based on a Redox-Sensitive Wound Dressing. Anal. Chem. 2018, 90, 6660–6665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, A.; Tam, P.K.H.; Lam, K.S.L.; Huang, B.; Liang, Y.; Lee, I.-K.; Wu, D.; Wang, Y. Upregulation of UCP2 by Adiponectin: The Involvement of Mitochondrial Superoxide and hnRNP K. PLoS ONE 2012, 7, e32349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, R.; Sipahi, H.; Dinc, O.; Kavaz, T.; Charehsaz, M.; Dimoglo, A.; Aydın, A. Toxicity, mutagenicity and stability assessment of simply produced electrolyzed water as a wound healing agent in vitro. Hum. Exp. Toxicol. 2020, 40, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Harada, G.; Nakamichi, N.; Kabayama, S.; Teruya, K.; Fugetsu, B.; Gong, W.; Sakata, I.; Shirahata, S. Electrochemically reduced water exerts superior reactive oxygen species scavenging activity in HT1080 cells than the equivalent level of hydrogen-dissolved water. PLoS ONE 2017, 12, e0171192. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. H2O2 inhibits the growth of human pulmonary fibroblast cells by inducing cell death, GSH depletion and G1 phase arrest. Mol. Med. Rep. 2013, 7, 1235–1240. [Google Scholar] [CrossRef] [Green Version]
- Tochigi, M.; Inoue, T.; Suzuki-Karasaki, M.; Ochiai, T.; Ra, C.; Suzuki-Karasaki, Y. Hydrogen peroxide induces cell death in human TRAIL-resistant melanoma through intracellular superoxide generation. Int. J. Oncol. 2013, 42, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Ruddock, L.W.; Klappa, P. Oxidative stress: Protein folding with a novel redox switch. Curr. Biol. 1999, 9, R400–R402. [Google Scholar] [CrossRef] [Green Version]
- Go, Y.-M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta BBA Gen. Subj. 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.-P.; Peng, Y.-B.; Zhang, Y.-F.; Wang, Y.; Yu, W.-R.; Yao, M.; Fu, X.-J. Reactive Oxygen Species Mediated Prostaglandin E2 Contributes to Acute Response of Epithelial Injury. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcos-Tejedor, F.; Sánchez-Rodríguez, R.; Mayordomo, R.; Martínez-Nova, A. The bacteriostatic effect of controlled-flux electrolyzed acidic solution on healthy hallucal skin. J. Tissue Viability 2019, 29, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Daliri, E.B.-M.; Oh, D.-H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]



| Strain | CFEAS (%) | PHMB (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95 | 50 | 25 | 12.5 | 6.2 | 3.2 | 1.5 | 0.7 | 0.3 | 1.5 | 0.7 | 0.3 | |
| Gram-positive | ||||||||||||
| S. aureus | − | + | + | + | + | + | + | + | + | − | − | − |
| E. faecalis | − | + | + | + | + | + | + | + | + | − | − | − |
| Gram-negative | ||||||||||||
| E. coli | − | + | + | + | + | + | + | + | + | − | − | − |
| K. pneumoniae | − | + | + | + | + | + | + | + | + | − | − | − |
| E. cloacae | − | + | + | + | + | + | + | + | + | − | − | − |
| A. baumannii | − | + | + | + | + | + | + | + | + | − | − | − |
| P. aeruginosa | − | + | + | + | + | + | + | + | + | − | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Wrooman, A.; Ortega-Peña, S.; Salgado, R.M.; Sandoval-Cuevas, B.; Krötzsch, E. Antiseptic Effects and Biosafety of a Controlled-Flow Electrolyzed Acid Solution Involve Electrochemical Properties, Rather than Free Radical Presence. Microorganisms 2022, 10, 745. https://doi.org/10.3390/microorganisms10040745
Cabrera-Wrooman A, Ortega-Peña S, Salgado RM, Sandoval-Cuevas B, Krötzsch E. Antiseptic Effects and Biosafety of a Controlled-Flow Electrolyzed Acid Solution Involve Electrochemical Properties, Rather than Free Radical Presence. Microorganisms. 2022; 10(4):745. https://doi.org/10.3390/microorganisms10040745
Chicago/Turabian StyleCabrera-Wrooman, Alejandro, Silvestre Ortega-Peña, Rosa M. Salgado, Belinda Sandoval-Cuevas, and Edgar Krötzsch. 2022. "Antiseptic Effects and Biosafety of a Controlled-Flow Electrolyzed Acid Solution Involve Electrochemical Properties, Rather than Free Radical Presence" Microorganisms 10, no. 4: 745. https://doi.org/10.3390/microorganisms10040745







