Photosynthesis, Respiration, and Growth of Five Benthic Diatom Strains as a Function of Intermixing Processes of Coastal Peatlands with the Baltic Sea
Abstract
:1. Introduction
2. Materials and Methods
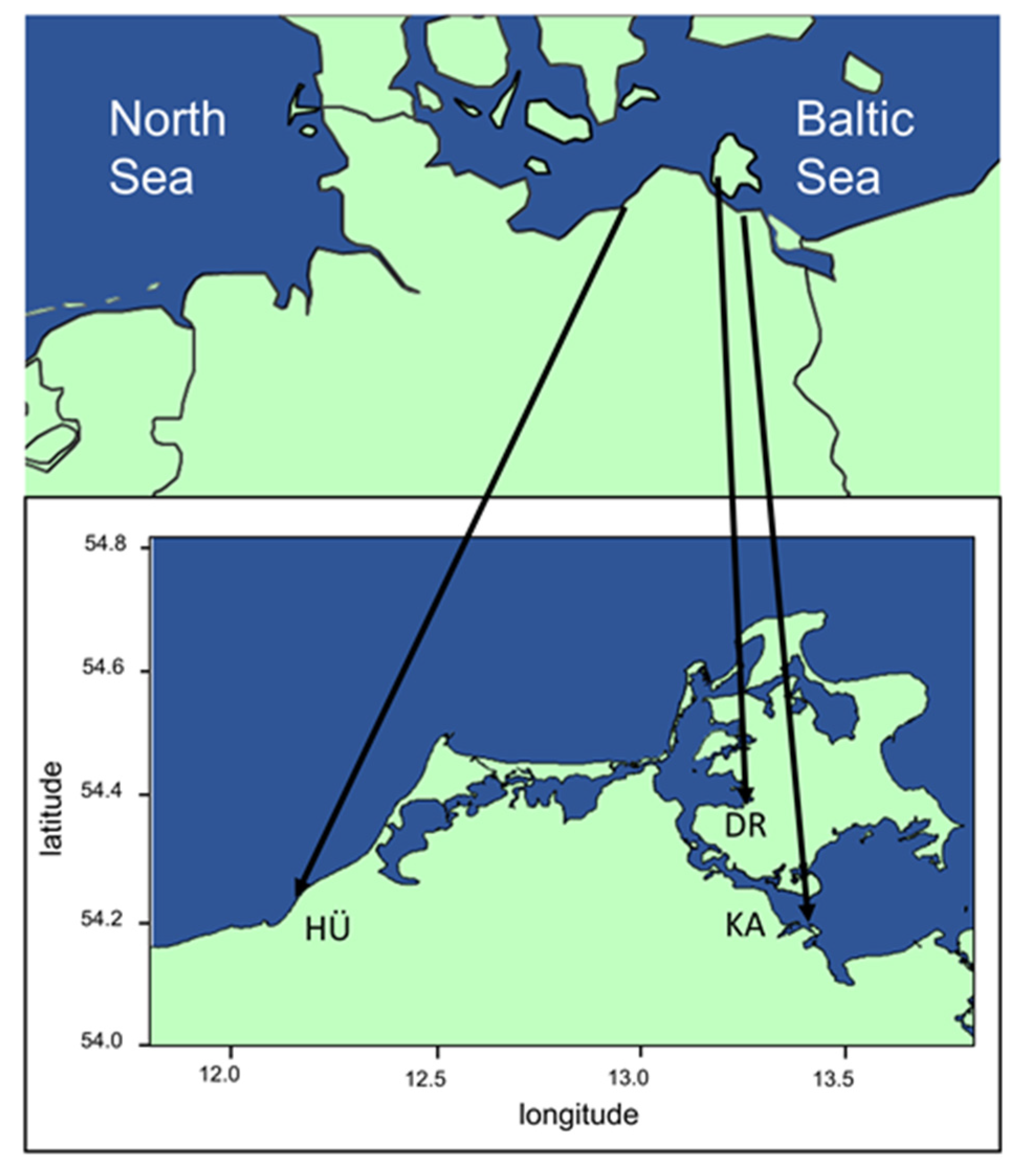
2.1. Study Site
2.2. Culture Establishment and Culture Conditions
2.3. Specific Growth Rates
2.4. Light Response Curves (PI-Curves)
2.5. Temperature Dependence of Photosynthesis and Respiration
2.6. Statistical Analysis
3. Results
3.1. Species Identification
3.2. Growth
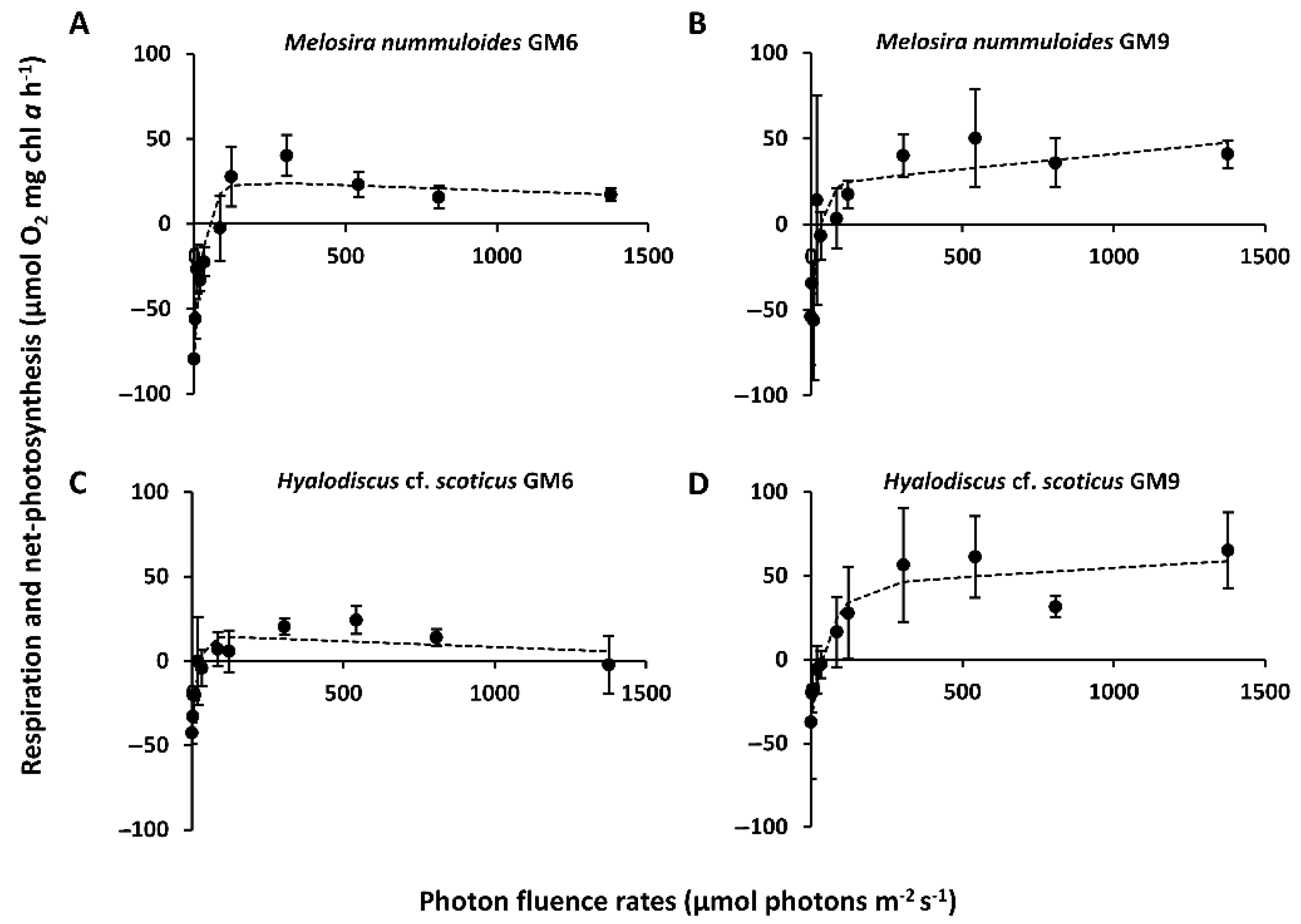
3.3. PI-Curves
3.4. Effect of Temperature on Photosynthesis and Respiration
4. Discussion
4.1. Growth
4.2. Light
4.3. Temperature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HELCOM. Climate Change in the Baltic Sea Area. In HELCOM Thematic Assessment in 2007, Baltic Sea Environment Proceedings; Helsinki Commission: Helsinki, Finland, 2007; Volume 111, p. 10. [Google Scholar]
- Lehmann, A.; Krauss, W.; Hinrichsen, H.H. Effects of remote and local atmospheric forcing on circulation and upwelling in the Baltic Sea. Tellus A Dyn. Meteorol. Oceanogr. 2002, 54, 299–316. [Google Scholar] [CrossRef]
- Gräwe, U.; Klingbeil, K.; Kelln, J.; Dangendorf, S. Decomposing mean sea level rise in a semi-enclosed basin, the Baltic Sea. J. Clim. 2019, 32, 3089–3108. [Google Scholar] [CrossRef]
- PSMSL (Permanent Service for Mean Sea Level), 7.12.2020, Relative Sea Level Trends. Available online: https://www.psmsl.org/products/trends (accessed on 3 February 2022).
- Johansson, M.M.; Kahma, K.K.; Boman, H.; Launiainen, J. Scenarios for sea level on the Finnish coast. Boreal Environ. Res. 2004, 9, 153–166. [Google Scholar]
- Jurasinski, G.; Janssen, M.; Voss, M.; Böttcher, M.E.; Brede, M.; Burchard, H.; Forster, S.; Gosch, L.; Gräwe, U.; Gründling-Pfaff, S.; et al. Understanding the coastal ecocline: Assessing sea-land-interactions at non-tidal, low-lying coasts through interdisciplinary research. Front. Mar. Sci. 2018, 5, 342. [Google Scholar] [CrossRef] [Green Version]
- Parish, F.; Sirin, A.; Charman, D.; Joosten, H.; Minaeva, T.; Silvius, M. Assessment on Peatlands, Biodiversity and Climate Change; Global Environment Centre, Kuala Lumpur and Wetlands International: Wageningen, The Netherlands, 2008. [Google Scholar]
- Vasander, H.; Tuittila, E.-S.; Lode, E.; Lundin, L.; Ilomets, M.; Sallantaus, T.; Heikkilä, R.; Pitkänen, M.-L.; Laine, J. Status and restoration of peatlands in northern Europe. Wetl. Ecol. Manag. 2003, 11, 51–63. [Google Scholar] [CrossRef]
- BSH (Bundesamt für Seeschifffahrt und Hydrographie), 2.1.2019, Sturmflut vom 02.01.2019. Available online: https://www.bsh.de/DE/THEMEN/Wasserstand_und_Gezeiten/Sturmfluten/_Anlagen/Downloads/Ostsee_Sturmflut_20190102.pdf;jsessionid=ED722161633F3DCAF23AAA4E4CFC0EA0.live11314?__blob=publicationFile&v=5 (accessed on 3 February 2022).
- Seiberling, S.; Stock, M.; Thapa, P.P. Renaturierung von Salzgrasland bzw. Salzwiesen der Küsten. In Renaturierung von Ökosystemen in Mitteleuropa; Zerbe, S., Wiegleb, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 183–208. [Google Scholar] [CrossRef]
- Janssen, M.; Böttcher, M.; Brede, M.; Burchard, H.; Forster, S.; Karsten, U.; Leinweber, P.; Lennartz, B.; Rehder, G.; Schubert, H.; et al. The Baltic TRANSCOAST approach—Investigating shallow coasts as terrestrial-marine interface of water and matter fluxes. EarthArXiv 2019. Preprint. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahoon, L.B. The role of benthic microalgae in neritic ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 1999, 37, 47–86. [Google Scholar]
- Cartaxana, P.; Ruivo, M.; Hubas, C.; Davidson, I.; Serôdio, J.; Jesus, B. Physiological versus behavioral photoprotection in intertidal epipelic and epipsammic benthic diatom communities. J. Exp. Mar. Biol. Ecol. 2011, 405, 120–127. [Google Scholar] [CrossRef]
- Blommaert, L.; Lavaud, J.; Vyverman, W.; Sabbe, K. Behavioural versus physiological photoprotection in epipelic and epipsammic benthic diatoms. Eur. J. Phycol. 2018, 53, 146–155. [Google Scholar] [CrossRef]
- Beninger, P.G.; Cuadrado, D.; van de Koppel, J. Sedimentary and biological patterns on mudflats. In Mudflat Ecology; Beninger, P., Ed.; Springer: Cham, Switzerland, 2018; Volume 7. [Google Scholar]
- Bricaud, A.; Morel, A.; Prieur, L. Absorption by dissolved organic matter of the sea (yellow substance) in the UV and visible domains. Limnol. Oceanogr. 1981, 26, 43–53. [Google Scholar] [CrossRef]
- Woelfel, J.; Schoknecht, A.; Schaub, I.; Enke, N.; Schuhmann, R.; Karsten, U. Growth and photosynthesis characteristics of three benthic diatoms from the brackish southern Baltic Sea in relation to varying environmental conditions. Phycologia 2014, 53, 639–651. [Google Scholar] [CrossRef] [Green Version]
- Cohn, S.A.; Warnick, L.; Timmerman, B. Photophobic Responses of Diatoms–Motility and Inter-Species Modulation. Diatom Glid. Motil. 2021, 2021, 111–134. [Google Scholar]
- Lippert, H.; Weigelt, R.; Glaser, K.; Krauss, R.; Bastrop, R.; Karsten, U. Teredo navalis in the baltic sea: Larval dynamics of an invasive wood-boring bivalve at the edge of its distribution. Front. Mar. Sci. 2017, 4, 331. [Google Scholar] [CrossRef] [Green Version]
- Prelle, L.R.; Graiff, A.; Gründling-Pfaff, S.; Sommer, V.; Kuriyama, K.; Karsten, U. Photosynthesis and respiration of Baltic Sea benthic diatoms to changing environmental conditions and growth responses of selected species as affected by an adjacent peatland (Hütelmoor). Front. Microbiol. 2019, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Prelle, L.R.; Albrecht, M.; Karsten, U.; Damer, P.; Giese, T.; Jähns, J.; Müller, S.; Schulz, L.; Viertel, L.; Glaser, K. Ecophysiological and Cell Biological Traits of Benthic Diatoms from Coastal Wetlands of the Southern Baltic Sea. Front. Microbiol. 2021, 12, 796. [Google Scholar] [CrossRef]
- Mortain-Bertrand, A.; Descolas-Gros, C.; Jupin, H. Growth, photosynthesis and carbon metabolism in the temperate marine diatom Skeletonema costatum adapted to low temperature and low photon-flux density. Mar. Biol. 1988, 100, 135–141. [Google Scholar] [CrossRef]
- Admiraal, W. The ecology of estuarine sediment inhabiting diatoms. Prog. Phycol. Res. 1984, 3, 269–322. [Google Scholar]
- Risgaard-Petersen, N.; Rysgaard, S.; Nielsen, L.P.; Revsbech, N.P. Diurnal variation of denitrification and nitrification in sediments colonized by benthic microphytes. Limnol. Oceanogr. 1994, 39, 573–579. [Google Scholar] [CrossRef]
- Worrall, F.; Clay, G.D.; Moody, C.S.; Burt, T.P.; Rose, R. The effective oxidation state of a peatland. J. Geophys. Res. Biogeosci. 2016, 121, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Villanova, V.; Fortunato, A.E.; Singh, D.; Bo, D.D.; Conte, M.; Obata, T.; Jouhet, J.; Fernie, A.R.; Marechal, E.; Falciatore, A.; et al. Investigating mixotrophic metabolism in the model diatom Phaeodactylum tricornutum. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 1728. [Google Scholar] [CrossRef] [Green Version]
- Guillard, R.R.L.; Ryther, J.H. Studies or marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Abarca, N.; Jahn, R.; Zimmermann, J.; Enke, N. Does the cosmopolitan diatom gomphonema parvulum (Kützing) Kützing have a biogeography? PLoS ONE 2014, 9, e086885. [Google Scholar] [CrossRef]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom flora of marine coasts I. In Iconographia Diatomologica 7: 1–925, 219 pls with 4504 figs; Gantner Verlag: Pforzheim, Germany, 2000. [Google Scholar]
- Karsten, U.; Klimant, I.; Holst, G. A new in vivo fluorimetric technique to measure growth of adhering phototrophic microorganisms. Appl. Environ. Microbiol. 1996, 62, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustavs, L.; Schumann, R.; Eggert, A.; Karsten, U. In vivo growth fluorometry: Accuracy and limits of microalgal growth rate measurements in ecophysiological investigations. Aquat. Microb. Ecol. 2009, 55, 95–104. [Google Scholar] [CrossRef] [Green Version]
- HELCOM. Annex C-4. Phytoplankton Chlorophyll a. HELCOM Combine; HELCOM: Helsinki, Finland, 2015; pp. 257–263. Available online: https://helcom.fi/media/publications/Manual-for-Marine-Monitoring-in-the-COMBINE-Programme-of-HELCOM.pdf (accessed on 3 February 2022).
- Walsby, A.E. Numerical integration of phytoplankton photosynthesis through time and depth in a water column. New Phytol. 1997, 136, 189–209. [Google Scholar] [CrossRef]
- Karsten, U.; Lütz, C.; Holzinger, A. Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Charophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. Phycologia 2010, 46, 1187–1197. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A. An equation for modelling the temperature response of plants using only the cardinal temperatures. Ann. Bot. 1999, 84, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Admiraal, W. Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar. Biol. 1977, 39, 1–9. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Res. 2012, 27, 65–73. [Google Scholar] [CrossRef]
- Spilling, K.; Ylöstalo, P.; Simis, S.; Seppälä, J. Interaction effects of light, temperature and nutrient limitations (N, P and Si) on growth, stoichiometry and photosynthetic parameters of the cold-water diatom Chaetoceros wighamii. PLoS ONE 2015, 10, e0126308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Balzano, S.; Sarno, D.; Kooistra, W.H. Effects of salinity on the growth rate and morphology of ten Skeletonema strains. J. Plankton Res. 2011, 33, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Trobajo, R.; Rovira, L.; Mann, D.G.; Cox, E.J. Effects of salinity on growth and on valve morphology of five estuarine diatoms. Phycol. Res. 2011, 59, 83–90. [Google Scholar] [CrossRef]
- Crawford, R.M. The organic component of the cell wall of the marine diatom Melosira nummuloides (Dillw.) C. Ag. Br. Phycol. J. 1973, 8, 257–266. [Google Scholar] [CrossRef]
- Beardall, J.; Young, E.; Roberts, S. Approaches for determining phytoplankton nutrient limitation. Aquat. Sci. 2001, 63, 44–69. [Google Scholar] [CrossRef]
- Brinkmann, N.; Friedl, T.; Mohr, K.I. Diatoms. In Encyclopedia of Geobiology; Reitner, H.J., Thiel, V., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 231. [Google Scholar]
- Racasa, E.D.; Lennartz, B.; Toro, M.; Janssen, M. Submarine Groundwater Discharge from Non-Tidal Coastal Peatlands Along the Baltic Sea. Front. Earth Sci. 2021, 9, 625. [Google Scholar] [CrossRef]
- Fransner, F.; Nycander, J.; Mörth, C.-M.; Humborg, C.; Meier, H.E.M.; Hordoir, R.; Gustafsson, E.; Deutsch, B. Tracing terrestrial DOC in the Baltic Sea-A 3-D model study. Glob. Biogeochem. Cycles 2016, 30, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Marella, T.K.; Bhattacharjya, R.; Tiwari, A. Impact of organic carbon acquisition on growth and functional biomolecule production in diatoms. Microb. Cell Factories 2021, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taraldsvik, M.; Myklestad, S.M. The effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum. Eur. J. Phycol. 2000, 35, 189–194. [Google Scholar] [CrossRef]
- Thomson, A.; Manoylov, K. Benthic Diatom Motility in Estuarine Mudflats and Coastal Sands of the Atlantic Ocean Coast. Int. J. Mar. Biol. Res. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Blommaert, L.; Chafai, L.; Bailleul, B. The fine-tuning of NPQ in diatoms relies on the regulation of both xanthophyll cycle enzymes. Sci. Rep. 2021, 11, 12750. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Karsten, U.; Herburger, K.; Holzinger, A. Dehydration, temperature, and light tolerance in members of the aeroterrestrial green algal genus Interfilum (Streptophyta) from biogeographically different temperate soils. J. Phycol. 2014, 50, 804–816. [Google Scholar] [CrossRef]
- Pierangelini, M.; Glaser, K.; Mikhailyuk, T.; Karsten, U.; Holzinger, A. Light and dehydration but not temperature drive photosynthetic adaptations of basal streptophytes (Hormidiella, Streptosarcina and Streptofilum) living in terrestrial habitats. Microb. Ecol. 2019, 77, 380–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleich, S.J.; Plough, L.V.; Gilbert, P.M. Photosynthetic efficiency and nutrient physiology of the diatom Thalassiosira pseudonana at three growth temperatures. Mar. Biol. 2020, 167, 124. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Karsten, U.; Herburger, K.; Holzinger, A. Living in biological soil crust communities of African deserts—Physiological traits of green algal Klebsormidium species (Streptophyta) to cope with desiccation, light and temperature gradients. J. Plant Physiol. 2016, 194, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Tcherkez, G.G.B.; Farquhar, G.D.; Andrews, J.T. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. USA 2006, 103, 7246–7251. [Google Scholar] [CrossRef] [Green Version]






| Medium | Water Base | Salinity (SA) | Nutrient Addition | pH |
|---|---|---|---|---|
| GM1 | Baltic Sea | 15 | f/2 + metasilicate | 7.6 |
| GM2 | Baltic Sea | 15 | - | 7.4 |
| GM3 | Baltic Sea | 15 | 219 µM N + 27 µM P | 7.9 |
| GM4 | 49/50 Baltic Sea + 1/50 peatland | 15 | 219 µM N + 27 µM P (in the proportional peatland water) | 7.6 |
| GM5 | 9/10 Baltic Sea + 1/10 peatland | 14.5 | 219 µM N + 27 µM P (in the proportional peatland water) | 7.4 |
| GM6 | ½ Baltic Sea + ½ Peatland | 8 | 219 µM N + 27 µM P (in the proportional peatland water) | 7.2 |
| GM7 | Peatland | 0.3 | 219 µM N + 27 µM P (in the proportional peatland water) | 7.8 |
| GM8 | Peatland | 0.3 | f/2 + metasilicate | 7.4 |
| GM9 | Peatland | 15 | 219 µM N + 27 µM P (in the proportional peatland water) | 7.1 |
| GM10 | Peatland | 15 | f/2 + metasilicate | 7.3 |
| Isolates | NPPmax (µmol O2 mg−1 chl a h−1) | Respiration (µmol O2 mg−1 chl a h−1) | α (µmol O2 mg−1 chl a h−1) (µmol Photons m−2 s−1)−1 | β (µmol O2 mg−1 chl a h−1) (µmol Photons m−2 s−1)−1 | Ik (µmol Photons m−2 s−1) | Ic (µmol Photons m−2 s−1) | NPPmax: Respiration |
|---|---|---|---|---|---|---|---|
| Melosira nummuloides GM6 | 23.31 ± 14.55 a | −79.45 ± 21.32 cd | 3.23 ± 2.57 a | −0.1 ± 0.01 a | 32.13 ± 21.40 ac | 46.19 ± 25.69 a | 0.31 ± 0.12 d |
| Melosira nummuloides GM9 | 23.47 ± 18.07 a | −53.90 ± 4.81 bd | 2.78 ± 2.38 a | 0.02 ± 0.01 a | 27.86 ± 22.03 abc | 32.58 ± 19.60 a | 0.44 ± 0.32 acd |
| Hyalodiscus cf. scoticus GM6 | 14.12 ± 11.22 a | −42.62 ± 4.66 ab | 2.89 ± 1.26 a | −0.01 ± 0.02 a | 19.61 ± 7.33 a | 26.99 ± 26.75 a | 0.33 ± 0.30 cd |
| Hyalodiscus cf. scoticus GM9 | 43.75 ± 27.66 a | −37.29 ± 5.83 ab | 1.32 ± 0.81 a | 0.01 ± 0.01 a | 61.39 ± 16.49 abc | 37.28 ± 22.63 a | 1.17 ± 0.54 acd |
| Planothidium sp. (st. 2) GM6 | 23.88 ± 13.26 a | −27.89 ± 4.70 ab | 2.07 ± 0.27 a | 0.00 ± 0.1 a | 25.07 ± 8.18 a | 19.33 ± 1.94 a | 0.86 ± 0.34 acd |
| Planothidium sp. (st. 2) GM9 | 79.58 ± 19.51 b | −30.48 ± 0.27 ab | 1.62 ± 0.47 a | 0.01 ± 0.00 a | 68.03 ± 7.06 abc | 21.95 ± 5.58 a | 2.61 ± 0.60 e |
| Nitzschia filiformis GM6 | 38.54 ± 8.42 a | −40.68 ± 11.32 ab | 1.78 ± 1.22 a | 0.01 ± 0.01 a | 44.50 ± 40.43 abc | 31.61 ± 26.22 a | 0.95 ± 022 acd |
| Nitzschia filiformis GM9 | 46.62 ± 23.12 ab | −30.32 ± 8.12 ab | 1.89 ± 1.12 a | −0.01 ± 0.02 a | 40.63 ± 9.98 abc | 20.33 ± 13.04 a | 1.54 ± 0.51 ab |
| Planothidium sp. (st. 1) GM6 | 29.60 ± 1.99 a | −28.95 ± 2.79 ab | 1.88 ± 0.42 a | −0.01 ± 0.00 a | 31.07 ± 7.68 ac | 21.10 ± 4.13 a | 1.02 ± 0.14 acd |
| Planothidium sp. (st. 1) GM9 | 44.01 ± 3.90 a | −23.86 ± 2.34 ab | 2.07 ± 0.73 a | −0.01 ± 0.00 a | 32.77 ± 6.05 a | 14.19 ± 1.97 a | 1.84 ± 0.20 abc |
| Melosira nummuloides GM6 | Melosira nummuloides GM9 | Hyalodiscus cf. scoticus GM6 | Hyalodiscus cf. scoticus GM9 | |||
|---|---|---|---|---|---|---|
| Photosynthesis | maximal photosynthetic rate | 144.5 | 125.9 | 74.1 | 148.8 | |
| optimum temperature (°C) | 9.0 | 6.3 | 4.2 | 18.2 | ||
| maximum temperature (°C) | 34.4 | 33.8 | 22.5 | 36.4 | ||
| residual sum-square | 24,794 | 28,054 | 7083 | 158,658 | ||
| temperature (°C) range for | optimal photosynthesis (80% photosynthetic rate) | 2.5–18.8 | 1.1–16.1 | 0.7–10.6 | 10.1–26.4 | |
| photosynthesis (20% photosynthetic rate) | 0.0–31.3 | 0.0–29.9 | 0.0–19.9 | 1.9–34.5 | ||
| Respiration | maximal respirational rate | −100.9 | −64.5 | −90.6 | −42.5 | |
| optimum temperature (°C) | 34.6 | 36.6 | 33.3 | 33.7 | ||
| maximum temperature (°C) | 44.6 | 47.7 | 43.3 | 45.0 | ||
| residual sum-square | 5415 | 4764 | 7797 | 1704 | ||
| Temperature (°C) range for | optimal respiration (80% respirational rate) | 28.1–39.6 | 29.4–42.1 | 26.8–38.3 | 26.6–39.3 | |
| respiration (20% respirational rate) | 16.4–45.8 | 16.4–46.7 | 15.0–42.4 | 14.1–43.9 | ||
| Planothidium sp. (st. 2) GM6 | Planothidium sp. (st. 2) GM9 | Nitzschia filiformis GM6 | Nitzschia filiformis GM9 | Planothidium sp. (st. 1) GM6 | Planothidium sp. (st. 1) GM9 | |||
|---|---|---|---|---|---|---|---|---|
| Photosynthesis | maximal photosynthetic rate | 57.1 | 76.1 | 87.9 | 138.6 | 93.1 | 113.3 | |
| optimum temperature (°C) | 20.6 | 20.1 | 22.1 | 18.4 | 25.7 | 26.1 | ||
| maximum temperature (°C) | 40.7 | 42.4 | 45.3 | 42.2 | 39.7 | 40.7 | ||
| residual sum-square | 2699 | 7837 | 30,816 | 95,423 | 2833 | 4240 | ||
| temperature (°C) range for | optimal photosynthesis (80% photosynthetic rate) | 11.5–29.6 | 10.6–29.9 | 11.9–32.4 | 8.9–28.7 | 17.9–32.4 | 18.0–33.0 | |
| photosynthesis (20% photosynthetic rate) | 2.3–38.6 | 1.7–40.0 | 2.1–42.9 | 1.1–39.5 | 6.7–38.4 | 6.6–39.3 | ||
| Respiration | maximal respirational rate | −45.5 | −35.9 | −75.7 | −49.8 | −47.1 | −44.4 | |
| optimum temperature (°C) | 33.0 | 34.1 | 35.4 | 33.8 | 35.4 | 34.2 | ||
| maximum temperature (°C) | 45.4 | 47.1 | 45.4 | 44.6 | 49.1 | 45.5 | ||
| residual sum-square | 1143 | 1062 | 3299 | 670 | 610 | 429 | ||
| temperature (°C) range for | optimal respiration (80% respirational rate) | 25.3–39.1 | 26.1–40.4 | 28.8–40.4 | 26.9–39.1 | 27.0–42.1 | 26.9–39.8 | |
| respiration (20% respirational rate) | 12.5–44.3 | 12.7–45.9 | 14.6–43.4 | 14.6–43.6 | 132.−47.8 | 14.3–44.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prelle, L.R.; Karsten, U. Photosynthesis, Respiration, and Growth of Five Benthic Diatom Strains as a Function of Intermixing Processes of Coastal Peatlands with the Baltic Sea. Microorganisms 2022, 10, 749. https://doi.org/10.3390/microorganisms10040749
Prelle LR, Karsten U. Photosynthesis, Respiration, and Growth of Five Benthic Diatom Strains as a Function of Intermixing Processes of Coastal Peatlands with the Baltic Sea. Microorganisms. 2022; 10(4):749. https://doi.org/10.3390/microorganisms10040749
Chicago/Turabian StylePrelle, Lara R., and Ulf Karsten. 2022. "Photosynthesis, Respiration, and Growth of Five Benthic Diatom Strains as a Function of Intermixing Processes of Coastal Peatlands with the Baltic Sea" Microorganisms 10, no. 4: 749. https://doi.org/10.3390/microorganisms10040749
APA StylePrelle, L. R., & Karsten, U. (2022). Photosynthesis, Respiration, and Growth of Five Benthic Diatom Strains as a Function of Intermixing Processes of Coastal Peatlands with the Baltic Sea. Microorganisms, 10(4), 749. https://doi.org/10.3390/microorganisms10040749






