Staphylococcus schweitzeri—An Emerging One Health Pathogen?
Abstract
:1. Introduction
2. The S. aureus Complex
3. Prevalence of S. schweitzeri
| Source | Prevalence (n/Total n (%)) | Country | Year | Setting | Antimicrobial Resistance | Ref. |
|---|---|---|---|---|---|---|
| Fomites | 2/239 (0.8%) | Nigeria | 2015–2016 | Contamination | None detected | [31] |
| Fruit bat (Eidolon helvum) | 11/250 (4.4%) | Nigeria | 2015–2016 | Fecal colonization | None detected | [8] |
| Fruit bat (Rousettus aegyptiacus) | 2/55 (4%) | Gabon | 2015 | Colonization | None detected | [32] |
| Human | 1/500 (0.2%) | Gabon | 2009 | Colonization | None detected | [33] |
| Human | 2/1014 (0.2%) | Gabon | 2012–2013 | Colonization | Not stated | [30] |
| Monkeys | 1/12 (8%) | Côte d’Ivoire | 2012 | Colonization | None detected | [9] |
| Monkeys | 17/71 (24%) | Gabon | 2010–2013 | Colonization | None detected | [9] |
| Monkeys | 6/10 (60%) | DR Congo | 2011 | Colonization | None detected | [9] |
| Wildlife | 0/2855 (0%) | Austria, Germany, Sweden | Not stated | Colonization and infection | Not applicable since no S. schweitzeri was found | [34] |
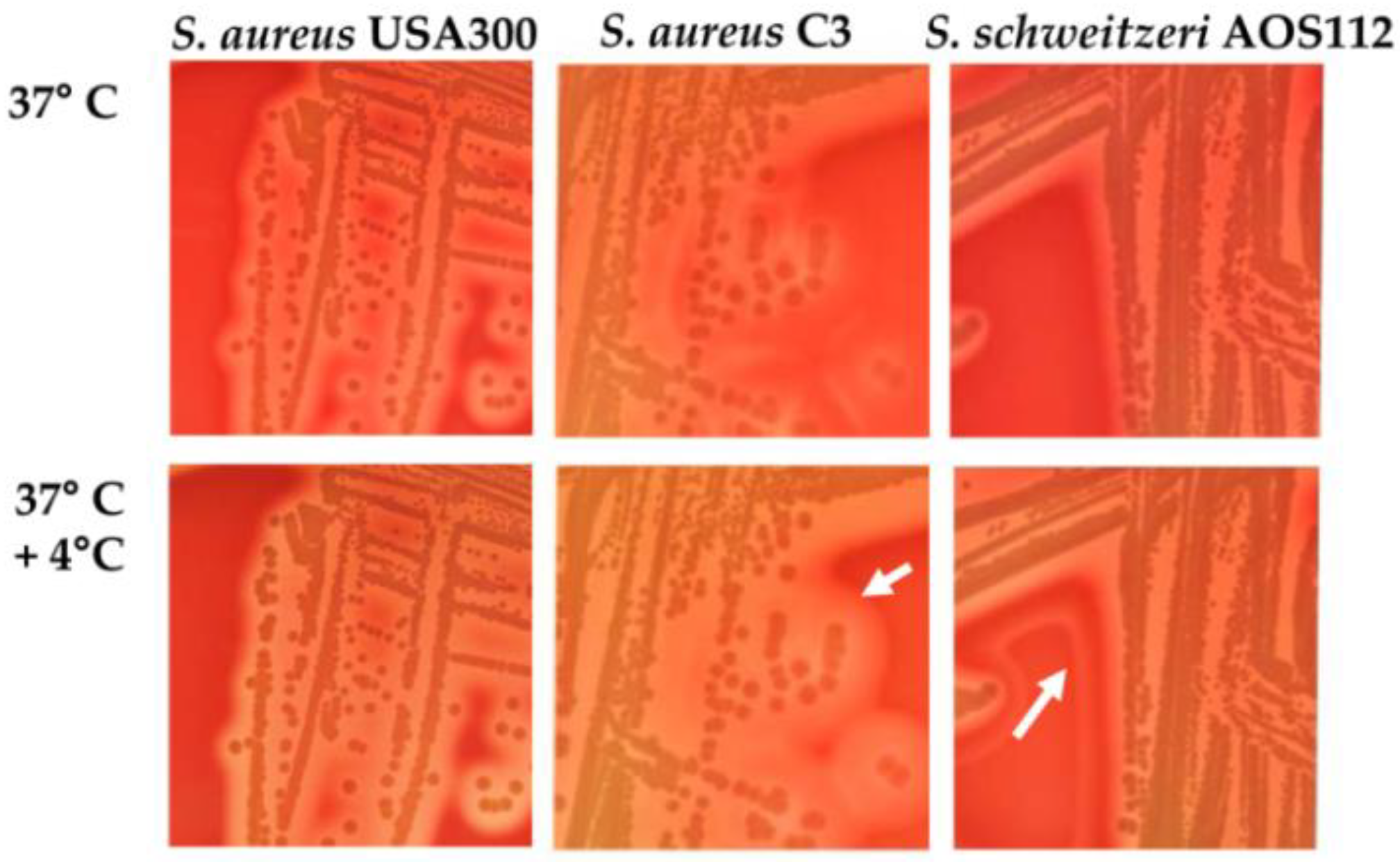
4. Identification
4.1. PCR
4.2. Sequencing
5. Antibiotic Resistance
6. Pathogenicity
7. Animal Adaption
8. Knowledge Gaps and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullahi, I.N.; Fernandez-Fernandez, R.; Juarez-Fernandez, G.; Martinez-Alvarez, S.; Eguizabal, P.; Zarazaga, M.; Lozano, C.; Torres, C. Wild Animals Are Reservoirs and Sentinels of Staphylococcus aureus and MRSA Clones: A Problem with “One Health” Concern. Antibiotics 2021, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Benic, M.; Macesic, N.; Cvetnic, L.; Habrun, B.; Cvetnic, Z.; Turk, R.; Duricic, D.; Lojkic, M.; Dobranic, V.; Valpotic, H.; et al. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Becker, K.; Schaumburg, F.; Kearns, A.; Larsen, A.R.; Lindsay, J.A.; Skov, R.L.; Westh, H. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: A position paper of members of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS). Clin. Microbiol. Infect. 2019, 25, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: The non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.L.; Octavia, S.; Lai, D.; Lin, R.T.P.; Teo, J.W.P. Staphylococcus singaporensis sp. nov., a new member of the Staphylococcus aureus complex, isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 2021, 71, 005067. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.H.J.; Strepis, N.; Zandijk, W.H.A.; Bexkens, M.L.; Bode, L.G.M.; Klaassen, C.H.W. Characterization of Staphylococcus roterodami sp. nov., a new species within the Staphylococcus aureus complex isolated from a human foot infection. Int. J. Syst. Evol. Microbiol. 2021, 71, 004996. [Google Scholar] [CrossRef]
- Ng, J.W.; Holt, D.C.; Lilliebridge, R.A.; Stephens, A.J.; Huygens, F.; Tong, S.Y.; Currie, B.J.; Giffard, P.M. Phylogenetically distinct Staphylococcus aureus lineage prevalent among indigenous communities in northern Australia. J. Clin. Microbiol. 2009, 47, 2295–2300. [Google Scholar] [CrossRef] [Green Version]
- Olatimehin, A.; Shittu, A.O.; Onwugamba, F.C.; Mellmann, A.; Becker, K.; Schaumburg, F. Staphylococcus aureus Complex in the Straw-Colored Fruit Bat (Eidolon helvum) in Nigeria. Front. Microbiol. 2018, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Schaumburg, F.; Pauly, M.; Anoh, E.; Mossoun, A.; Wiersma, L.; Schubert, G.; Flammen, A.; Alabi, A.S.; Muyembe-Tamfum, J.J.; Grobusch, M.P.; et al. Staphylococcus aureus complex from animals and humans in three remote African regions. Clin. Microbiol. Infect. 2015, 21, 345-e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, A.; Frobose, N.J.; Mellmann, A.; Alabi, A.S.; Schaumburg, F.; Niemann, S. An in vitro study on Staphylococcus schweitzeri virulence. Sci. Rep. 2021, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Cvetnic, L.; Samardzija, M.; Duvnjak, S.; Habrun, B.; Cvetnic, M.; Jaki Tkalec, V.; Duricic, D.; Benic, M. Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia. Microorganisms 2021, 9, 725. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, M.; Dougall, A.; Holt, D.; Huygens, F.; Oppedisano, F.; Giffard, P.M.; Inman-Bamber, J.; Stephens, A.J.; Towers, R.; Carapetis, J.R.; et al. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote aboriginal communities. J. Clin. Microbiol. 2006, 44, 3720–3727. [Google Scholar] [CrossRef] [Green Version]
- Argudin, M.A.; Dodemont, M.; Vandendriessche, S.; Rottiers, S.; Tribes, C.; Roisin, S.; de Mendonca, R.; Nonhoff, C.; Deplano, A.; Denis, O. Low occurrence of the new species Staphylococcus argenteus in a Staphylococcus aureus collection of human isolates from Belgium. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Chantratita, N.; Wikraiphat, C.; Tandhavanant, S.; Wongsuvan, G.; Ariyaprasert, P.; Suntornsut, P.; Thaipadungpanit, J.; Teerawattanasook, N.; Jutrakul, Y.; Srisurat, N.; et al. Comparison of community-onset Staphylococcus argenteus and Staphylococcus aureus sepsis in Thailand: A prospective multicentre observational study. Clin. Microbiol. Infect. 2016, 22, 458-e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradigaravand, D.; Jamrozy, D.; Mostowy, R.; Anderson, A.; Nickerson, E.K.; Thaipadungpanit, J.; Wuthiekanun, V.; Limmathurotsakul, D.; Tandhavanant, S.; Wikraiphat, C.; et al. Evolution of the Staphylococcus argenteus ST2250 Clone in Northeastern Thailand Is Linked with the Acquisition of Livestock-Associated Staphylococcal Genes. MBio 2017, 8, e00802-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigaill, J.; Grattard, F.; Grange, S.; Forest, F.; Haddad, E.; Carricajo, A.; Tristan, A.; Laurent, F.; Botelho-Nevers, E.; Verhoeven, P.O. Community-Acquired Staphylococcus argenteus Sequence Type 2250 Bone and Joint Infection, France, 2017. Emerg. Infect. Dis. 2018, 24, 1958–1961. [Google Scholar] [CrossRef] [Green Version]
- Schuster, D.; Rickmeyer, J.; Gajdiss, M.; Thye, T.; Lorenzen, S.; Reif, M.; Josten, M.; Szekat, C.; Melo, L.D.R.; Schmithausen, R.M.; et al. Differentiation of Staphylococcus argenteus (formerly: Staphylococcus aureus clonal complex 75) by mass spectrometry from S. aureus using the first strain isolated from a wild African great ape. Int. J. Med. Microbiol. 2017, 307, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Kubota, H.; Ono, H.K.; Kobayashi, M.; Murauchi, K.; Kato, R.; Hirai, A.; Sadamasu, K. Food poisoning outbreak in Tokyo, Japan caused by Staphylococcus argenteus. Int. J. Food Microbiol. 2017, 262, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Thaipadungpanit, J.; Amornchai, P.; Nickerson, E.K.; Wongsuvan, G.; Wuthiekanun, V.; Limmathurotsakul, D.; Peacock, S.J. Clinical and molecular epidemiology of Staphylococcus argenteus infections in Thailand. J. Clin. Microbiol. 2015, 53, 1005–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monecke, S.; Stieber, B.; Roberts, R.; Akpaka, P.E.; Slickers, P.; Ehricht, R. Population structure of Staphylococcus aureus from Trinidad & Tobago. PLoS ONE 2014, 9, e89120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.F.; Zhi, X.Y.; Zhang, J.; Paoli, G.C.; Cui, Y.; Shi, C.; Shi, X. Preliminary comparative genomics revealed pathogenic potential and international spread of Staphylococcus argenteus. BMC Genom. 2017, 18, 808. [Google Scholar] [CrossRef] [Green Version]
- Dupieux, C.; Blonde, R.; Bouchiat, C.; Meugnier, H.; Bes, M.; Laurent, S.; Vandenesch, F.; Laurent, F.; Tristan, A. Community-acquired infections due to Staphylococcus argenteus lineage isolates harbouring the Panton-Valentine leucocidin, France, 2014. Eurosurveillance 2015, 20, 21154. [Google Scholar] [CrossRef]
- Lebughe, M.; Phaku, P.; Niemann, S.; Mumba, D.; Peters, G.; Muyembe-Tamfum, J.J.; Mellmann, A.; Strauss, L.; Schaumburg, F. The Impact of the Staphylococcus aureus Virulome on Infection in a Developing Country: A Cohort Study. Front. Microbiol. 2017, 8, 1662. [Google Scholar] [CrossRef]
- Rentinck, M.N.; Kruger, R.; Hoppe, P.A.; Humme, D.; Niebank, M.; Pokrywka, A.; Stegemann, M.; Kola, A.; Hanitsch, L.G.; Leistner, R. Skin infections due to Panton-Valentine leukocidin (PVL)-producing S. aureus-Cost effectiveness of outpatient treatment. PLoS ONE 2021, 16, e0253633. [Google Scholar] [CrossRef]
- Holt, D.C.; Holden, M.T.; Tong, S.Y.; Castillo-Ramirez, S.; Clarke, L.; Quail, M.A.; Currie, B.J.; Parkhill, J.; Bentley, S.D.; Feil, E.J.; et al. A very early-branching Staphylococcus aureus lineage lacking the carotenoid pigment staphyloxanthin. Genome Biol. Evol. 2011, 3, 881–895. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.A.; Bartels, M.D.; Hogh, S.V.; Dons, L.E.; Pedersen, M.; Jensen, T.G.; Kemp, M.; Skov, M.N.; Gumpert, H.; Worning, P.; et al. Whole Genome Sequencing of Danish Staphylococcus argenteus Reveals a Genetically Diverse Collection with Clear Separation from Staphylococcus aureus. Front. Microbiol. 2017, 8, 1512. [Google Scholar] [CrossRef]
- Okuda, K.V.; Toepfner, N.; Alabi, A.S.; Arnold, B.; Belard, S.; Falke, U.; Menschner, L.; Monecke, S.; Ruppelt-Lorz, A.; Berner, R. Molecular epidemiology of Staphylococcus aureus from Lambarene, Gabon. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1963–1973. [Google Scholar] [CrossRef]
- Shittu, A.O.; Mellmann, A.; Schaumburg, F. Molecular characterization of Staphylococcus aureus complex from fomites in Nigeria. Infect. Genet. Evol. 2020, 85, 104504. [Google Scholar] [CrossRef]
- Held, J.; Gmeiner, M.; Mordmuller, B.; Matsiegui, P.B.; Schaer, J.; Eckerle, I.; Weber, N.; Matuschewski, K.; Bletz, S.; Schaumburg, F. Bats are rare reservoirs of Staphylococcus aureus complex in Gabon. Infect. Genet. Evol. 2017, 47, 118–120. [Google Scholar] [CrossRef]
- Ngoa, U.A.; Schaumburg, F.; Adegnika, A.A.; Kosters, K.; Moller, T.; Gaus, E.; Fernandes, J.F.; Alabi, A.; Issifou, S.; Becker, K.; et al. Epidemiology and population structure of Staphylococcus aureus in various population groups from a rural and semi urban area in Gabon, Central Africa. Acta Trop. 2012, 124, 42–47. [Google Scholar] [CrossRef]
- Monecke, S.; Gavier-Widen, D.; Hotzel, H.; Peters, M.; Guenther, S.; Lazaris, A.; Loncaric, I.; Muller, E.; Reissig, A.; Ruppelt-Lorz, A.; et al. Diversity of Staphylococcus aureus Isolates in European Wildlife. PLoS ONE 2016, 11, e0168433. [Google Scholar] [CrossRef] [Green Version]
- Shittu, A.O.; Taiwo, F.F.; Frobose, N.J.; Schwartbeck, B.; Niemann, S.; Mellmann, A.; Schaumburg, F. Genomic analysis of Staphylococcus aureus from the West African Dwarf (WAD) goat in Nigeria. Antimicrob. Resist. Infect. Control 2021, 10, 122. [Google Scholar] [CrossRef]
- Strobel, M.; Pfortner, H.; Tuchscherr, L.; Volker, U.; Schmidt, F.; Kramko, N.; Schnittler, H.J.; Fraunholz, M.J.; Loffler, B.; Peters, G.; et al. Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain. Clin. Microbiol. Infect. 2016, 22, 799–809. [Google Scholar] [CrossRef] [Green Version]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.F.; Xu, X.; Song, Q.; Bai, Y.; Zhang, Y.; Song, M.; Shi, C.; Shi, X. Identification of Staphylococcus argenteus in Eastern China based on a nonribosomal peptide synthetase (NRPS) gene. Future Microbiol. 2016, 11, 1113–1121. [Google Scholar] [CrossRef]
- O’Hara, F.P.; Suaya, J.A.; Ray, G.T.; Baxter, R.; Brown, M.L.; Mera, R.M.; Close, N.M.; Thomas, E.; Amrine-Madsen, H. spa Typing and Multilocus Sequence Typing Show Comparable Performance in a Macroepidemiologic Study of Staphylococcus aureus in the United States. Microb. Drug Resist. 2016, 22, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Schaumburg, F.; Pauly, M.; Schubert, G.; Shittu, A.; Tong, S.; Leendertz, F.; Peters, G.; Becker, K. Characterization of a novel thermostable nuclease homolog (NucM) in a highly divergent Staphylococcus aureus clade. J. Clin. Microbiol. 2014, 52, 4036–4038. [Google Scholar] [CrossRef] [Green Version]
- Pickering, A.C.; Yebra, G.; Gong, X.; Goncheva, M.I.; Wee, B.A.; MacFadyen, A.C.; Muehlbauer, L.F.; Alves, J.; Cartwright, R.A.; Paterson, G.K.; et al. Evolutionary and Functional Analysis of Coagulase Positivity among the Staphylococci. mSphere 2021, 6, e0038121. [Google Scholar] [CrossRef]
- Guidry, A.J.; Oliver, S.P.; Squiggins, K.E.; Erbe, E.F.; Dowlen, H.H.; Hambleton, C.N.; Berning, L.M. Effect of anticapsular antibodies on neutrophil phagocytosis of Staphylococcus aureus. J. Dairy Sci. 1991, 74, 3360–3369. [Google Scholar] [CrossRef]
- Schaumburg, F.; Alabi, A.S.; Kock, R.; Mellmann, A.; Kremsner, P.G.; Boesch, C.; Becker, K.; Leendertz, F.H.; Peters, G. Highly divergent Staphylococcus aureus isolates from African non-human primates. Environ. Microbiol. Rep. 2012, 4, 141–146. [Google Scholar] [CrossRef]
- Schaumburg, F.; Kock, R.; Friedrich, A.W.; Soulanoudjingar, S.; Ngoa, U.A.; von Eiff, C.; Issifou, S.; Kremsner, P.G.; Herrmann, M.; Peters, G.; et al. Population structure of Staphylococcus aureus from remote African Babongo Pygmies. PLoS Negl. Trop. Dis. 2011, 5, e1150. [Google Scholar] [CrossRef] [Green Version]
- Schaumburg, F.; Ngoa, U.A.; Kosters, K.; Kock, R.; Adegnika, A.A.; Kremsner, P.G.; Lell, B.; Peters, G.; Mellmann, A.; Becker, K. Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin. Microbiol. Infect. 2011, 17, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Boyer, L.; Doye, A.; Rolando, M.; Flatau, G.; Munro, P.; Gounon, P.; Clement, R.; Pulcini, C.; Popoff, M.R.; Mettouchi, A.; et al. Induction of transient macroapertures in endothelial cells through RhoA inhibition by Staphylococcus aureus factors. J. Cell Biol. 2006, 173, 809–819. [Google Scholar] [CrossRef]
- Mariutti, R.B.; Souza, T.A.; Ullah, A.; Caruso, I.P.; de Moraes, F.R.; Zanphorlin, L.M.; Tartaglia, N.R.; Seyffert, N.; Azevedo, V.A.; Le Loir, Y.; et al. Crystal structure of Staphylococcus aureus exfoliative toxin D-like protein: Structural basis for the high specificity of exfoliative toxins. Biochem. Biophys. Res. Commun. 2015, 467, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Rautelin, H.; Kaden, R. Staphylococcus argenteus and Staphylococcus schweitzeri are cytotoxic to human cells in vitro due to high expression of alpha-hemolysin Hla. Virulence 2019, 10, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz-Linek, U.; Hook, M.; Potts, J.R. The molecular basis of fibronectin-mediated bacterial adherence to host cells. Mol. Microbiol. 2004, 52, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and let die. Front. Cell Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldovan, A.; Fraunholz, M.J. In or out: Phagosomal escape of Staphylococcus aureus. Cell Microbiol. 2019, 21, e12997. [Google Scholar] [CrossRef] [Green Version]
- Stelzner, K.; Boyny, A.; Hertlein, T.; Sroka, A.; Moldovan, A.; Paprotka, K.; Kessie, D.; Mehling, H.; Potempa, J.; Ohlsen, K.; et al. Intracellular Staphylococcus aureus employs the cysteine protease staphopain A to induce host cell death in epithelial cells. PLoS Pathog. 2021, 17, e1009874. [Google Scholar] [CrossRef] [PubMed]
- Ruffing, U.; Alabi, A.; Kazimoto, T.; Vubil, D.C.; Akulenko, R.; Abdulla, S.; Alonso, P.; Bischoff, M.; Germann, A.; Grobusch, M.P.; et al. Community-Associated Staphylococcus aureus from Sub-Saharan Africa and Germany: A Cross-Sectional Geographic Correlation Study. Sci. Rep. 2017, 7, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choby, J.E.; Buechi, H.B.; Farrand, A.J.; Skaar, E.P.; Barber, M.F. Molecular Basis for the Evolution of Species-Specific Hemoglobin Capture by Staphylococcus aureus. MBio 2018, 9, e01524-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohmer, C.; Wolz, C. The Role of hlb-Converting Bacteriophages in Staphylococcus aureus Host Adaption. Microb. Physiol. 2021, 31, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Van Wamel, W.J.; Rooijakkers, S.H.; Ruyken, M.; van Kessel, K.P.; van Strijp, J.A. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senghore, M.; Bayliss, S.C.; Kwambana-Adams, B.A.; Foster-Nyarko, E.; Manneh, J.; Dione, M.; Badji, H.; Ebruke, C.; Doughty, E.L.; Thorpe, H.A.; et al. Transmission of Staphylococcus aureus from Humans to Green Monkeys in The Gambia as Revealed by Whole-Genome Sequencing. Appl. Environ. Microbiol. 2016, 82, 5910–5917. [Google Scholar] [CrossRef] [Green Version]
- Loffler, B.; Hussain, M.; Grundmeier, M.; Bruck, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef]
- Perelman, S.S.; James, D.B.A.; Boguslawski, K.M.; Nelson, C.W.; Ilmain, J.K.; Zwack, E.E.; Prescott, R.A.; Mohamed, A.; Tam, K.; Chan, R.; et al. Genetic variation of staphylococcal LukAB toxin determines receptor tropism. Nat. Microbiol. 2021, 6, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lopez, E.A.; Rivera, G.; Cruz-Hernandez, M.A.; Martinez-Vazquez, A.V.; Castro-Escarpulli, G.; Flores-Magallon, R.; Vazquez, K.; Cruz-Pulido, W.L.; Bocanegra-Garcia, V. Identification and Characterization of the CRISPR/Cas System in Staphylococcus aureus Strains From Diverse Sources. Front. Microbiol. 2021, 12, 656996. [Google Scholar] [CrossRef] [PubMed]



| Knowledge Gap | Research Agenda |
|---|---|
| Epidemiology | To understand the geographic dispersal of S. schweitzeri, surveillance studies should be performed. These should include:
|
| Horizontal gene transfer | The acquisition of genes by horizontal gene transfer can improve the adaptation of S. schweitzeri to humans and can also lead to antimicrobial resistance. It should be investigated whether gene transfer of resistance genes or virulence factors, e.g., via bacteriophages, from S. aureus to S. schweitzeri is possible. |
| Pathogenicity in vitro | In vitro experiments on the interaction of S. schweitzeri with immune cells as well as on the survival of the pathogens in whole blood (from humans, monkeys, bats) could provide information on the adaptation of S. schweitzeri to its natural hosts. Neutrophil transmigration under the influence of S. schweitzeri isolates could also be investigated to obtain information on neutrophil recruitment. |
| Pathogenicity in vivo | Pathogenicity should be tested in animal models to determine whether S. schweitzeri can cause infections. Special attention should be paid to the model; humanized mouse models, bats and monkeys appear adequate. |
| Capacity building | In order to gain accurate knowledge about the prevalence and distribution of S. schweitzeri, but also of the other members of the S. aureus complex, it is necessary to familiarise scientists in Africa with the subject and train them to distinguish between the staphylococci. The necessary scientific equipment for this must also be available in laboratories in Africa. |
| Improved diagnostics | Culture-based detection and confirmation are needed to improve the species identification of S. schweitzeri in resource-limited settings. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akoua-Koffi, C.; Kacou N’Douba, A.; Djaman, J.A.; Herrmann, M.; Schaumburg, F.; Niemann, S. Staphylococcus schweitzeri—An Emerging One Health Pathogen? Microorganisms 2022, 10, 770. https://doi.org/10.3390/microorganisms10040770
Akoua-Koffi C, Kacou N’Douba A, Djaman JA, Herrmann M, Schaumburg F, Niemann S. Staphylococcus schweitzeri—An Emerging One Health Pathogen? Microorganisms. 2022; 10(4):770. https://doi.org/10.3390/microorganisms10040770
Chicago/Turabian StyleAkoua-Koffi, Chantal, Adèle Kacou N’Douba, Joseph Allico Djaman, Mathias Herrmann, Frieder Schaumburg, and Silke Niemann. 2022. "Staphylococcus schweitzeri—An Emerging One Health Pathogen?" Microorganisms 10, no. 4: 770. https://doi.org/10.3390/microorganisms10040770
APA StyleAkoua-Koffi, C., Kacou N’Douba, A., Djaman, J. A., Herrmann, M., Schaumburg, F., & Niemann, S. (2022). Staphylococcus schweitzeri—An Emerging One Health Pathogen? Microorganisms, 10(4), 770. https://doi.org/10.3390/microorganisms10040770






