Effects of Mercury Contamination on Microbial Diversity of Different Kinds of Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Setup
2.2. Extraction, Sequencing and Processing of Soil Microbial DNA
2.3. Data Statistics and Analysis
3. Results
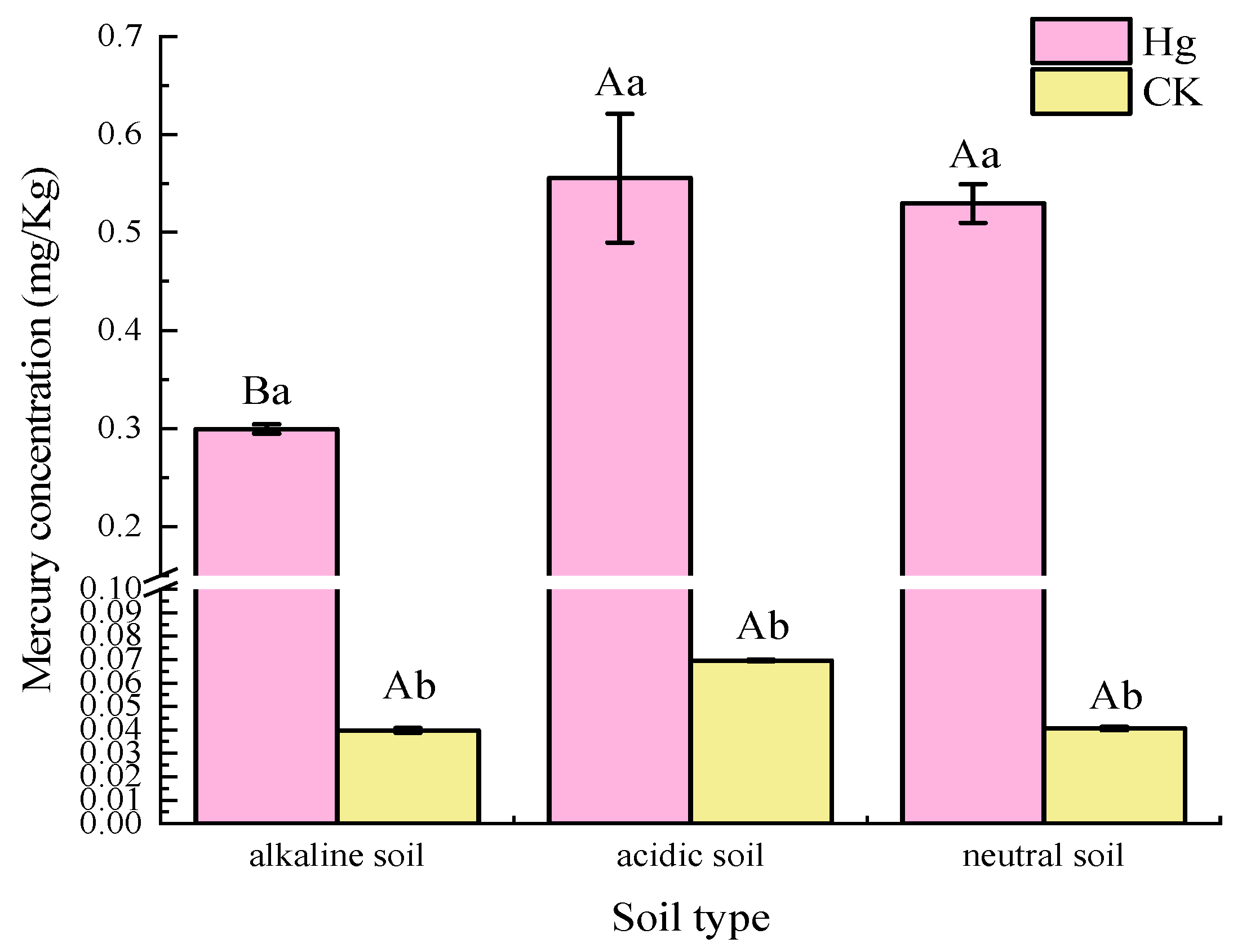
3.1. Differences in Soil Properties
3.2. The Impact of Mercury Pollution on the Diversity of Soil Bacteria and Fungi with Different Properties
3.3. The Impact of Mercury Pollution on the Composition of Soil Microbial Communities with Different Properties
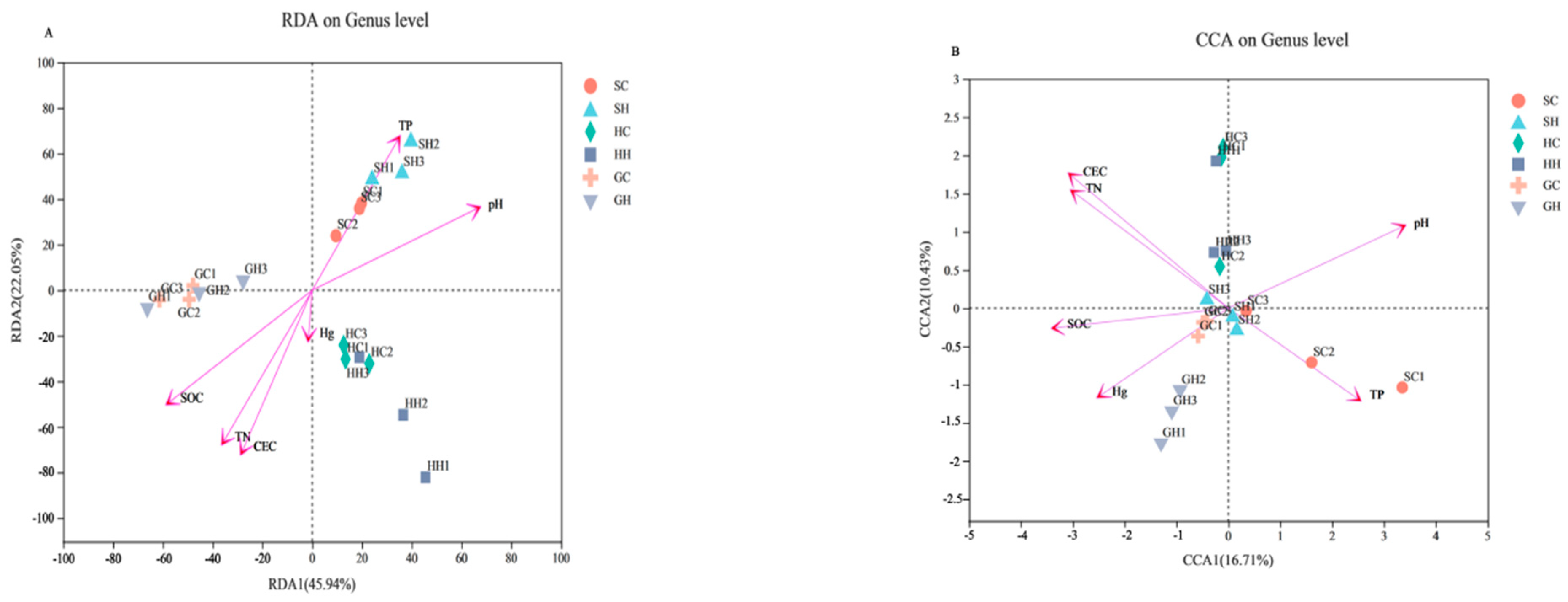
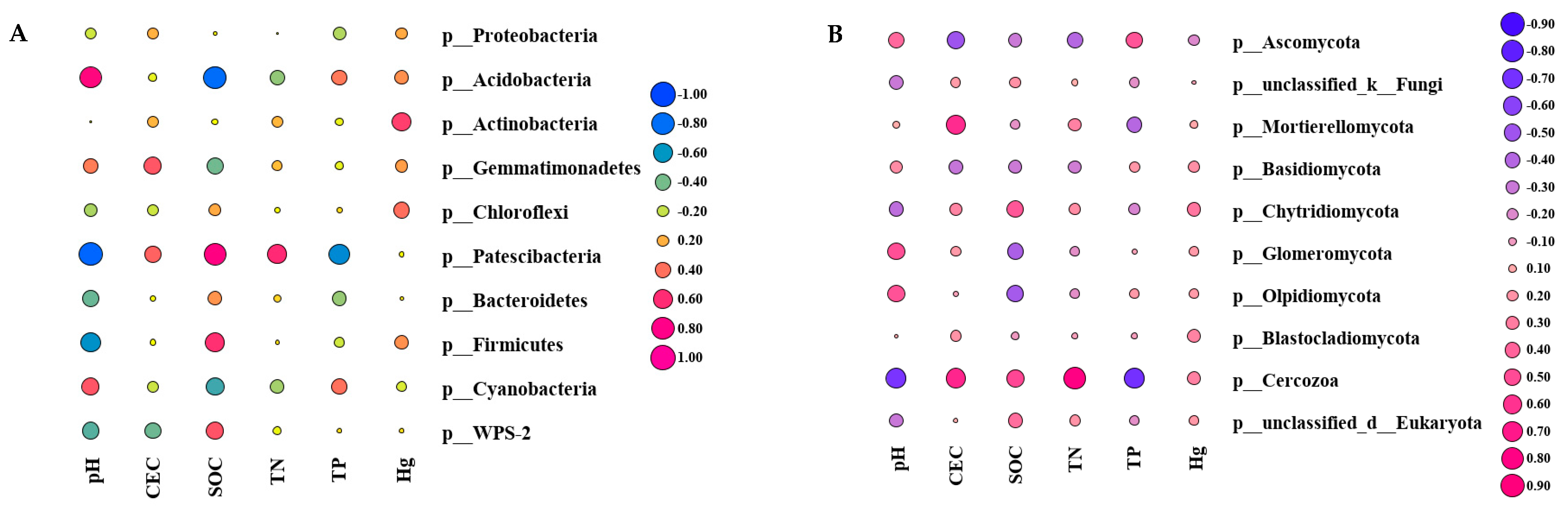
3.4. The Relationship between Soil Environmental Factors and Microorganisms
4. Discussion
4.1. Response of Soil Bacterial Diversity and Community Structure to Mercury Pollution
4.2. Response of Soil Fungal Diversity and Community Structure to Mercury Pollution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, A.B.; Zevenhoven, R.; Brodersen, J.; Hylander, L.D.; Bhattacharya, P. Mercury in waste in the European Union: Sources, disposal methods and risks. Resour. Conserv. Recycl. 2004, 42, 155–182. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Wang, D.X.; Zhu, L.N.; Lan, Y.L.; Cheng, M.; Zhang, L.M.; Chu, J.Q.; Li, X.Z.; Kong, D.M. Dinuclear HgII tetracarbene complex-triggered aggregation-induced emission for rapid and selective sensing of Hg2+ and organomercury species. Chem. Sci. 2019, 10, 4220–4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoo, E.M.; Valente, J.G.; Grattan, L.; Schmidt, S.L.; Platt, I.; Silbergeld, E.K. Low level methylmercury exposure affects neuropsychological function in adults. Environ. Health 2003, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Jiang, X.; Zhao, S.; Zheng, X.; Lan, J.; Wang, H.; Ng, T.B. A polysaccharide-peptide with mercury clearance activity from dried fruiting bodies of maitake mushroom Grifola frondosa. Sci. Rep. 2018, 8, 17630–17639. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yuan, J.; Sonke, J.E.; Zhang, Y.; Zhang, T.; Zheng, W.; Chen, S.; Meng, M.; Chen, J.; Liu, Y.; et al. Methylmercury produced in upper oceans accumulates in deep Mariana Trench fauna. Nat. Commun. 2020, 11, 3389. [Google Scholar] [CrossRef]
- Yeh, M.-J.; Yuan, C.-S.; Hung, K.-N.; Ie, I.-R.; Lee, C.-E.; Chiang, K.-C.; Soong, K.-Y. Temporal variation and potential origins of atmospheric speciated mercury at a remote island in South China Sea based on two-year field measurement data. Sci. Rep. 2021, 11, 5678. [Google Scholar] [CrossRef]
- Meng, D.; Wang, N.; Ai, J.C.; Zhang, G.; Liu, X.J. Distribution and assessment of residual mercury from gold mining in Changbai Mountain Range Northeastern China. IOP Conf. Ser. Earth Environ. Sci. 2016, 39, 12007. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.C.; Wang, L.A.; Ding, S.M. The absorption condition of mercury in mercury-contaminated soils by opuntia stricta. Fresenius Environ. Bull. 2018, 27, 3439–3443. [Google Scholar]
- Pignataro, A.; Moscatelli, M.C.; Mocali, S.; Grego, S.; Benedetti, A. Assessment of soil microbial functional diversity in a coppiced forest system. Appl. Soil Ecol. A Sect. Agric. Ecosyst. Environ. 2012, 62, 115–123. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Drenkhan, R.; Pritsch, K.; Buegger, F.; Padari, A.; Hagh-Doust, N.; Mikryukov, V.; Gohar, D.; et al. Regional-Scale In-Depth Analysis of Soil Fungal Diversity Reveals Strong pH and Plant Species Effects in Northern Europe. Front. Microbiol. 2020, 11, 1953. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I. Fluorescent Pseudomonas -FAP2 and Bacillus licheniformis interact positively in biofilm mode enhancing plant growth and photosynthetic attributes. Sci. Rep. 2019, 9, 4547. [Google Scholar] [CrossRef]
- Jiao, S.; Xu, Y.; Zhang, J.; Hao, X.; Lu, Y. Core Microbiota in Agricultural Soils and Their Potential Associations with Nutrient Cycling. mSystems 2019, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, S.; Worrich, A.; Banitz, T.; Harms, H.; Kästner, M.; Miltner, A.; Wick, L.Y.; Frank, K.; Thullner, M.; Centler, F. Functional Resistance to Recurrent Spatially Heterogeneous Disturbances Is Facilitated by Increased Activity of Surviving Bacteria in a Virtual Ecosystem. Front. Microbiol. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgari Lajayer, B.; Ghorbanpour, M.; Nikabadi, S. Heavy metals in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Frey, B.; Rieder, S.R. Biochemistry: Response of forest soil bacterial communities to mercury chloride application. Soil Biol. Biochem. 2013, 65, 329–337. [Google Scholar] [CrossRef]
- Liu, Y.R.; Wang, J.J.; Zheng, Y.M.; Zhang, L.M.; He, J.Z.J.M.E. Patterns of Bacterial Diversity Along a Long-Term Mercury-Contaminated Gradient in the Paddy Soils. Microb. Ecol. 2014, 68, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Zhang, H.; Yu, S.; Chen, C.; Huang, C. Effects of Cadmium and Mercury alone and in Combination on the Soil Microbial Community Structural Diversity. In Molecular Environmental Soil Science at the Interfaces in the Earth’s Critical Zone; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Xie, X.; Liao, M.; Ma, A.; Zhang, H. Effects of contamination of single and combined cadmium and mercury on the soil microbial community structural diversity and functional diversity. Chin. J. Geochem. 2011, 30, 366–374. [Google Scholar] [CrossRef]
- Frossard, A.; Hartmann, M.; Frey, B. Tolerance of the forest soil microbiome to increasing mercury concentrations. Soil Biol. Biochem. 2017, 105, 162–176. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, Y.; Bararunyeretse, P.; Li, H. Characterization of microbial communities of soils from gold mine tailings and identification of mercury-resistant strain. Ecotoxicol. Environ. Saf. 2018, 165, 182–193. [Google Scholar] [CrossRef]
- Hu, H.; Li, M.; Wang, G.; Drosos, M.; Li, Z.; Hu, Z.; Xi, B. Water-soluble mercury induced by organic amendments affected microbial community assemblage in mercury-polluted paddy soil. Chemosphere 2019, 236, 124405. [Google Scholar] [CrossRef]
- Frossard, A.; Donhauser, J.; Mestrot, A.; Gygax, S.; Bååth, E.; Frey, B. Long- and short-term effects of mercury pollution on the soil microbiome. Soil Biol. Biochem. 2018, 120, 191–199. [Google Scholar] [CrossRef]
- Rajapaksha, R.M.C.P.; Tobor-KapŁOn, M.A.; Baath, E. Metal Toxicity Affects Fungal and Bacterial Activities in Soil Differently. Appl. Environ. Microbiol. 2004, 70, 2966–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, S.R.; Brunner, I.; Daniel, O.; Liu, B.; Frey, B. Methylation of mercury in earthworms and the effect of mercury on the associated bacterial communities. PLoS ONE 2013, 8, e61215. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557–558, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef]
- Dash, H.R.; Das, S. Bioremediation of mercury and the importance of bacterial mer genes. Int. Biodeterior. Biodegrad. 2012, 75, 207–213. [Google Scholar] [CrossRef]
- Li, B.I.; Jizheng, H.E.; Zhang, L.; Liu, Y. Microbial transformations of mercury in the environment. Environ. Chem. 2018, 37, 2358–2367. [Google Scholar]
- Yang, J.; Zhu, W.; Qu, W.; Yang, Z.; Wang, J.; Zhang, M.; Li, H. Selenium Functionalized Metal–Organic Framework MIL-101 for Efficient and Permanent Sequestration of Mercury. Environ. Sci. Technol. 2019, 53, 2260–2268. [Google Scholar] [CrossRef]
- Yao, X.-f.; Zhang, J.-m.; Tian, L.; Guo, J.-h. The effect of heavy metal contamination on the bacterial community structure at Jiaozhou Bay, China. Braz. J. Microbiol. 2017, 48, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Mesa, J.; Mateos-Naranjo, E.; Pajuelo, E.; Caviedes, M.Á.; Rodríguez-Llorente, I.D. Heavy Metal Pollution Structures Soil Bacterial Community Dynamics in SW Spain Polluted Salt Marshes. Water Air Soil Pollut. 2016, 227, 466. [Google Scholar] [CrossRef]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of Heavy Metals Pollution on Soil Microbial Diversity and Bermudagrass Genetic Variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.-a.; Zhan, X.; Huang, Y.; Wang, J.; Wang, X. Response mechanism of microbial community to the environmental stress caused by the different mercury concentration in soils. Ecotoxicol. Environ. Saf. 2020, 188, 109906. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Soil Agrochemcial Analysis Method; China Agriculture Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reith, F.; Dennis, P.G.; Hamonts, K.; Powell, J.R.; Young, A.; Brajesh, K.S.; Bissett, A. Ecological drivers of soil microbial diversity and soil biological networks in the Southern Hemisphere. Ecology 2018, 99, 583–596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhang, Q.; Fan, M.; Liu, X. Comparison of Bacterial Diversity in Forest Soils of Hippophae rhamnoides Growing in Two Different Sites. J. Northwest For. Univ. 2020, 35, 32–39. [Google Scholar]
- Ma, Y.; Wang, Y.; Chen, Q.; Li, Y.; Guo, D.; Nie, X.; Peng, X. Assessment of heavy metal pollution and the effect on bacterial community in acidic and neutral soils. Ecol. Indic. 2020, 117, 106626. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, Y.; Chen, H.; Hu, Y. Response of Bacterial and Fungal Soil Communities to Chinese Fir (Cunninghamia lanceolate) Long-Term Monoculture Plantations. Front. Microbiol. 2020, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, C.; Yu, W.; Turak, A.; Chen, D.; Huang, Y.; Ao, J.; Jiang, Y.; Huang, Z. Effects of Nitrogen and Phosphorus Inputs on Soil Bacterial Abundance, Diversity, and Community Composition in Chinese Fir Plantations. Front. Microbiol. 2018, 9, 1543. [Google Scholar] [CrossRef]
- Niu, S.; Ren, L.; Song, L.; Duan, Y.; Huang, T.; Han, X.; Hao, W. Plant stoichiometry characteristics and relationships with soil nutrients in Robinia pseudoacacia communities of different planting ages. Acta Ecol. Sin. 2017, 37, 355–362. [Google Scholar] [CrossRef]
- Wang, Y.; Osman, J.R.; DuBow, M.S. Bacterial Communities on the Surface of the Mineral Sandy Soil from the Desert of Maine (USA). Curr. Microbiol. 2020, 77, 1429–1437. [Google Scholar] [CrossRef]
- Wei, H.; Chen, X.; He, J.; Zhang, J.; Shen, W. Exogenous Nitrogen Addition Reduced the Temperature Sensitivity of Microbial Respiration without Altering the Microbial Community Composition. Front. Microbiol. 2017, 8, 2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongdong, H.; Zhenyu, H.; Guanghai, G.; Yingying, W. Ecological function of oligotrophic bacteria and their Applications in the environment. Microbiol. China 2012, 39, 526–535. [Google Scholar]
- Almås, Å.R.; Mulder, J.; Bakken, L.R. Trace Metal Exposure of Soil Bacteria Depends on Their Position in the Soil Matrix. Environ. Sci. Technol. 2005, 39, 5927–5932. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Smolders, E. Background zinc concentrations in soil affect the zinc sensitivity of soil microbial processes-a rationale for a metalloregion approach to risk assessments. Environ. Toxicol. Chem. 2001, 20, 2639–2643. [Google Scholar] [CrossRef]
- Mertens, J.; Springael, D.; De Troyer, I.; Cheyns, K.; Wattiau, P.; Smolders, E. Long-term exposure to elevated zinc concentrations induced structural changes and zinc tolerance of the nitrifying community in soil. Environ. Microbiol. 2006, 8, 2170–2178. [Google Scholar] [CrossRef]
- Baaaath, E. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, T.; Yang, X. Study on assessment methods of heavy metal pollution in river sediments. J. Hefei Univ. Technol. 2005, 28, 1419–1423. [Google Scholar]
- Rieder, S.R.; Frey, B. Methyl-mercury affects microbial activity and biomass, bacterial community structure but rarely the fungal community structure. Soil Biol. Biochem. 2013, 64, 164–173. [Google Scholar] [CrossRef]
- Schneider, A.R.; Gommeaux, M.; Duclercq, J.; Fanin, N.; Conreux, A.; Alahmad, A.; Lacoux, J.; Roger, D.; Spicher, F.; Ponthieu, M.; et al. Response of bacterial communities to Pb smelter pollution in contrasting soils. Sci. Total Environ. 2017, 605–606, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Fritze, H.; Pennanen, T.; Kitunen, V. Characterization of dissolved organic carbon from burned humus and its effects on microbial activity and community structure. Soil Biol. Biochem. 1998, 30, 687–693. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, Y.; Hu, Y.; Shi, H. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol. Environ. Saf. 2019, 180, 557–564. [Google Scholar] [CrossRef]
- Schuster, E. The behavior of mercury in the soil with special emphasis on complexation and adsorption processes—A review of the literature. Water Air Soil Pollut. 1991, 56, 667–680. [Google Scholar] [CrossRef]





| Chemical Properties | ||||||
|---|---|---|---|---|---|---|
| Soil Types | Treatments | pH | CEC | SOC | TN | TP |
| Alkaline soil | SH | 8.347 ± 0.088Aa | 10.520 ± 0.058Ca | 13.373 ± 0.204Ca | 0.989 ± 0.039Ba | 0.931 ± 0.024Aa |
| SC | 8.320 ± 0.153Aa | 10.533 ± 0.233Ca | 14.510 ± 0.196Ca | 1.000 ± 0.0377Ba | 0.893 ± 0.043Aa | |
| Acidic soil | GH | 4.950 ± 0.153Ca | 19.213 ± 0.095Ba | 44.970 ± 0.950Aa | 1.927 ± 0.0241Aa | 0.563 ± 0.021Ba |
| GC | 4.880 ± 0.116Db | 19.247 ± 0.419Ba | 44.937 ± 0.825Aa | 2.030 ± 0.0.022Aa | 0.550 ± 0.018Ba | |
| Neutral soil | HH | 6.783 ± 0.088Ba | 20.677 ± 0.514Aa | 33.890 ± 0.215Ba | 2.001 ± 0.014Aa | 0.524 ± 0.002Ba |
| HC | 6.813 ± 0.088Ba | 20.270 ± 0.158Aa | 33.667 ± 0.665Ba | 2.009 ± 0.044Aa | 0.572 ± 0.012Ba | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Cao, H.; Liu, B.; Zhang, M.; Zhang, C.; Chen, P.; Yang, B. Effects of Mercury Contamination on Microbial Diversity of Different Kinds of Soil. Microorganisms 2022, 10, 977. https://doi.org/10.3390/microorganisms10050977
Zheng X, Cao H, Liu B, Zhang M, Zhang C, Chen P, Yang B. Effects of Mercury Contamination on Microbial Diversity of Different Kinds of Soil. Microorganisms. 2022; 10(5):977. https://doi.org/10.3390/microorganisms10050977
Chicago/Turabian StyleZheng, Xiangqun, Haoyu Cao, Bo Liu, Man Zhang, Chunxue Zhang, Peizhen Chen, and Bo Yang. 2022. "Effects of Mercury Contamination on Microbial Diversity of Different Kinds of Soil" Microorganisms 10, no. 5: 977. https://doi.org/10.3390/microorganisms10050977





